Abstract
Background The burden on social and economic development caused by cirrhosis, a common terminal pathological process in most liver diseases, is substantial. The liver, being the outstanding immune organ in the body, thus emphasizing the critical role of immune cells in the pathogenesis and progression of cirrhosis.However, there is a paucity of studies investigating the correlation between immunophenotype and cirrhosis. The Mendelian randomization(MR) study was employed to explore relationships between these factors, aiming to provide new directions and insights for clinical research. Methods The genetic data associated with cirrhosis from genome-wide association studies (GWAS) were obtained from the FinnGen R10 database.Inverse variance weighting (IVW) completing with MR-Egger, weighted mode, simple mode, and weighted median were adopted to enhance the robustness of our analysis. Finally, the potential association between immunophenotypes and cirrhosis was clarified. Results Fourteen immunophenotypes closely associated with cirrhosis were identified. Specifically, there were four distinct types of B cells, seven distinct types of Myeloid cells, one classical dendritic cell (cDC), CCR2 on CD62L + myeloid DC; one type of Treg cell, CD39 on CD39 + secreting Treg; one type of TBNK cells, SSC-A on NK. The robustness of the MR Study was further confirmed through comprehensive evaluations of pleiotropy and heterogeneity. Conclusion The MR study is the pioneering effort to offer informative and comprehensive data supporting the association between immunophenotypes and cirrhosis. These findings have significant implications for tailoring individualized treatment strategies, optimizing cirrhosis management, and enhancing overall survival.
Similar content being viewed by others
Introduction
Cirrhosis represents the final stage in the progression of chronic liver disease, characterized by diffuse liver fibrosis, pseudolobular formation, regenerative nodules, and intrahepatic vessel proliferation resulting from chronic liver inflammation1. The main clinical manifestations included compensatory symptoms such as abdominal pain, anorexia, nausea and fatigue; as well as decompensated manifestations including hepatomegaly, splenomegaly, jaundice, ascites, portal hypertension, spider nevus formation and palmar erythema2. The mortality rate of cirrhosis is approximately 1 million per year, accounting for 2% of global deaths, exerting a significant impact on patients’ daily lives and imposing a substantial burden on social and economic development3. Currently, the primary approach to managing cirrhosis involves addressing underlying factors, safeguarding liver function, and providing symptomatic and supportive care. Liver transplantation represents the most efficacious treatment for this condition; however, its widespread adoption is hindered by objective factors such as donor scarcity, high costs, and immune rejection4. Consequently, strategies targeting early screening and diagnosis of cirrhosis can significantly alleviate symptoms, mitigate complications, and reduce mortality rates5.
The liver is strategically positioned not only to receive the dual blood supply via both the portal vein and hepatic artery, but also to facilitate bidirectional communication between the liver and gut through the bile duct, as well as portal and systemic pathways6. The role of inflammation is pivotal in the progression of cirrhosis, with immune cells releasing inflammatory cytokines that serve as crucial mediators in facilitating communication between the liver and the immune system7. Cirrhosis is commonly associated with immune alterations, known as cirrhosis-related immune dysfunction (CAID), which include impaired neutrophil phagocytosis function, reduced natural killer cell activity, expansion of TNF-producing monocytes, and elevated levels of circulating proinflammatory cytokines8. Interactions between the immune system and the liver can potentially impact liver homeostasis and contribute to disease.The current body of evidence, however, heavily relies on observational studies, which are prone to limitations in sample size, flawed study design, subjective inclination and confounding factors9. As a result, obtaining an exact association between immune cells and cirrhosis remains challenging.
The use of genetic variants strongly associated with exposure factors as instrumental variables (IVs) in MR study allows for inference of relationship between exposure factors and outcomes, resembling randomized controlled study (RCT)10,11. The challenges of expensive, time-consuming, unsatisfactory compliance, and ethical limitations associated with RCTs can be effectively addressed through the utilization of MR techniques, enabling the establishment of relationship between exposures and outcome from observational data12,13,14. The MR study, in contrast to traditional observational studies, follows the principles of Mendel’s second law by employing random assignment processes and prioritizing genetic mutations preceding disease phenotype15,16,17. This design effectively attenuates the influence of confounding variables on the analysis and eliminates concerns regarding reverse causality. In this investigation, we aimed to investigate the relationship between immunophenotypes and cirrhosis using a two-sample MR analysis.
Materials and methods
Study design
The relationship between 731 immunophenotypes in seven groups and cirrhosis was evaluated using two-sample MR analysis in our study (Table S1). Quality control procedures was employed to select immunophenotypes-related SNPs as appropriate instrumental variables (IVs), immunophenotypes poist as exposure, and cirrhosis function as outcome18,19.
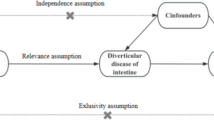
The MR study must satisfy three key assumptions for ensuring the reliability of results: (1) Strong association between IVs and immunophenotypes; (2) The association between IVs and potential confounders was not observed; and (3) IVs don’t affect cirrhosis via alternative pathways other than exposure to immunophenotypes15,20. A comprehensive illustration of the relationship between immunophenotypes and cirrhosis in our MR study (Fig. 1).
Data sources regarding exposure and outcome
We gathered GWAS summary statistics for SNP-related immunophenotypes from the GWAS Catalog (Accession Number range from GCST0001391 to GCST0002121).A total of 731 immunophenotypes, encompassing various cell categories such as B cells, CDCs, TBNK cells, myeloid cells, and Treg cells. These phenotypes were distinguished based on relative count (RC), absolute count (AC), morphological parameters (MP), and median fluorescence intensity (MFI) (Table S4)21,22,23. The data regarding cirrhosis in the GWAS statistics was acquired from the FinnGen R10 database (https://storage.googleapis.com/finngen-public-data-r10/summary_stats/finngen_R10_CIRRHOSIS_BROAD.gz). This analysis emcompassed 412,181 individuals from 4,380 Cirrhosis cases and 407,801 controls.
Selection of IVs
In accordance with recent research, we selected appropriate IVs, which exhibited a statistically significant association associated with immunophenotypes via quality control procedure (p < 1 × 10−5)21. The screening criterion for P values was adjusted from P < 5 × 10−8 days to P < 1 × 10−5pure due to the limited number of SNPs meeting the P < 5 × 10−8 substitution criterion, which was insufficient to provide adequate support for subsequent studies20. The linkage Disequilibrium (LD), serving as a non-random association between alleles of different loci, is evaluated via two parameters, r2 and kb.The value of r2 spans a range from 0 to 1, and the smaller value of r2, implying a higher degree of proximity to complete linkage equilibrium among SNPs, concomitant with a fully random allocation14. The SNPs were independently aggregated based on the European 1000 Genome reference panel, with a condition of r2 < 0.001 and a clump distance > 10,000 kb.
Additionally, the F-statistics were calculated to ensure a robust association between the IVs and exposure factors. Weak IVs(F < 10) were excluded in order to mitigate potential bias20. Then, PhenoScannerV2 database(http://www.phenoscanner.medschl.cam.ac.uk/) was utilized to exclude confounding determinants or risks that may influence the outcomes or other liver disease.Ultimately, SNPs meeting the criteria for cirrhosis were identified (Table S3).
MR statistical analysis and quality assessment
The objective of MR statistical analysis is to yield dependable results and investigate the relationship between immunophenotypes and cirrhosis.We performed statistical analyses using R software (version 4.2.2) and further refined the results with the TwosampleMR software package (version 0.56).IVW completing with simple mode, MR-Egger, weighted mode, and weighted median were adopted to enhance the robustness of our analysis10,23. The IVW method employs the Wald ratio approach to initially establish a correlation between individual SNPs.It then selects either the fixed effect model or the random effect model to analyze the impact on multiple sites, ensuring the provision of compelling estimation and remains the determinant analytical technique for MR investigation (P < 0.05)24. The findings are presented as odds ratios (ORs) with 95% confidence intervals (CIs) greater than 1 were indicating a positive association with an increased risk, while those less than 1 were associated with a protective effect.
We conducted a comprehensive analysis of heterogeneity using Cochran’s Q test to evaluate the presence of heterogeneity, and employed forest plots to visually represent the effect size of each SNP (Figs. 2 and 3). In order to detect potential horizontal pleiotropy among these SNPs, both the MR-Egger intercept and the MR-PRESSO Global test were employed to verify sensitivity analyses for MR studies.Among them, MR-Egger intercept recognizes horizontal pleiotropy via evaluating the intercept term into the regression analysis.The MR-PRESSO global test is designed to address horizontal pleiotropy by reassessing causality through the identification and exclusion of outliers and biases12 (Table S4). The process of SNPs detection employed a systematic “Leave-one-out”procedure to guarantee the reliability of the study by emliminating SNPs with potential heterogeneity. “Leave-one-out”sensitivity analysis, funnel plots, scatter plots, and forest plots (Fig. 2) further corroborate the observed heterogeneity and robustness(Figure S1-S3 and S4-S13).
Liver fibrosis is a necessary stage of various chronic liver diseases25. Studies have shown that lactylation-associated immune infiltration is inseparable in the progression of liver fibrosis to HCC26. The stage of liver fibrosis usually includes grade 0 to 4.F0, no fibrosis; F1, mild fibrosis distributed in the sinusoid or portal area; F2, significant liver fibrosis with portal fibrosis and a small amount of bridging between portal areas or hepatic veins; F3, severe bridging fibrosis with structural distortion; F4, showing cirrhosis with fibrosis of regenerating nodules27.
Approval of ethical
The study utilized publicly available data. Each GWAS study obtained approval from its respective ethics review board to ensure that informed consent had been acquired from participants, designated caregivers, or legal guardians10.
Results
Selection of instrumental variables
To explore high-quality IVs, our study indentified 14 immunophenotypes with IVs ranging from 14 to 28 SNPs through stringent measures.The correlation P values of the IVW method were all below 0.05, confirming the reliability of our study.Each retained SNP exhibited an F-statistic exceeding the threshold of 10, indicating a minimal likelihood of weak IVs.Comprehensive sensitivity analysis was conducted on all MR results, including assessment of heterogeneity using Cochran’s Q test and investigation potential pleiotropy via MR-Egger intercept and MR-Presso Global test, with P-values exceeding 0.05.
Exploration of the relationship of immunophenotypes on cirrhosis
The IVW method was considered the predominant research instrument, consistent with the weighted median estimates, MR-Egger, simple mode and weighted mode to investigate the effect of cirrhosis on immunophenotypes.After adjusting for multiple tests, significant associations were observed between 14 immunophenotypes and cirrhosis (Table S2). Then, the 14 immunophenotypes were divided into two groups according to the OR value of IVW completing with MR-Egger, Weighted-mode, Weighted-Median, and Simple-mode. The OR value exceeding 1 was considered a significant risk factor (Fig. 3), whereas it will be deemed as protective factors (Fig. 4).
Specially speaking, the increased number of CD33br HLA DR + CD14dim AC(ORIVW=1.03, 95%Cl = 1.00–1.06, PIVW=0.040), CD33br HLA DR + CD14dim %CD33br HLA DR+(ORIVW=1.05, 95% Cl = 1.01–1.09, PIVW=0.023), CD25 on IgD- CD38dim(ORIVW=1.06, 95%Cl = 1.00–1.13, PIVW=0.042), CD25 on memory B cell (ORIVW=1.05, 95% Cl = 1.01–1.10,PIVW=0.017), CD38 on CD20-(ORIVW=1.09, 95%Cl = 1.00–1.20, PIVW= 0.049), BAFF-R on CD20-(ORIVW=1.07, 95% Cl = 1.01–1.14, PIVW=0.032), CD33 on CD66b + + myeloid cell (ORIVW=1.04, 95% Cl = 1.01–1.08,PIVW=0.012), CD33 on CD33dim HLA DR- (ORIVW=1.05, 95% Cl = 1.02–1.09,PIVW=0.001), CD33 on basophil (ORIVW=1.03, 95% Cl = 1.01–1.05, PIVW=0.011), CD33 on Im MDSC (ORIVW=1.06, 95% Cl = 1.02–1.09,PIVW=0.01) were correlated with an elevated level of risk (Fig. 3), while CCR2 on CD62L + myeloid DC(ORIVW=0.91, 95% Cl = 0.85–0.97, PIVW=0.006), CD39 on CD39 + secreting Treg (ORIVW=0.97, 95% Cl = 0.94–0.99, PIVW=0.015), SSC-A on NK(ORIVW=0.93, 95%Cl = 0.89–0.98, PIVW=0.010) and HLA DR on CD33dim HLA DR + CD11b+ (ORIVW=0.96, 95%Cl = 0.93–099, PIVW=0.008) manifested protective effects across different subtypes (Fig. 4).
Due to the potential bias inherent in the IVW analysis, we further conduct investigations into heterogeneity and pleiotropy.The Cochran Q test, MR-Egger intercept test and MR-PRESSO Global test yield P-vaules that exceed the threshold of 0.05 (Fig. 2), indicating no evidence of heterogeneity and pleiotropy in the research (Figs. 3 and 4).
Discussion
Cirrhosis is a chronic and progressive hepatic disorder characterized by hepatic dysfunction, portal hypertension, and an array of complications in the advanced stages of the disease.The pathogenesis of cirrhosis is multifactorial and complex.The liver is not only a central organ in the gut-liver axis but also a vital immune organ. Under healthy physiological conditions, the intestinal epithelial and vascular barriers, gut microbiota, liver, and immune system establish intricate interactions to maintain tolerance to harmless stimuli while coordinating systemic immunity. However, in decompensated cirrhosis, this balance is disrupted, leading toCAID, characterized by systemic inflammation and host immune deficiency25,28. Studies indicated that the gut-liver axis plays a pivotal role in the pathogenesis of CAID.
Compelling experimental and clinical data have irrefutably established that gut-liver-immune interactions are mediated through pattern recognition receptors (e.g., TLR4), microbial metabolites, and hepatic stellate cell activation, constituting a critical axis in hepatic pathophysiology29,30. Intestinal barrier provides resistance against inflammation and maintains gut immune homeostasis, intestinal epithelial cells and gut-associated lymphoid tissues activate downstream signaling pathways—mediated by pattern recognition receptors and the aryl hydrocarbon receptor —that enhance epithelial barrier function29. The gut microbiota and its metabolites, such as short-chain fatty acids (SCFAs) and bile acids, can also enhance epithelial integrity and stimulate enterocyte proliferation through immune regulation, and reduce LPS translocation, thereby suppressesing macrophage activation, proinflammatory cytokine production, and neutrophil infiltration, ultimately mitigating liver injury in experimental rat models31,32. However, uring the progression of cirrhosis, gut dysbiosis and disruption of the gut-liver axis lead to CAID29,33, so the gut-liver-immune axis may play a central role in the pathogenesis of cirrhosis.
In our research, multiple immunophenotypes were found to be associated with the disease progression of liver cirrhosis.The myeloid-derived suppressor cells (MDSCs) are characterized by their heterogeneity and possesses potent immunosuppressive functions, encompassing distinct monocytic and granulocytic subtypes.MDSCs typically express CD11b, CD33, and exhibit low levels of human leukocyte antigen DR in humans. The immunohistochemical results indicated that a significant upregulation in CD33 expression among clinical HCC patients and colon cancer patients with liver metastasis34. Elevated levels of CD33 exhibit a positive correlation with an unfavorable prognosis and shorter survival time in HCC patients.Furthermore, there is a significantly higher enrichment of CD33 in fibrotic tissues compared to non-fibrotic tissues35,36. CAID can be divided into mild systemic inflammation and severe systemic inflammation. The latter often leads to immune paralysis and persistent impairment of the immune response to ongoing bacterial challenge. CD36 + CAFs derived from HSCs enhance the immunosuppressive ability of CD33 + MDSCs through lipid peroxidation/p38/CEBPs axis, which mediates oxidized low-density lipoprotein uptake dependent expression of MIF, and recruitment of CD33 + myeloid-derived suppressor cells in a MIF−and CD74-dependent manner. In contrast, CD36 inhibitors can restore antitumor T cell responses in HCC, thereby synergistically enhancing the efficacy of anti-programmed death-1 immunotherapy37. In preclinical models of colorectal cancer, the anaerobic Gram-negative bacterium Fusobacterium nucleatum has been shown to recruit MDSCs and tumor-associated macrophages into the tumor microenvironment, thereby suppressing antitumor immune response38. Thus, the critical role of CD33 in regulating gut-liver-immune homoeostatic interactions during the progression of chronic liver disease is evident. Our MR analysis showed that CD33br HLA DR + CD14dim AC, CD33br HLA DR + CD14-%CD33br HLA DR+, and the increased proportion of CD33 on CD66b + + myeloid cells, CD33dim HLA DR-, basophil and Im MDSC are associated with an heightened risk of cirrhosis.Therefore, strategies that focus on inhibiting the activation of CD33 offer promising therapeutic approaches for cirrhosis management.
B cells have been proofed an integral component of the liver Immune microenvironment, encompassing naive mature B cells, memory B cells, and plasma cells. Studies have revealed heightened expression of IgD by naive mature B cells among patients afflicted with cirrhosis39. CD38, which is secreted by plasma cells, becomes activated in the context of inflammation and facilitates cellular migration to sites of inflammation40. Furthermore, the surface marker CD27+, indicative of memory B cells, exhibits a significant reduction in individuals with decompensated cirrhosis and showed reduced responsiveness to TLR9 activation, thereby impairing antigen presentation and ultimately leading to portal hypertension and hepatic dyssynthesis41.
The primary biliary cholangitis (PBC) is an autoimmune disease affecting the intrahepatic bile ducts, and it represents a significant etiology of cirrhosis. Rituximab, an immune agent, effectively eliminates B cells by specifically binding to CD20 receptors on the surface of B cells and inhibiting the expansion of pdc-e2 reactive CD8 + T cells, providing an efficacious approach for treating early PBC42,43. However, treatment with anti-CD20 alone resulted in the depletion of regulatory B cells (Breg), leading to a partial alleviation of liver inflammation in PSC but exacerbation of colitis.The presence of serum B cell activating factor of the TNF-family (BAFF) is responsible for this phenomenon, as it has been demonstrated to exhibit a positive correlation with the severity of cirrhosis and facilitates B cell survival through BAFF receptors44. Similarly, compared to patients with simple steatosis, those with NASH exhibit higher serum BAFF levels. In a methionine-choline-deficient (MCD) diet-induced NASH mouse model, antibody-mediated BAFF neutralization alleviates hepatocyte injury and lobular inflammation45. Additionally, it transmits costimulatory signals to T cells via the Th1/Th17 pathway in order to promote an inflammatory response46. Combination therapy with anti-CD20 and anti-BAFF can effectively preserve the functionality of Breg cells, efficiently eradicate circulating and tissue B cells, and ameliorate hepatic inflammation47. In CCl4-induced liver fibrosis mice, B cells promote liver fibrosis. The induction of liver fibrosis by CCl4 is significantly attenuated in B cell-deficient mice, with reduced collagen deposition, decreased immune cell infiltration, and diminished HSCs activation, suggesting that B cells are essential in the development of liver fibrosis48. The aforementioned findings hightlight the pivotal role of B cells in the development and progression of liver disease, aligning with our own investigaton. Specifically, we have observed that CD38 on CD20-B cells, and BAFF-R on CD20-B cells are associated with an elevated risk of cirrhosis.The serologic marker alpha-fetoprotein (AFP) is widely recognized for hepatocellular carcinoma (HCC) screening, and ongoing research aims to identify alternative tests that can replace AFP49. Severity studies have confirmed that the severity of HCC has been positively correlated with CD25 levels, suggesting its potential as an effective tool for early HCC screening50. Our study also found that the increased number of CD25 on IgD-CD38-B cells and memory B cells was correlated with an elevated risk of cirrhosis. These findings emphasize the detrimental involvement of B cells (excluding CD27 + memory B cells) in the pathogenesis of cirrhosis.
NK cells serve a crucial role not only in the human tumor immune surveillance system but hold significant potential for treating fibrotic diseases51. In the context of liver fibrosis, NK cells exhibit potent anti-fibrosis effects by directly eliminating hepatic stellate cells(HSCs) at various stage through cell-cell adhesion mechanisms. And impaired NK cell activity has been found in both advanced liver fibrosis patients and mouse models52,53. Tao et al.54discovered that E-prostanoid 3 receptor (EP3) expression was significantlly down-regulated in NK cells from mice and patients with cirrhosis. They also observed that the absence of EP3 resulted in decrease in the cytotoxicity displayed by NK cells against activated HSCs. In conclusion, EP3 facilitates the translocation of Itga4 to the nucleus in NK cells through PKC-mediated Spic protein phosphorylation at T191, thereby upregulating Itga4 expression and promoting NK cell activation to protect LF mice55. The NKT cells, a subset of effector lymphocytes, exhibit rapid cytokine upon activation, including interferon (IFN)-γ and interleukin (IL)−4.This subsequently stimulates downstream NK cells. In an MR study investigating the correlation between side scatter area (SSC-A) on NKT cells and 28-day mortality in patients with septic, it was demonstrated that NKT cells exerted a protective effect against sepsis55. The role of chronic inflammation in fibrosis progression is significant, and NK cells, as downstream counterparts of NKT cells, possess similar functional properties to NKT cells. Our study further confirmed the protective effect of SSC-A on NK cell against cirrhosis, highlighting their potential antifibrotic ability.
The population of Treg cells accounts for approximately 5–10% of the CD4 + T lymphocytes and plays a indispensable role in maintaining immune homeostasis in the intestines and regulating infections56.Our study also demonstrated that the increased number of CD39 on CD39 + secreting Treg cells appears to confer a protective effect against cirrhosis.The gut-liver axis has displayed as a prominent research focus in recent years, offering a viable approach for alleviating chronic liver disease through the administration of oral antibiotics, probiotics, and biological agents.Bifidobacterium longum subspecies infantis 35,624 (B infantis) serves a remarkable role in the management of intestinal inflammation in both humans and mice by activating DCs within the intestines and inducing the expression of Treg cells in the mucosal lining57. Additionally, it significantly inhibits the pro-inflammatory NF-κb pathway induced by lipopolysaccharide(LPS), thereby improving immune homeostasis58.Treatment with fluoroquinolone antibiotics leads to an elevation in the quantity of peripheral Treg cells, which is facilitated by DCs and contributes to the mitigation of the proinflammatory environment associated with cirrhosis59.
The cell surface receptor HLA-DR, which belongs to the MHC class II family, is expressed on various immune cells and lymphoid tissues. The downregulation of HLA-DR, which are commonly observed among myeloid cells including monocytes, dendritic cells, neutrophils, expression in monocytes is associated with an elevated susceptibility to liver diseases, such as viral hepatitis, cirrhosis, and acute-on-chronic liver failure (ACLF)60. The expression of HLA-DR gradually diminishes as liver disease progresses, and is closely related with the development of complications and unfavorable prognosis in chronic liver disease61. Meanwhile, the expression of CCR2 is observed on CD14high/CD16−classical monocytes, and its level in monocytes was significantly reduced in individuals with HCV and NAFLD compared to healthy controls61. These findings imply that HLA-DR and CCR2 function as protective factors against inflammation and fibrosis.In our findings, the increased number of CCR2 on CD62L + myeloid dendritic cells and HLA DR on CD33dim HLA DR + cells demonstrated a significant correlation with a decreased risk of cirrhosis, which aligns with previous researchs.
In summary, our study has made significant strides in comprehending the relationship of immunophenotypes on cirrhosis, primarily utilizing IVW combined with weighted median, weighted mode, MR-Egger, and simple mode approaches to investigate relationship of 731 immunophenotypes on cirrhosis. Our findings were consistent with horizontal pleiotropy, which effectively minimized confounding factors, and all F-statistics exceeded 10, thereby ensuring the robust statistical power of the MR Study and mitigating instrument bias.However, our study also has certain limitations. Firstly, our data source primarily consisted of adults with European ancestry, which may compromise the generalizability and accuracy of the researchs. Therefore, it is necessary to validate these results in larger and more ethnically diverse patient cohorts in the future. Secondly, although we rigorously screened 731 immunophenotypes using robust methods, certain phenotypes were unable to be analyzed due to the limitations in the available data. Moreover, the selection criteria for IVs in our study were relatively permissive (p < 1 × 10 − 5), potentially resulting in an elevated risk of false positives. However, it is worth noting that all IVs exhibited a robust F-statistic > 10, thereby mitigating potential biases arising from weak instrument bias.Finally, the relationship between immunophenotypes and cirrhosis was solely investigated, without delving into their underlying mechanisms.
Conclusion
This study unveils the association between immunophenotypes and cirrhosis.Following this study,4 immunophenotypes suggest potential protective effects against cirrhosis, while 10 immunophenoytpes were associated with an elevated risk.CD33 and B-cell-associated immunophenotypes are closely correlated with the susceptibility to cirrhosis and warrant particular attention.
These findings shed light on the intricate interplay between immunophenotypes and cirrhosis, thereby providing valuable references and recommendations for clinical practice. In the future, further academic research is warranted to delve into the impact of immunophenotypes on the progression of cirrhosis, while animal and molecular experiments are necessary to unveil underlying molecular mechanisms.
Data availability
The original contributions presented in the study are included in the article Supplementary Material.Further inquiries can be directed to the corresponding authors.
References
Roehlen, N., Crouchet, E. & Baumert, T. F. Liver fibrosis: mechanistic concepts and therapeutic perspectives. Cells 9(4). (2020).
Wilson, R. & Williams, D. M. Cirrhosis. Med. Clin. N. Am. 106 (3), 437–446 (2022).
Devarbhavi, H. et al. Global burden of liver disease: 2023 update. J. Hepatol. 79 (2), 516–537 (2023).
Ge, P. S. & Runyon, B. A. Treatment of patients with cirrhosis. N Engl. J. Med. 375 (8), 767–777 (2016).
Ginès, P. et al. Liver cirrhosis. Lancet 398 (10308), 1359–1376 (2021).
Zhao, J. et al. Single-cell RNA sequencing reveals the heterogeneity of liver-resident immune cells in human. Cell. Discovery. 6, 22 (2020).
Lario, M. et al. Defective thymopoiesis and poor peripheral homeostatic replenishment of T-helper cells cause T-cell lymphopenia in cirrhosis. J. Hepatol. 59 (4), 723–730 (2013).
Albillos, A. et al. Cirrhosis-associated immune dysfunction. Nat. Rev. Gastroenterol. Hepatol. 19 (2), 112–134 (2022).
Ni, Y., Wang, W., Liu, Y. & Jiang, Y. Causal associations between liver traits and colorectal cancer: a Mendelian randomization study. BMC Med. Genom. 16 (1), 316 (2023).
Wang, K. et al. Causal relationship between gut microbiota and risk of esophageal cancer: evidence from Mendelian randomization study. Aging 16 (4), 3596–3611 (2024).
Cao, W. et al. Causal relationship between immune cells and risk of heart failure: evidence from a Mendelian randomization study. Front. Cardiovasc. Med. 11, 1473905 (2024).
Larsson, S. C., Butterworth, A. S. & Burgess, S. Mendelian randomization for cardiovascular diseases: principles and applications. Eur. Heart J. 44 (47), 4913–4924 (2023).
Wang, S., Wang, K., Chen, X. & Lin, S. The relationship between autoimmune thyroid disease, thyroid nodules and sleep traits: a Mendelian randomization study. Front. Endocrinol. 14, 1325538 (2023).
Wang, K. et al. The causal relationship between gut microbiota and biliary tract cancer: comprehensive bidirectional Mendelian randomization analysis. Front. Cell. Infect. Microbiol. 14, 1308742 (2024).
Wang, K. et al. Causal relationship between gut microbiota and risk of gastroesophageal reflux disease: a genetic correlation and bidirectional Mendelian randomization study. Front. Immunol. 15, 1327503 (2024).
Wang, S., Wang, K., Chen, X., Chen, D. & Lin, S. Autoimmune thyroid disease and myasthenia gravis: a study bidirectional Mendelian randomization. Front. Endocrinol. 15, 1310083 (2024).
Wang, K. et al. Causal link between gut microbiota and four types of pancreatitis: a genetic association and bidirectional Mendelian randomization study. Front. Microbiol. 14, 1290202 (2023).
Nian, S. et al. Causal associations between immune cell phenotypes and varicose veins: A Mendelian randomization analysis. Ann. Vasc. Surg. 114, 126–132 (2025).
Mo, L., Pan, W., Cao, W., Wang, K. & Huang, L. Immune cells and intracerebral hemorrhage: A causal investigation through Mendelian randomization. Brain Behav. 15 (1), e70263 (2025).
Chen, Y. et al. Relationship between fatty acid intake and aging: a Mendelian randomization study. Aging 16 (6), 5711–5739 (2024).
Hu, Y. et al. Causal relationship between immune cell phenotypes and risk of biliary tract cancer: evidence from Mendelian randomization analysis. Front. Immunol. 15, 1430551 (2024).
Liu, R. et al. A causal relationship between distinct immune features and acute or chronic pancreatitis: results from a Mendelian randomization analysis. Pancreatology 24 (8), 1219–1228 (2024).
Cao, W. et al. Causal relationship between immune cells and risk of myocardial infarction: evidence from a Mendelian randomization study. Front. Cardiovasc. Med. 11, 1416112 (2024).
Gill, D. & Burgess, S. The evolution of Mendelian randomization for investigating drug effects. PLoS Med. 19 (2), e1003898 (2022).
Chen, Y. et al. Gut microbe and hepatic macrophage polarization in non-alcoholic fatty liver disease. Front. Microbiol. 14, 1285473 (2023).
Li, L. N., Li, W. W., Xiao, L. S. & Lai, W. N. Lactylation signature identifies liver fibrosis phenotypes and traces fibrotic progression to hepatocellular carcinoma. Front. Immunol. 15, 1433393 (2024).
Petitclerc, L., Gilbert, G., Nguyen, B. N. & Tang, A. Liver fibrosis quantification by magnetic resonance imaging. Top. Magn. Reson. Imaging: TMRI. 26 (6), 229–241 (2017).
Hernandez-Gea, V. & Friedman, S. L. Pathogenesis of liver fibrosis. Annu. Rev. Pathol. 6, 425–456 (2011).
Tranah, T. H., Edwards, L. A., Schnabl, B. & Shawcross, D. L. Targeting the gut-liver-immune axis to treat cirrhosis. Gut 70 (5), 982–994 (2021).
Bozward, A. G., Ronca, V., Osei-Bordom, D. & Oo, Y. H. Gut-Liver immune traffic: Deciphering immune-Pathogenesis to underpin translational therapy. Front. Immunol. 12, 711217 (2021).
Park, J. et al. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 8 (1), 80–93 (2015).
Liu, B. et al. Butyrate protects rat liver against total hepatic ischemia reperfusion injury with bowel congestion. PLoS One. 9 (8), e106184 (2014).
Hang, S. et al. Bile acid metabolites control T(H)17 and T(reg) cell differentiation. Nature 576 (7785), 143–148 (2019).
Ham, B. et al. TNF Receptor-2 facilitates an immunosuppressive microenvironment in the liver to promote the colonization and growth of hepatic metastases. Cancer Res. 75 (24), 5235–5247 (2015).
Liu, M. et al. Targeting monocyte-intrinsic enhancer reprogramming improves immunotherapy efficacy in hepatocellular carcinoma. Gut 69 (2), 365–379 (2020).
Osman, H. A. et al. Peripheral mononuclear cells surface markers evaluation in different stages of hepatocellular carcinoma; in a trial for early and accurate diagnosis in patients with Post-Hepatitis liver cirrhosis and unremarkable Raised AFP. Int. J. Gen. Med. 16, 1047–1058 (2023).
Zhu, G. Q. et al. CD36(+) cancer-associated fibroblasts provide immunosuppressive microenvironment for hepatocellular carcinoma via secretion of macrophage migration inhibitory factor. Cell. Discovery. 9 (1), 25 (2023).
Sakamoto, Y. et al. Relationship between Fusobacterium nucleatum and antitumor immunity in colorectal cancer liver metastasis. Cancer Sci. 112 (11), 4470–4477 (2021).
Doi, H. et al. Enhanced B-cell differentiation driven by advanced cirrhosis resulting in hyperglobulinemia. J Gastroenterol. Hepatol (2018).
Piedra-Quintero, Z. L., Wilson, Z., Nava, P. & Guerau-de-Arellano, M. CD38: an Immunomodulatory molecule in inflammation and autoimmunity. Front. Immunol. 11, 597959 (2020).
Jhun, J. Y. et al. B-cell-associated immune profiles in patients with decompensated cirrhosis. Scand. J. Gastroenterol. 50 (7), 884–891 (2015).
Myers, R. P., Swain, M. G., Lee, S. S., Shaheen, A. A. & Burak, K. W. B-cell depletion with rituximab in patients with primary biliary cirrhosis refractory to ursodeoxycholic acid. Am. J. Gastroenterol. 108 (6), 933–941 (2013).
Moritoki, Y. et al. B-cell depletion with anti-CD20 ameliorates autoimmune cholangitis but exacerbates colitis in transforming growth factor-beta receptor II dominant negative mice. Hepatology 50 (6), 1893–1903 (2009).
Rosser, E. C. & Mauri, C. Regulatory B cells: origin, phenotype, and function. Immunity 42 (4), 607–612 (2015).
Barrow, F., Khan, S., Wang, H. & Revelo, X. S. The emerging role of B cells in the pathogenesis of NAFLD. Hepatology 74 (4), 2277–2286 (2021).
Uzzan, M., Colombel, J. F., Cerutti, A., Treton, X. & Mehandru, S. B cell-Activating factor (BAFF)-Targeted B cell therapies in inflammatory bowel diseases. Dig. Dis. Sci. 61 (12), 3407–3424 (2016).
Zhang, W. et al. Dual B-cell targeting therapy ameliorates autoimmune cholangitis. J. Autoimmun. 132, 102897 (2022).
Thapa, M. et al. Liver fibrosis occurs through dysregulation of MyD88-dependent innate B-cell activity. Hepatology 61 (6), 2067–2079 (2015).
Tayob, N., Kanwal, F., Alsarraj, A., Hernaez, R. & El-Serag, H. B. The performance of AFP, AFP-3, DCP as biomarkers for detection of hepatocellular carcinoma (HCC): A phase 3 biomarker study in the united States. Clin. Gastroenterol. Hepatology: Official Clin. Pract. J. Am. Gastroenterological Association. 21 (2), 415–423e414 (2023).
Abdelfattah, S. N., Haseeb, A. F., Tawfik, M. M., Khalil, D. M. & Attia, D. Soluble CD25 as a predictor of hepatocellular carcinoma compared with alpha-fetoprotein. Clin. Experimental Hepatol. 5 (2), 140–146 (2019).
Tognarelli, S., Jacobs, B., Staiger, N. & Ullrich, E. Flow Cytometry-based assay for the monitoring of NK cell functions. J. Vis. Exp. JoVE 116 (2016).
Melhem, A. et al. Anti-fibrotic activity of NK cells in experimental liver injury through killing of activated HSC. J. Hepatol. 45 (1), 60–71 (2006).
Mele, D. et al. Adaptive natural killer cell functional recovery in hepatitis C virus cured patients. Hepatology 73 (1), 79–90 (2021).
Tao, X. et al. EP3 enhances adhesion and cytotoxicity of NK cells toward hepatic stellate cells in a murine liver fibrosis model. J Exp. Med 219(5). (2022).
Liu, Q. et al. Mendelian randomization and transcriptomic analysis reveal the protective role of NKT cells in Sepsis. J. Inflamm. Res. 17, 3159–3171 (2024).
Ajith, A. et al. Immune regulation and therapeutic application of T regulatory cells in liver diseases. Front. Immunol. 15, 1371089 (2024).
Konieczna, P. et al. Bifidobacterium infantis 35624 administration induces Foxp3 T regulatory cells in human peripheral blood: potential role for myeloid and plasmacytoid dendritic cells. Gut 61 (3), 354–366 (2012).
O’Mahony, C. et al. Commensal-induced regulatory T cells mediate protection against pathogen-stimulated NF-kappaB activation. PLoS Pathog. 4 (8), e1000112 (2008).
Juanola, O. et al. Selective intestinal decontamination with Norfloxacin enhances a regulatory T cell-mediated inflammatory control mechanism in cirrhosis. Liver Int. 36 (12), 1811–1820 (2016).
Irvine, K. M., Ratnasekera, I., Powell, E. E. & Hume, D. A. Causes and consequences of innate immune dysfunction in cirrhosis. Front. Immunol. 10, 293 (2019).
Gadd, V. L. et al. Altered peripheral blood monocyte phenotype and function in chronic liver disease: implications for hepatic recruitment and systemic inflammation. PLoS One. 11 (6), e0157771 (2016).
Acknowledgements
We express our sincere gratitude to the MiBioGen Consortium and the GWAS Catalog database for generously sharing their GWAS summary data, which greatly facilitated our research endeavors.Furthermore, we extend our appreciation to Figdraw (www.figdraw.com) for their professional assistance in chart production.
Author information
Authors and Affiliations
Contributions
YC, KW, YPL, HLZ, and HL contributed to the concept and design of this study. YC, KW were responsible for statistical analysis and writing of the report, YPL revalidated the experimental methodology, overall grasp of the structure of the article, and made revise to the article, HLZ assisted in statistical analysis, YC, JHK, SJW and JZH reviewed the article and provided critical feedback to improve and structure the report.The authors JWW who were removed were primarily devoted to literature indexing. YC, KW and YPL are regarded as co-first authors with the same degree of contribution. JHK, SJW and JZH are the corresponding authors. Corresponding author All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
All data utilized in this study are publicly accessible and fall within the public ___domain. Informed consent was obtained from all participants, and the study protocol received approval from the local ethics committee.
Consent for publication
Consensus among all authors was achieved regarding the manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, Y., Wang, K., Liu, Y. et al. The role of immune cells in cirrhosis: evidence from a two-sample Mendelian randomization study. Sci Rep 15, 20737 (2025). https://doi.org/10.1038/s41598-025-07325-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-07325-7