Abstract
The development of highly efficient and stable electrocatalysts with minimized noble metal loading is crucial for advancing oxygen reduction reaction (ORR) technology in alkaline fuel cells. Herein, we fabricated a quaternary catalyst comprising PdPt nanoalloys on CoNi mixed oxide matrix (denoted as PdPt-CoNi) with an ultra-low Pt content of ∼ 2 wt%. As-prepared PdPt-CoNi catalyst achieves a remarkable mass activity of 3250 mA mgPt–1 at 0.85 V vs. RHE and 635 mA mgPt–1 at 0.90 V vs. RHE in alkaline ORR (0.1 M KOH), surpassing the performance of the commercial J.M.-Pt/C (20 wt%) catalyst by approximately 48 and 28 times, respectively, at 0.85 V vs. RHE and 0.90 V vs. RHE. More importantly, the PdPt-CoNi catalyst demonstrates remarkable long-term stability, retaining 100% of its initial activity even after 20,000 accelerated durability test (ADT) cycles, showcasing its long-term durability under the harsh redox environment. Utilizing in-situ X-ray absorption spectroscopy at the Co, Ni, Pd, and Pt edges, we revealed that the exceptional ORR performance of the PtPd-CoNi catalyst stems from the strong metal-support interaction between PtPd nanoalloys and the CoNi mixed-oxide support, where Co and Ni domains serve as the electron donor to the surface Pd/Pt sites. Comparative studies reveal that carbon-supported PdPt nanoalloys with a similar metal loading suffer a significant decline in ORR activity, showing reductions of approximately 68% at 0.85 V and 80% at 0.90 V vs. RHE. This work highlights the synergistic effects of PdPt nanoalloys and CoNi-mixed oxides in promoting ORR kinetics while ensuring outstanding stability, paving the way for next-generation electrocatalysts with minimal noble metal utilization.
Similar content being viewed by others
Introduction
The oxygen reduction reaction (ORR) is a fundamental electrochemical process that significantly influences the performance of fuel cells. Given its inherently sluggish kinetics, developing efficient ORR electrocatalysts remains one of the primary challenges in advancing clean energy technologies. Platinum (Pt)-based catalysts have long been the benchmark for ORR due to their superior catalytic activity.1–2 However, their high cost, scarcity, and susceptibility to degradation have impeded large-scale commercialization3. Consequently, researchers have been actively exploring strategies to reduce Pt usage while enhancing the activity and durability of ORR electrocatalysts4. While it is acknowledged that M–N–C catalysts exhibit commendable ORR activity in alkaline media under laboratory-scale conditions5,6,7,8, their long-term operational stability remains a significant concern. These catalysts often suffer from metal leaching, carbon corrosion, and structural degradation, particularly under the harsh redox environment of practical fuel cell systems. In contrast, Pt-based catalysts, despite their higher cost, continue to be the benchmark due to their superior intrinsic activity, well-understood reaction mechanisms, and excellent durability. Therefore, our study focuses on improving Pt utilization and reducing loading, which not only retain the advantages of Pt but also address cost and performance limitations making them more viable for real-world alkaline fuel cell applications. One widely adopted approach to improve ORR electrocatalysts involves alloying Pt with other transition metals9. Alloying can enhance catalytic activity by modifying the electronic structure of Pt, thereby optimizing its binding affinity with oxygenated species and improving overall reaction kinetics. Among these alloyed materials, PdPt nanoalloys have garnered significant attention due to their unique structural and electronic properties, which can potentially rival or even surpass the catalytic performance of pure Pt10. However, conventional carbon-supported PdPt nanoalloy catalysts often suffer from particle agglomeration and support corrosion during prolonged operation, leading to diminished performance and stability. To overcome these limitations, researchers have turned to mixed metal oxides as alternative support materials11. Mixed oxides not only provide enhanced stability compared to carbon-based supports but also introduce electronic and structural synergies that can further boost catalytic activity12. Transition metal oxides, particularly those composed of nickel (Ni) and cobalt (Co), have shown promise as support materials due to their high conductivity, stability, and ability to modulate electronic interactions with supported noble metals.13–14 The combination of these oxides with PdPt nanoalloys offers a promising route to develop highly active and durable ORR electrocatalysts.
In this study, we introduce a novel PdPt-CoNi catalyst, wherein PdPt nanoalloys are supported on a CoNi-mixed oxide framework. This structural design aims to enhance ORR performance by leveraging the synergistic interactions between noble metal nanoalloys and the mixed oxide support. The CoNi-mixed oxide serves as a robust structural foundation that not only stabilizes the PdPt nanoparticles but also facilitates electron transfer, thereby improving catalytic efficiency. Electrochemical performance evaluations of the PdPt-CoNi catalyst reveal a remarkable mass activity of 3250 mA mgPt–1 at 0.85 V vs. RHE and 635 mA mgPt–1 at 0.90 V vs. RHE in alkaline ORR (0.1 M KOH). These values surpass the performance of the commercial J.M.-Pt/C (20 wt%) catalyst by approximately 48 and 28 times, respectively. Such an extraordinary enhancement can be attributed to the unique interaction between the PdPt nanoalloys and the CoNi-mixed oxide, which facilitates efficient electron transfer to Pd/Pt domains. Comparative studies highlight the critical role of the CoNi-mixed oxide support in improving catalyst performance. Carbon-supported PdPt nanoalloys with a similar metal loading exhibit a drastic decline in ORR activity, with reductions of approximately 68% at 0.85 V and 80% at 0.90 V vs. RHE. This stark contrast underscores the significance of the mixed oxide support in preserving the stability and functionality of the PdPt nanoalloys. Beyond catalytic activity, durability remains a crucial parameter in evaluating ORR electrocatalysts. The PdPt-CoNi catalyst demonstrates remarkable stability, retaining 100% of its initial activity even after 20,000 accelerated durability test (ADT) cycles. Such exceptional durability positions the PdPt-CoNi catalyst as a promising candidate for next-generation fuel cell applications. From a broader perspective, this work highlights the potential of mixed metal oxides as effective support materials for noble metal catalysts in electrochemical applications. The successful integration of PdPt nanoalloys with a CoNi-mixed oxide framework not only reduces noble metal usage but also maximizes catalytic efficiency and stability. This strategy aligns with ongoing efforts to develop cost-effective, high-performance electrocatalysts that can drive the commercialization of fuel cells and other clean energy technologies.
Experimental section
Synthesis of CoNi-mixed oxide supported PdPt nanoalloys
The CoNi-mixed oxide-supported PdPt nanoalloys were synthesized through a meticulously controlled multi-step procedure, incorporating ion chemisorption, a self-aligned wet chemical reduction approach, and ambient annealing. Careful regulation of the elemental molar ratios and reaction time during heterogeneous nucleation and crystal growth enabled precise control over the catalyst’s structural composition and distribution. For catalyst preparation, 3.06 g of 0.1 M cobalt(II) chloride (CoCl₂, 99%, Sigma-Aldrich) and 3.06 g of 0.1 M nickel(II) chloride hexahydrate (NiCl₂·6 H₂O, Showa Chemical Co. Ltd.) solutions were mixed in 20 ml of DI water. The resulting mixture was stirred at 600 rpm for six hours to ensure effective mixing of Co2+ and Ni2+, forming solution A. This solution contained 0.306 mmol (18 mg) of each Co and Ni metal ions. In the next step, 0.22 g of sodium borohydride (NaBH₄, 99%, Sigma-Aldrich) was dissolved in 5.0 ml of deionized (DI) water and rapidly introduced into solution A while maintaining stirring at 600 rpm for 10 s. This step reduced Co2+ and Ni2+ ions, forming CoNi mixed-oxide nanoparticles (solution-B). Subsequently, a mixed solution of 1.53 g of 0.1 M palladium chloride (PdCl₂, 99%, Sigma-Aldrich) and 0.153 g of 0.1 M hexachloroplatinic acid (H₂PtCl₆·6 H₂O, 99%, Sigma-Aldrich) was introduced into solution B. Since an excess amount of NaBH₄ remained in the solution from the previous step, Pd2+ and Pt2+ ions were sequentially reduced. After the addition of Pd and Pt precursors into solution B, the stirring was continued up to 10 min to ensure the complete reduction of Pd2+ and Pt2+ ions. This controlled process led to the formation of PdPt nanoalloys on the CoNi mixed-oxide support, denoted as PdPt-CoNi. The resulting product was thoroughly washed with acetone and DI water, followed by centrifugation and drying at 70 °C. For comparison the PdPt nanoalloys were directly fabricated on the CNT support with a similar procedure. For preparing the CNT-supported PdPt nanoalloys, the CoNi mixed-oxide support was replaced by the multi-walled carbon nanotubes (MWCNTs; Sino Applied Technology Taiwan), while the rest of the protocol is the same as the previous one. Prior to catalyst synthesis, multi-walled carbon nanotubes were functionalized via acid treatment to enhance surface adsorption sites by introducing defects and ligands. This treatment facilitated the efficient anchoring of metal ions onto the CNT surface. Hereafter, the CoNi mixed-oxide and CNT supported PdPt nanoalloys, respectively denoted as PdPt@CoNi and PdPt@CNT.
Physical characterizations
The elemental composition of the as-prepared catalyst was analyzed using an inductively coupled plasma-atomic emission spectrometer (ICP-AES, Jarrell-Ash, ICAP 9000). Various microscopy and X-ray techniques were employed to examine its structural and electronic properties. Aberration-corrected scanning transmission electron microscopy (AC-STEM) and high-resolution transmission electron microscopy (HRTEM) imaging were conducted at the Electron Microscopy Center of National Sun Yat-sen University, Taiwan. For sample preparation, the catalyst powder was dispersed in isopropanol (IPA) through ultrasonication, drop-cast onto 200-mesh copper grids, and dried at 120 °C for 48 h. Prior to insertion into the TEM chamber, plasma cleaning was performed to eliminate surface contaminants. X-ray diffraction (XRD) measurements were carried out at the BL-01C2 beamline of the National Synchrotron Radiation Research Center (NSRRC), Taiwan, using an incident X-ray wavelength of 0.6888 Å (18.0 KeV) to obtain structural insights. X-ray absorption spectroscopy (XAS) was used to study the electronic states and atomic arrangements, with spectra recorded at Pt L₃-edge, Pd K-edge, Co K-edge, and Ni K-edge in fluorescence mode at beamlines BL-17 C and 01C1 of NSRRC. X-ray photoelectron spectroscopy (XPS) was also performed at beamline BL-24A1 of NSRRC to determine oxidation states and surface compositions.
Electrochemical analysis
Electrochemical measurements were carried out at a stable room temperature using a CH Instruments Model 600B potentiostat, configured with a three-electrode system. The catalyst slurry for the oxygen reduction reaction (ORR) experiments was prepared by dispersing 5 mg of catalyst powder in 1.0 ml of isopropanol (IPA), along with 50 µL of Nafion-117 (99%, Sigma-Aldrich Co.) as a conductive binder. This mixture was subjected to ultrasonication for 30 min to ensure uniform dispersion before the ORR test. For electrode preparation, 10.0 µL of the catalyst slurry was drop-cast onto a glassy carbon rotating disk electrode (RDE) with a surface area of 0.196 cm² and subsequently air-dried. This RDE served as the working electrode. A saturated Hg/HgCl₂ electrode in KCl aqueous solution, calibrated with a potential shift of 0.242 V to align with the reversible hydrogen electrode (RHE), was used as the reference electrode, while a graphite rod was employed as the counter electrode to eliminate the possibility of Pt contamination. Cyclic voltammetry (CV) and linear sweep voltammetry (LSV) were conducted at scan rates of 0.02 V s–1 and 0.001 V s–1, respectively. CV was performed within a potential range of 0.1 V to 1.3 V (vs. RHE), whereas LSV was recorded over 0.4 V to 1.1 V (vs. RHE) in a 0.1 M KOH alkaline electrolyte (pH 13). The electrode rotation speed during LSV varied between 400 and 3600 rpm to evaluate kinetic behavior. Nitrogen (N₂) was used to purge the electrolyte during CV measurements, while oxygen (O₂) was employed for LSV analysis. The long-term electrochemical stability of the CPNP catalyst was assessed through an accelerated durability test (ADT) by applying potential cycling between 0.5 V and 1.0 V (vs. RHE) at a scan rate of 0.05 V s–1 in an oxygen-saturated environment across multiple ADT cycles.
Results and discussion
Structural characterization
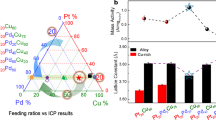
The elemental composition of the PdPt-CoNi catalyst, as determined by inductively coupled plasma atomic emission spectroscopy (ICP-AES), consists of Co/Ni/Pd/Pt in a weight% of 9.64/5.70/9.44/2.47. To gain insight into the structural and morphological characteristics of the synthesized material, advanced electron microscopy techniques, including aberration-corrected scanning transmission electron microscopy (AC-STEM) and high-resolution transmission electron microscopy (HRTEM), were employed. The high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) image of the PdPt-CoNi catalyst, presented in Fig. 1a, provides a detailed visualization of its nanostructure. Additionally, the corresponding energy-dispersive X-ray spectroscopy (EDS) elemental mapping of Co, Ni, Pd, and Pt (shown as insets) confirms the successful deposition of PdPt nanoalloys onto the CoNi mixed-oxide support. To further examine the crystalline structure, HRTEM analysis was conducted. As depicted in Fig. 1b, the presence of multi-faceted twin boundaries (indicated by red arrows) and variations in intensity (highlighted by white circles) strongly suggest the integration of Pt atoms within the Pd matrix, leading to the formation of PdPt nanoalloys. The observed twin boundaries play a crucial role in reducing the total energy of the nanoparticles by mitigating strain or lowering surface energy. This is further supported by the reduced lattice spacing of 0.205 nm in the PdPt-CoNi catalyst, indicating increased lattice strain when compared to the standard Pd (111) plane, which typically exhibits a d-spacing of 0.225 nm15. Moreover, the fast Fourier transform (FFT) pattern reveals distinct twin bright spots with intensity variations, providing additional confirmation of the incorporation of Pt atoms within the Pd lattice. Furthermore, the presence of a relatively amorphous region, as indicated by yellow arrows, is attributed to the CoNi mixed-oxide support, which contributes to the overall stability and performance of the catalyst. For a fair comparison, the high- and low-resolution TEM images of CNT-supported PdPt nanoalloys (i.e., PdPt@CNT) have been depicted in Figure S1. Similar to the PdPt@CoNi, the PdPt@CNT nanoparticles also exhibit significant variations in intensity (shown by white circles), indicating the incorporation of Pt atoms within the Pd crystal, resulting in the formation of PdPt nanoalloys. More importantly, instead of the formation of twin boundaries in PdPt@CoNi due to strong interaction at the surface of CoNi-mixed oxide, the PdPt nanoalloys preferentially grow unidirectionally.
The crystal structure of the PdPt-CoNi catalyst was further analyzed using X-ray diffraction (XRD). Figure 2 presents the XRD patterns of the PdPt-CNT and PdPt-CoNi catalysts, with the diffraction patterns of control samples (Pt-AC and Pd-AC) included for comparison. The characteristic diffraction peaks labeled “P” and “Q” correspond to the (111) and (200) planes of the face-centered cubic (fcc) structures of Pd and/or Pt. Interestingly, the PdPt-CoNi catalyst exhibits distinct variations in the open (111) and closed (200) planes. Compared to the Pd-CNT catalyst, the (111) and (200) diffraction peaks of PdPt-CoNi are notably broadened and shifted. Specifically, the (111) peak shifts to a higher angle, indicating a decrease in the lattice constant, whereas the (200) peak shifts to a lower angle, signifying an increase in the lattice constant. This observation suggests the presence of both expansive and compressive strain in different crystallographic planes16. A more detailed examination reveals that the PdPt-CoNi catalyst displays significantly broader diffraction peaks, accompanied by an enhanced background signal (HB), particularly in the regions marked R1-R2 (highlighted by red squares). This phenomenon can be attributed to the higher surface roughness due to the influence of the CoNi-mixed oxide support, which contributes to the structural modifications observed in the catalyst17.
The local atomic and electronic structure of PdPt-CoNi catalyst is determined by cross-referencing results of Pt L3-edge, and Pd K-edge XAS analysis. Figure 3a compares the normalized Pt L3-edge X-ray absorption near edge spectra (XANES) of the PdPt-CoNi catalyst with Pt-CNT and PdPt-CNT. In a Pt L3-edge spectrum, adsorption edge intensity (HA) corresponds to the unoccupied density of Pt-5d orbitals (i.e., with the decreasing HA, the number of electrons in the occupied d band increases) and the amount of surface chemisorption of oxygen18. Whereas the position of the inflection point (arrow X) indicates their corresponding threshold energy (E0) for 2p to 5d electron transition and is generally proportional to the oxidation state of Pt atoms18. It is evident from Fig. 3a that, among experimental samples under investigation, the lowest HA and downshift of inflection peak X indicates the strongest electron relocation from CoNi mixed-oxide support to Pt atoms (due to significant electronegativity difference) and a severe atomic structure disordering around Pt atoms due to alloy formation in PdPt-CoNi catalyst19. The Fourier-transformed extended X-ray absorption fine structure (FT-EXAFS) spectra of the corresponding samples at the Pt-L3 edge are shown in the inset of Fig. 3a. Accordingly, the PdPt-CNT and PdPt-CoNi samples exhibit a reduced bond length of the Pt-Pt bond pair as compared to Pt-CNT, which is evident from the left shift of the radial peak corresponding to the Pt-Pt bond pair in those samples as compared to Pt-CNT. Such a scenario can be attributed integration of Pt into the Pd matrix and is in good agreement with the former HRTEM results.
Furthermore, the results of XANES analysis at Pd K-edge are shown in Fig. 3b. Accordingly, two absorption peaks “P” and “Q” are typical features of 1s ◊ 5p-4f transitions in a K-edge spectrum and varies of intensity between them correspond to a charge relocation between Pd and neighboring atoms20. Whereas the position of inflection point (X) mainly elucidates the oxidation state of Pd-atoms. Interestingly, the intensities of absorption peaks “P” and “Q” follow the same trend as that of Pt L3-edge, indicating the highest density of electron density in Pd 5p/4f orbitals in the PdPt-CoNi catalyst. Along with the XANES results of Pt L3-edge, such a scenario confirms the electron relocation from CoNi mixed-oxide support to Pd and Pt atoms, indicating the strong metal-support interaction between PdPt nanoalloys and CoNi mixed-oxide support. Moreover, the FT-EXAFS spectra at Pd K-edge (inset of Fig. 3b) exhibit similar features as those of Pt L3-edge, where PdPt-CNT and PdPt-CoNi samples exhibit significantly reduced Pd-Pd bond length as compared to the Pd-CNT.
Figure 4a presents a comparison of the cyclic voltammetry (CV) curves for the PdPt-CoNi catalyst alongside Pd-CNT, Pt-CNT, and PdPt-CNT. The overlaid CV curves reveal three distinct potential regions. The first region, known as the hydrogen underpotential deposition (HUPD) region, appears below 0.40 V vs. RHE and corresponds to the adsorption (reverse sweep) and desorption (forward sweep) of hydrogen. This is followed by the double-layer region, spanning from 0.40 V to 0.55 V vs. RHE, which is associated with OH⁻ ion adsorption. Beyond 0.55 V vs. RHE, the oxide formation (forward sweep) and subsequent oxide reduction (backward sweep) occur21. For Pd-CNT, the broad peak profile in the HUPD region suggests a strong affinity for H⁺ ion adsorption on the Pd surface22. Interestingly, no significant current signal related to oxide formation (i.e., oxygenated species adsorption on Pd) is observed up to approximately 0.7 V vs. RHE. This absence can be attributed to the presence of electrochemically inactive oxygenated species on the Pd surface, indicating surface oxidation of Pd. The distinct EOdes peak in the backward sweep corresponds to oxide reduction from the Pd surface. Since the position of this peak is directly correlated with the binding energy of oxygenated intermediate species, the pronounced oxide reduction peak and lowest onset potential observed for Pd-CNT suggest the highest energy barrier for oxide reduction23. Pt-CNT exhibits a similar CV profile to Pd-CNT, except for a shift in the EOdes peak position. Notably, Pt-CNT displays a more positive onset potential for oxide reduction compared to Pd-CNT, implying a lower energy barrier for the oxygen reduction reaction (ORR). This behavior is attributed to the optimized electronic structure of Pt. Meanwhile, the PdPt-CNT catalyst features an EOdes peak position similar to that of Pd-CNT, indicating a comparable energy barrier for oxygen reduction. However, the peak is broader and more pronounced, which can be attributed to the increased availability of active reaction sites due to Pt incorporation. Among all samples, the PdPt-CoNi catalyst demonstrates the highest positive ORR onset potential for the oxide reduction peak (EOdes), signifying the lowest energy barrier and, consequently, the highest ORR activity. A closer analysis reveals that the PdPt-CoNi catalyst exhibits distinct and sharp peaks (Ha/Ha*) at approximately 0.12 V vs. RHE in both the forward and backward sweeps within the HUPD region, indicative of strong hydrogen (H₂) evolution activity. Notably, this strong H₂ evolution activity has been widely reported in the literature as a characteristic feature of atomic-level alloying, confirming the presence of PtPd nanoalloys in the PdPt-CoNi catalyst24,25,26. Furthermore, the PdPt-CNT catalyst displays relatively smeared Ha/Ha* peaks, suggesting that the PdPt species are predominantly accommodated at defect sites within the CNT matrix. Figure 4b compares Nyquist plots of samples under investigation. Accordingly, the PdPt-CoNi shows the lowest charge transfer resistance (RCT) and thus the highest ORR kinetics.
Figure 5a compares the oxygen reduction reaction (ORR) polarization curves of the PdPt-CoNi catalyst with those of Pd-CNT, Pt-CNT, and PdPt-CNT. Among these, the PdPt-CoNi catalyst exhibits the highest half-wave potential (E₁/₂) of 0.917 V vs. RHE and an onset potential (VOC) of 0.975 V vs. RHE (Fig. 5b), outperforming its counterparts. These results indicate that the PdPt-CoNi catalyst possesses the lowest energy barrier and the highest reaction kinetics for ORR. Notably, the E₁/₂ and VOC values correspond closely to the oxide reduction peak (EOdes) observed in the CV curves, where the PdPt-CoNi catalyst features the highest EOdes peak potential, further confirming its reduced energy barrier for ORR. Additionally, the mass activity (MA) of the synthesized catalysts is determined by normalizing the kinetic current densities (Jk) (Fig. 5c) at 0.85 V vs. RHE with respect to the Pt and Pd metal loadings (details of MA calculation have been given in supplementary note 1). As illustrated in Fig. 5d, the PdPt-CoNi catalyst achieves an initial MA of 3250 mA mgPt–1 at 0.85 V vs. RHE and 635 mA mgPt–1 at 0.90 V vs. RHE, significantly surpassing its counterparts despite an ultra-low Pt loading of approximately 2.5 wt%. For a fair comparison, the ORR performance of the PdPt-CoNi catalyst is evaluated against commercial J.M.-Pt/C and reference samples (Figure S2). Collectively, the superior values of E₁/₂, VOC, Jk, and MA for the PdPt-CoNi catalyst, in contrast to those of the PtPd-CNT catalyst, unequivocally demonstrate that its enhanced reaction kinetics arise from the strong electronic interactions between the PdPt nanoalloys and the CoNi mixed-oxide support (Table S1). Finally, the Koutecky–Levich (K.L.) plots suggest the 4− - electron transfer pathway for all the catalysts. (Figure S3). Furthermore, the ORR mass activity at 0.85 V (vs. RHE) and 0.90 V (vs. RHE) of PdPt-CNT and PdPt-CoNi catalysts are calculated with respect to Pd + Pt metal loading and shown in Figure S4. Finally, the ORR performance of the PdPt-CoNi catalyst has been compared with literature in Table S2.
The PdPt-CoNi catalyst not only exhibited exceptional ORR performance but also demonstrated remarkable long-term durability. As shown in the representative ORR polarization curves (Fig. 6a), the PdPt-CoNi catalyst undergoes a positive shift in the half-wave potential (E₁/₂), indicating that its durability in the accelerated durability test (ADT) extends beyond 20,000 cycles. This enhanced stability can be attributed to surface restructuring, involving the redistribution of PdPt nanoalloys and the removal of surface oxides from the Pd/Pt surface27. This phenomenon is further corroborated by the cyclic voltammetry (CV) analysis after 20,000 ADT cycles (Fig. 6b), where the oxide reduction peak (Odes) in the backward sweep shifts to higher potentials with increasing ADT cycles. This shift signifies a reduced energy barrier for ORR, further reinforcing the catalyst’s superior durability. The HAADF-STEM image and the corresponding EDS elemental maps for Co, Ni, Pd, and Pt elements of post-stability PdPt-CoNi are shown in Figure S5. Accordingly, the PdPt-CoNi catalyst maintains its structure even after 20 K ADT cycles. In addition, the stability test results of commercial J.M.-Pt/C and PdPt-CNT catalysts are presented in Figure S6.
In-situ XAS investigations for disclosing the ORR pathways
The performance descriptors and associated ORR pathways on the surface of the PdPt-CoNi catalyst have been elucidated via in-situ XAS inspections at Pt-L3 edge, Pd-K, Co-K, and Ni-K edges. The in-situ XAS spectra at various edges were measured in fluorescence mode using a homemade electrochemical cell integrated with a potentiostat (CH Instruments Model 600B, CHI 600B) equipped with a three-electrode system. The in-situ XANES spectra of the PdPt-CoNi catalyst at Pt L3 and Pd K-edges are depicted in Fig. 7a and b, respectively. Accordingly, the similar positions of the inflection point (X) suggest an unchanged oxidation state of Pt and Pd under potential-driven and open circuit voltage (OCV) conditions. However, the PdPt-CoNi catalyst demonstrates progressively suppressing adsorption edge intensities HA and HM/N with increasing potentials, respectively for Pt L3 and Pd K-edges, indicating the increasing density of occupied orbitals for Pt and Pd domains18,20. Interestingly, the XANES spectra of Co K-edge (Fig. 7c) and Ni K-edge (Fig. 7d) exhibit the opposite behaviours as compared to Pt L3 edge and Pd Kedge XANES spectra, where progressively increasing adsorption edge intensities were observed with increasing applied potentials. Such a scenario confirms the severe electron relocation from CoNi mixed-support to Pt/Pd domains for enhanced ORR kinetics.
Conclusion
In summary, this study presents a novel PdPt-CoNi catalyst that achieves outstanding ORR performance with ultra-low noble metal loading. By supporting PdPt nanoalloys on a CoNi-mixed oxide framework, the catalyst delivers exceptional mass activity, reaching 3250 mA mgPt–1 at 0.85 V vs. RHE and 635 mA mgPt–1 at 0.90 V vs. RHE in 0.1 M KOH. This represents a significant enhancement, surpassing the commercial J.M.-Pt/C (20 wt%) catalyst by approximately 48 and 28 times, respectively. In contrast, carbon-supported PdPt nanoalloys with a similar metal composition exhibit a substantial decline in ORR activity, reinforcing the importance of the CoNi-mixed oxide support. Furthermore, the PdPt-CoNi catalyst demonstrates remarkable durability, maintaining 100% of its initial activity even after 20,000 ADT cycles. In-situ X-ray absorption spectroscopy confirms that the superior ORR performance is driven by strong metal-support interactions. Overall, this work underscores the synergistic effects of PdPt nanoalloys and CoNi-mixed oxides in improving ORR kinetics and long-term stability, offering a promising pathway for next-generation electrocatalysts with minimal noble metal usage.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Liu, M., Zhao, Z., Duan, X. & Huang, Y. Nanoscale structure design for high-performance Pt-based ORR catalysts. Adv. Mater. 31, 1802234. (2019).
Zhao, Z. et al. Pt-based nanocrystal for electrocatalytic oxygen reduction. Adv. Mater. 31, 1808115. (2019).
3 Ren, X. et al. Current progress and performance improvement of pt/c catalysts for fuel cells. J. Mater. Chem. A. 8, 24284–24306. https://doi.org/10.1039/D0TA08312G (2020).
Li, C. et al. Emerging Pt-based electrocatalysts with highly open nanoarchitectures for boosting oxygen reduction reaction. Nano Today. 21, 91–105. https://doi.org/10.1016/j.nantod.2018.06.005 (2018).
Chung, H. T. et al. Direct atomic-level insight into the active sites of a high-performance PGM-free ORR catalyst. Science 357, 479–484. https://doi.org/10.1126/science.aan2255 (2017).
Jiao, L. et al. Chemical vapour deposition of Fe–N–C oxygen reduction catalysts with full utilization of dense Fe–N4 sites. Nat. Mater. 20, 1385–1391. https://doi.org/10.1038/s41563-021-01030-2 (2021).
Xu, X. et al. Collective effect in a multicomponent ensemble combining single atoms and nanoparticles for efficient and durable oxygen reduction. Angew. Chem. Int. Ed. 63, e202400765. https://doi.org/10.1002/anie.202400765 (2024).
Sun, X. et al. Fine engineering of d-Orbital vacancies of ZnN4 via High-Shell metal and nonmetal Single-Atoms for efficient and Poisoning-Resistant ORR. Nano Lett. 24, 14602–14609. https://doi.org/10.1021/acs.nanolett.4c02830 (2024).
Greeley, J. et al. Alloys of platinum and early transition metals as oxygen reduction electrocatalysts. Nat. Chem. 1, 552–556. https://doi.org/10.1038/nchem.367 (2009).
Jia, Q. et al. Metal and metal oxide interactions and their catalytic consequences for oxygen reduction reaction. J. Am. Chem. Soc. 139, 7893–7903. https://doi.org/10.1021/jacs.7b02378 (2017).
Jukk, K., Kongi, N., Tammeveski, K., Solla-Gullón, J. & Feliu, J. M. Electroreduction of oxygen on PdPt alloy nanocubes in alkaline and acidic media. ChemElectroChem 4, 2547–2555. https://doi.org/10.1002/celc.201700588 (2017).
Zhai, Y., Zhu, Z. & Dong, S. Carbon-Based Nanostructures for Advanced Catalysis. ChemCatChem 7, 2806–2815, (2015). https://doi.org/10.1002/cctc.201500323
Hao, Y., Xu, Y., Liu, J. & Sun, X. Nickel–cobalt oxides supported on co/n decorated graphene as an excellent bifunctional oxygen catalyst. J. Mater. Chem. A. 5, 5594–5600. https://doi.org/10.1039/C7TA00299H (2017).
Yabesh, N. R. K., Marimuthu, S. & Maduraiveeran, G. Potent noble Metal–Nickel oxide interaction: A strategy for improved oxygen reduction reaction via engineering the electronic structure. J. Phys. Chem. C. 128, 11562–11571. https://doi.org/10.1021/acs.jpcc.4c03056 (2024).
Liu, H., Yan, X., Luo, W., Liu, J. & Ren, S. Effect of Pd crystal facet on the reaction of oxygen-promoted hydrogen evolution from formaldehyde driven by visible light. Colloids Surf., A. 673, 131820. https://doi.org/10.1016/j.colsurfa.2023.131820 (2023).
Bhalothia, D. et al. Potential synergy between Pt2Ni4 Atomic-Clusters, oxygen vacancies and adjacent Pd nanoparticles outperforms commercial Pt nanocatalyst in alkaline fuel cells. Chem. Eng. J. 483, 149421. https://doi.org/10.1016/j.cej.2024.149421 (2024).
Bhalothia, D. et al. Surface-decorated Sn-oxide and atomic Sn-metal clusters boost the oxygen reduction reaction performance of palladium nanoparticles. J. Alloys Compd. 1014, 178583. https://doi.org/10.1016/j.jallcom.2025.178583 (2025).
Bhalothia, D., Lin, C. Y., Yan, C., Yang, Y. T. & Chen, T. Y. H2 reduction annealing induced phase transition and improvements on redox durability of Pt Cluster-Decorated cu@pd electrocatalysts in oxygen reduction reaction. ACS Omega. 4, 971–982. https://doi.org/10.1021/acsomega.8b02896 (2019).
Bhalothia, D., Lin, C. Y., Yan, C., Yang, Y. T. & Chen, T. Y. Effects of Pt metal loading on the atomic restructure and oxygen reduction reaction performance of Pt-cluster decorated cu@pd electrocatalysts. Sustainable Energy Fuels. 3, 1668–1681. https://doi.org/10.1039/C9SE00074G (2019).
20 Watanabe, S. et al. Spectroscopic and first-principles calculation studies of the chemical forms of palladium ion in nitric acid solution for development of disposal of high-level radioactive nuclear wastes. AIP Adv. 8, 045221. https://doi.org/10.1063/1.5025778 (2018).
Rahul, R., Singh, R. K. & Neergat, M. Effect of oxidative heat-treatment on electrochemical properties and oxygen reduction reaction (ORR) activity of Pd–Co alloy catalysts. J. Electroanal. Chem. 712, 223–229 (2014).
Łukaszewski, M. & Czerwiński, A. Electrochemical behavior of palladium–gold alloys. Electrochim. Acta. 48, 2435–2445. https://doi.org/10.1016/S0013-4686(03)00270-6 (2003).
Bhalothia, D. et al. Pt-Mediated interface engineering boosts the oxygen reduction reaction performance of Ni Hydroxide-Supported Pd nanoparticles. ACS Appl. Mater. Interfaces. 15, 16177–16188. https://doi.org/10.1021/acsami.2c21814 (2023).
Wells, P. P. et al. Preparation, structure, and stability of Pt and Pd monolayer modified Pd and Pt electrocatalysts. Phys. Chem. Chem. Phys. 11, 5773–5781. https://doi.org/10.1039/B823504J (2009).
Wang, R. et al. Dispersing Pt atoms onto nanoporous gold for high performance direct formic acid fuel cells. Chem. Sci. 5, 403–409. https://doi.org/10.1039/C3SC52792A (2014).
Yang, T. et al. Surface-Limited synthesis of Pt nanocluster decorated Pd hierarchical structures with enhanced electrocatalytic activity toward oxygen reduction reaction. ACS Appl. Mater. Interfaces. 7, 17162–17170. https://doi.org/10.1021/acsami.5b04021 (2015).
Bhalothia, D. et al. Iridium single atoms to nanoparticles: nurturing the local synergy with Cobalt-Oxide supported palladium nanoparticles for oxygen reduction reaction. Adv. Sci. 11, 2404076. https://doi.org/10.1002/advs.202404076 (2024).
Acknowledgements
The authors thank the staff of the National Synchrotron Radiation Research Center (NSRRC), Hsinchu, Taiwan for helping in various synchrotron-based spectroscopies and diffraction analysis (BL-01C1, BL-01C2, BL-07 A, BL-16 A and BL-17 C). T.-Y. Chen acknowledges the funding support from the National Science and Technology Council, Taiwan (NSTC 113-2112-M-007-014-) and the industrial collaboration projects from the MA-tek (MA-tek 2024-T-018) and the Taiwan Space Agency (TASA-S-1120691). Dinesh Bhalothia acknowledges the funding support (Enhanced Seed Grant EF/2024-25/QE-04-08) from Manipal University Jaipur.
Funding
Open access funding provided by Manipal University Jaipur. Open access funding provided by Manipal University Jaipur. T.-Y. Chen acknowledges the funding support from the National Science and Technology Council, Taiwan (NSTC 113-2112-M-007-014-) and the industrial collaboration projects from the MA-tek (MA-tek 2024-T-018) and the Taiwan Space Agency (TASA-S-1120691). Dinesh Bhalothia acknowledges the funding support (Enhanced Seed Grant EF/2024-25/QE-04-08) from Manipal University Jaipur.
Author information
Authors and Affiliations
Contributions
D.B. writing original draft, data acquisition, and fundingAshima Bagaria: validationAmisha Beniwal: formal analysisT.-Y. C.: Formal Review.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Bhalothia, D., Bagaria, A., Beniwal, A. et al. Strong electronic coupling between CoNi mixed oxide support and PdPt nanoalloys enables highly active and durable oxygen reduction reaction. Sci Rep 15, 23403 (2025). https://doi.org/10.1038/s41598-025-07795-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-07795-9