Abstract
Hyperlipidemia, a metabolic disorder characterized by abnormal lipid levels, is closely linked to an increased risk of cardiovascular disease. In this study, we investigated the hypolipidemic properties of Paeoniae Radix Rubra and its regulatory effects on gut microbiota composition in a high-fat diet model. Using UHPLC-QE-MS/MS, we identified its chemical constituents and applied bioinformatics, network pharmacology, and molecular docking to virtually screen for bioactive compounds and molecular targets. Gelomulide N and (E)-5-[(1 S,4aR,8aR)-2-formyl-5,5,8a-trimethyl-1,4,4a,6,7,8-hexahydronaphthalen-1-yl]-3-(acetoxymethyl)pent-2-enoic acid were identified as potential active compounds. Paeoniae Radix Rubra exhibited notable hypolipidemic, hepatoprotective, and gut microbiota-restoring effects, potentially influencing the mevalonate pathway by interacting with proteins such as P53, HMGCR, and SREBP2, which may contribute to reduced cholesterol synthesis. These findings indicate that the Paeoniae Radix Rubra could serve as a potential therapeutic strategy for hyperlipidemia, possibly mediated through modulation of lipid metabolism pathways and gut microbiota remodeling.
Similar content being viewed by others
Introduction
Hyperlipidemia, a prevalent metabolic disorder, characterized by abnormally elevated levels of lipids, also referred to as dyslipidemia. Over the past three decades, the incidence of hyperlipidemia has steadily increased worldwide, especially in developing countries, where the prevalence of dyslipidemia among adults has been rising annually, with an increasingly younger population affected. This trend is largely attributable to changes in dietary patterns, as high-fat diets contribute to the elevation of blood lipid levels, further exacerbating hyperlipidemia1.
Hyperlipidemia is closely linked to cardiovascular pathologies including coronary atherosclerosis, stroke, and myocardial infarction2. Lipid accumulation in the liver promotes non-alcoholic fatty liver disease (NAFLD) progression through hepatic steatosis and injury3. High-fat diets inducing hyperlipidemia further disrupt gut microbiota homeostasis, exacerbating metabolic dysregulation and accelerating disease progression4. Currently, the primary clinical drugs have many side effects. Therefore, exploring new therapeutic strategies for hyperlipidemia is urgently needed.
Paeoniae Radix Rubra (PRR), derived from the dried roots of Paeonia lactiflora Pall. or Paeonia veitchii Lynch of the Ranunculaceae family. Modern pharmacology research has found that specific components of PRR can reduce blood viscosity and inhibit platelet aggregation, thereby exerting antithrombotic effects. Furthermore, studies have demonstrated that the administration of PRR significantly modulates lipid metabolism5,6. These findings suggest its potential therapeutic benefits in the prevention and management of metabolic diseases, including atherosclerosis, diabetes mellitus, and obesity.
The role of gut microbiota in regulating lipid metabolism is significant. A prolonged high-fat diet may disrupt the balance of gut microbiota, adversely affecting lipid metabolism and intestinal barrier function, which in turn leads to hyperlipidemia. Studies have shown that PRR has inhibitory effects on Helicobacter pylori, Bacillus subtilis, and certain Clostridia species7,8. However, studies on the use of PRR for the treatment of hyperlipidemia are still scarce, and the bioactive components of its efficacy are unknown. In addition, whether PRR can restore the dysregulated gut microbiota and subsequently alleviate the progression of hyperlipidemia phenotypes remains largely unexplored. Given the complexity and diversity of the components in PRR, bioinformatics techniques are indispensable for investigating its lipid-lowering effects. In this study, we employed an integrated approach combining network pharmacology, molecular docking, and data from the Gene Expression Omnibus (GEO) database. This strategy aimed to identify potential therapeutic targets associated with hyperlipidemia and comprehensively elucidate the underlying mechanisms through which PRR exerts its therapeutic effects. Additionally, the effects of PRR on lipid metabolism and gut microbiota were further investigated using 16 S rRNA high-throughput sequencing in mice fed a high-fat diet, providing deeper insights into its potential mechanisms of action.
Materials and methods
Materials and chemicals
The PRR used in this study was sourced from the Yanbian area in Jilin Province and was authenticated by Professor Lin Zhe from Changchun University of Chinese Medicine, in accordance with the 2020 edition of the Chinese Pharmacopoeia (sample number: 202211041249R). Simvastatin, was obtained from Hunan Mingrui Pharmaceutical Co., Ltd. Biochemical assay kits for total cholesterol (TC, A111-1-1), triglycerides (TG, A110-1-1), high-density lipoprotein cholesterol (HDL-C, A112-1-1), low-density lipoprotein cholesterol (LDL-C, A113-1-1), aspartate aminotransferase (AST, C010-2-1), alanine aminotransferase (ALT, C009-2-1), superoxide dismutase (SOD, A001-3-2), and malondialdehyde (MDA, A003-1-2) were all procured from Nanjing Jiancheng. The ELISA kit used to detect acetyl-CoA acetyltransferase (ACAT) levels in mice was purchased from Jiangsu Meimian Industrial Co., Ltd. Antibodies against P53 (10442-1-AP), ABCA1 (26564-1-AP), SREBP2 (28212-1-AP), MVD (15331-1-AP), MVK (12228-1-AP), and GAPDH (10494-1-AP) were sourced from Proteintech Biotechnology Co., Ltd., Hubei Province. The HMGCR antibody (#DF6518) was provided by Affinity Biosciences.
Composition analysis of aqueous extract of PRR
Untargeted component analysis of the aqueous extract of PRR was performed by Ultra-High Performance Liquid Chromatography-Quadrupole-Electrostatic Field Orbitrap Mass Spectrometry (UHPLC-QE-MS/MS) with a UPLC HSS T3 column (2.1 mm × 100 mm, 1.8 μm) coupled to Orbitrap Exploris 120 mass spectrometer (Orbitrap MS, Thermo). The mobile phase A consisted of water with 5 mmol/L ammonium acetate and 5 mmol/L acetic acid, while phase B was acetonitrile. The Orbitrap Exploris 120 mass spectrometer, controlled by the acquisition software (Xcalibur, Thermo), collected MS/MS spectra in an information-dependent acquisition (IDA) mode. The ESI source conditions were set as follows: sheath gas flow rate at 50 Arb, auxiliary gas flow rate at 15 Arb, capillary temperature at 320℃, full MS resolution at 60,000, MS/MS resolution at 15,000, collision energy at 10/30/60 (NCE mode), and spray voltages at 3.8 kV (positive) or −3.4 kV (negative).
Raw data were converted into mzXML format using ProteoWizard software, followed by XCMS-based data processing according to reference9:
(1)Peak Detection.
EIBPC chromatograms were generated through 0.1 m/z slicing. Second-derivative Gaussian filtering (σ = 13 s, FWHM = 30 s) enhanced peak morphology characteristics. Peaks were identified using SNR ≥ 10 threshold, with isotope interference removal via adjacent elimination algorithm.
(2) Peak Matching.
0.25 m/z binning enabled mass dimension clustering. Kernel density estimation (KDE, σ = 30 s) identified time-distributed “meta peaks” within each bin.
(3) Peak Alignment (Retention Time Alignment).
Stable chromatographic peaks were dynamically selected as internal standards. Nonlinear drift correction was achieved through LOESS regression (span = 0.3), with iterative KDE optimization (σ = 10 s) enhancing alignment precision.
(4) Peak Integration.
Missing peaks were backfilled using corrected retention time windows. Integration boundaries were defined via filtered zero-crossing points. Final peak areas were recalculated from raw spectral data.
Compound annotation was completed using a secondary mass spectrometry database (BiotreeDB V2.1, Biotree, Shanghai, China) with a threshold set at 0.3.
Screening of active compounds in the aqueous extract of PRR
Based on the components identified through UHPLC-QE-MS/MS analysis, active compounds were screened by evaluation on the SwissADME website (http://www.swissadme.ch/). Candidate components exhibited all six pharmaceutical parameters (Lipophility, SIZE, Polarity, Insolubility, Insaturation, Flexibility) within the optimal pink zone of the drug-likeness radar chart and if they satisfied at least three “Yes” parameters in the Druglikeness Section10,11. These criteria ensure that the selected compounds possess favorable oral bioavailability and drug-likeness, qualifying them as the medicinal components of the aqueous extract of PRR.
Targeted network Pharmacology analysis
Component screening of PRR
The SMILES codes of the screened active compounds were retrieved from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/) and subsequently imported into the SwissTargetPrediction platform (http://swisstargetprediction.ch/) for further target prediction12. Only targets with a “probability > 0” were selected, and compounds with no probable targets were excluded. The remaining compounds were considered as candidates for network pharmacology analysis. All identified targets were then consolidated, and duplicates were removed to establish the final list of medicinal targets for PRR.
Collection of hyperlipidemia targets
Using the keywords “Hyperlipidemia” and “Dyslipidemia,” the databases “OMIM,” (https://omim.org/) “DisGeNET,” (https://disgenet.com/) “GeneCards,” (https://www.genecards.org/) and the “Therapeutic Target Database” (https://db.idrblab.net/ttd/) were searched to retrieve and collect disease targets associated with hyperlipidemia, eliminating any duplicate targets. For the OMIM database, only targets marked with an asterisk (*) were selected; for DisGeNET, the targets with a “Score_gda > 0.1” were selected; and for GeneCards, the targets with a “Relevance score ≥ 0.5” were included. The non-standard target names obtained from these searches were then entered into the Uniprot database (https://www.uniprot.org/) to acquire the corresponding gene names for all targets13.
Additionally, two mRNA expression microarray datasets, GSE15653 and GSE111412, related to hyperlipidemia were obtained from the GEO database (https://www.ncbi.nlm.nih.gov/geo/). The GSE15653 dataset included clinical samples of liver tissue from 5 normal individuals and 4 obese patients, while the GSE111412 dataset comprised adipose tissue samples from 3 normal mice and 3 high-fat diet (HFD) mice. Gene expression differences were analyzed in Rstudio with the help of the “limma” package, with the screening criteria set at “|Log2FC| > 0.5” and “P < 0.05” to identify significant differentially expressed genes in both datasets. We then merged the gene targets obtained from the six databases, removing duplicates, to establish a comprehensive list of disease targets for hyperlipidemia.
Protein-Protein interaction (PPI) analysis
The common targets of PRR and hyperlipidemia were further pinpointed utilizing the Venny 2.1 web tool (https://bioinfogp.cnb.csic.es/tools/venny/). These intersection targets were considered potential therapeutic targets for PRR in treating hyperlipidemia, as the herb may exert its lipid-lowering effects through these targets. Next, We employed the STRING database (http://string-db.org/cgi/input.pl) to identify the common targets and built a protein-protein interaction (PPI) network, setting the confidence “score to ≥ 0.9”. The resulting PPI network was then imported into Cytoscape 3.7.0 for visualization. To further analyze the network, we utilized the CytoNCA14, MCODE15, and CytoHubba16 modules in Cytoscape. By intersecting the core targets identified by these three modules, we obtained the key therapeutic targets of PRR for treating hyperlipidemia.
GO and KEGG enrichment analysis
We upload the core targets to Metascape and setting the parameters to “H species,” the enrichment analysis results for GO Biological Processes (GO BP), Cellular Components (GO CC), Molecular Functions (GO MF), and KEGG signaling pathways were generated. The results collected were “P < 0.01,” with a minimum count of “3” and an enrichment factor greater than 1.5 (the enrichment factor is the ratio of observed counts to those expected by chance). These criteria were used to predict the potential mechanisms by which PRR exerts its therapeutic effects on hyperlipidemia.
Molecular Docking analysis
The molecular docking methods were employed to calculate and analyze the affinity and binding sites between the components of PRR and the core targets identified in Sect. “Protein-Protein interaction (PPI) analysis” at the molecular level. This analysis further explores the interactions between the compounds and biological molecules. The three-dimensional (3D) structures of the small-molecule ligands, which are components of PRR, were sourced from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/). Meanwhile, the crystal structures of the macromolecular receptors, representing the core targets, were retrieved from the RCSB PDB database (https://www.rcsb.org/)). Using the MOE (2022.02) software, the semi-flexible docking were performed. The ligands underwent energy minimization, and the receptor proteins were preprocessed by removing water molecules, protonation, and completing or correcting the crystal structures. Finally, the ligands and receptors were docked to predict their interactions. In general, binding energies below − 5.0 kcal/mol are indicative of good binding activity, whereas those below − 7.0 kcal/mol denote strong binding affinity17. It is generally accepted that the binding energy of the compound and the target is below − 5.0 kcal/mol suggesting good binding activity between the two, and below − 7.0 kcal/mol having strong binding activity18, we selected the docking results with binding energies below − 5.0 kcal/mol and visualized the results.
Animal experiments
Animals
72 SPF grade male mice, weighing 18–22 g, were provided by Liaoning Changsheng Biotechnology Co., Ltd. (Certificate No.: SCX (Liao) 2020-0001). The experiment was approved and supervised by the Experimental Animal Ethics Committee of Changchun University of Chinese Medicine (Ethics Approval No.: 2020262). Throughout the experiment, the mice were housed under controlled conditions with a temperature of 23 ± 2℃, humidity of 55 ± 10%, and a 12-hour light-dark cycle.
Grouping and administration
After one week of adaptive feeding, the mice were randomly divided into a normal control group (NC, n = 12) and a high-fat diet group (HFD, n = 60). The NC group was fed standard chow provided by Liaoning Changsheng Biotechnology Co., Ltd., while the high-fat diet administered to the HFD group was sourced from Yisi Experimental Animal Technology Co., Ltd., Changchun, Jilin Province (Ingredients: Crude Protein (20.34%), Crude Fat (14.89%), Carbohydrates (58.83%), Sugar (20.00%), number: 20230324), with free access to water. After 8 weeks of feeding, total cholesterol (TC) and triglyceride (TG) levels in the HFD group were measured using assay kits. The HFD group was then further subdivided into the following groups: high-fat diet group (HFD, n = 12), positive control group (simvastatin, SIM, 20 mg/kg, n = 12), and three dosage groups of PRR extract (high dose, CSH, 100 mg/kg; medium dose, CSM, 50 mg/kg; low dose, CSL, 25 mg/kg; n = 12). The SIM group and PRR extract groups were administered their respective doses by gavage, while the NC and HFD groups received distilled water by gavage in the same volume, once daily for 5 weeks. Food intake was recorded daily, and body weight was measured every three days.
Mouse fat content measurement
At the end of the 13 weeks feeding period, the day prior to euthanizing the mice, we administered anesthesia using 1% sodium pentobarbital (50 mg/kg). The fat content of the mice was measured using a small animal X-ray in vivo imaging system (Faxitron UltraFocus, USA).
Sample collection
After the final administration at the end of the 13th week, the mice were weighed, and blood samples were collected. After blood collection, the liver and perirenal fat were harvested and weighed to calculate the liver index (liver index = liver weight/body weight×100%). Formed feces from the colon were collected in sterile 1.5 mL EP tubes, rapidly frozen in liquid nitrogen, and stored at −80℃ for further analysis.
Biochemical index analysis
Serum levels of total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), aspartate aminotransferase (AST), alanine aminotransferase (ALT), superoxide dismutase (SOD), and malondialdehyde (MDA) and acyl coenzyme a-cholesterol acyltransferase (ACAT) were measured using respective assay kits.
Histopathological analysis
The harvested liver and perirenal fat were fixed in 4% paraformaldehyde for 24 h. The tissues were then processed for histological examination either by paraffin embedding for sectioning or by cryosectioning. Hematoxylin and eosin (HE) staining and Oil Red O staining were performed to observe the pathological changes in the mouse tissues.
Gut microbiota analysis
The DNA from the mouse fecal samples was isolated utilizing the TGuide S96 Magnetic Bead Stool DNA Extraction Kit. Next, the V3-V4 regions of the 16 S rRNA gene were amplified using the following primers: 338 F (5’-ACTCCTACGGGAGGCAGCA-3’) and 806R (5’-GGACTACHVGGGTWTCTAAT-3’). The resulting PCR products were sequenced on the Illumina NovaSeq 6000 platform (Beijing Biomarker Technologies Co., Ltd.).
Subsequent analysis included Alpha Diversity and Beta Diversity assessments, differential species analysis, and functional prediction analysis to investigate the impact of PRR on the gut microbiota of hyperlipidemic mice. The functional prediction analysis was conducted using the PICRUSt2 method. PICRUSt2 aligns the characteristic sequences (16 S rRNA) with reference sequences from the Integrated Microbial Genomes (IMG) database to construct a phylogenetic tree and identify the “nearest species” to the characteristic sequences. Based on the known gene types and abundance information of the species, we predicted the gene information of unknown species. By combining the gene information with KEGG pathway data, we predicted the functional pathways of the entire microbiota community. PICRUSt2 enables prediction of 16 S rRNA gene sequences using the KEGG (https://www.kegg.jp/)19 and COG (https://www.ncbi.nlm.nih.gov/COG/) functional databases.
Immunohistochemical (IHC) analysis of mouse liver
Immunohistochemical analysis was performed on the livers of mice from each group (Servicebio Technology Co., Ltd., Wuhan, China). Liver tissues fixed in 4% paraformaldehyde were processed for paraffin embedding and sectioning. After dewaxing and rehydration, antigen retrieval was conducted using citrate buffer. The sections were then treated with 3% hydrogen peroxide and PBS, followed by blocking with 3% BSA. The primary antibodies HMGCR (1:1500) and SREBP2 (1:200) were incubated with the sections overnight at 4℃. This was followed by incubation with a secondary antibody and then staining with diaminobenzidine (DAB) and hematoxylin. The sections were then dehydrated, mounted, and observed under a microscope. The positive staining rates of HMGCR and nuclear SREBP2 were calculated.
Western blot analysis of mouse liver
A 0.1 g liver tissue sample was finely minced and lysed in a pre-chilled lysis buffer. The tissue was homogenized on ice and centrifuged at 13,000 rpm for 20 min at 4℃. The supernatant was collected, and the protein concentration was determined using a BCA protein assay kit. The supernatant was then diluted to the same concentration with PBS and mixed with Loading Buffer (6×) at a 1:5 ratio, followed by boiling for 5 min. After electrophoresis, the samples were transferred to a membrane and incubated overnight at 4℃ with primary antibodies P53 (1:10000), ABCA1 (1:1000), SREBP2 (1:4000), MVD (1:1000), MVK (1:2000), and GAPDH (1:10000). The next day, they were incubated with secondary antibodies at room temperature for 2 h. The next day, the membrane was incubated with the corresponding secondary antibodies for 2 h at room temperature. Chemiluminescence detection was performed using the Alliance Q9 fluorescence imaging system. The images were scanned, and grayscale analysis was conducted to quantify and evaluate the experimental results.
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics 27. Data are presented as mean ± standard deviation (SD). Statistical significance was assessed with two-tailed Student’s t-tests. One-way analysis of variance (ANOVA) was used for comparisons among three or more groups. All P-values were two-sided, and a P-value<0.05 were deemed statistically significant.
Statement
All experiments followed ARRIVE guidelines.
Results
Analysis of components of PRR by UHPLC-QE-MS/MS
To explore the potential bioactive compounds in PRR, the aqueous decoction were conducted to analysis by UHPLC-QE-MS/MS. The characteristic ions of each substance were screened and the major components were analyzed. Following the exclusion of non-botanical constituents through the Lotus database (https://lotus.naturalproducts.net/), a total of 1146 compounds were identified, categorized into 13 major classes. The distribution of these classes is as follows: Alkaloids and derivatives (0.893%), Benzenoids (15.179%), Hydrocarbon derivatives (0.446%), Lignans, neolignans, and related compounds (0.893%), Lipids and lipid-like molecules (22.545%), Nucleosides, nucleotides, and analogues (2.455%), Organic acids and derivatives (9.821%), Organic nitrogen compounds (0.670%), Organic oxygen compounds (13.393%), Organoheterocyclic compounds (16.071%), Organooxygen compounds (0.223%), Phenylpropanoids and polyketides (15.402%), and Others (2.009%). The positive and negative total ion chromatograms obtained by UHPLC-QE-MS/MS are shown in Supplementary Figure (SF) 1 A and 1B, respectively, the specific classifications and their proportions mentioned above are shown in SF.1 C.
After screening these compounds on the SwissADME website, 18 compounds were identified as candidates for targeted network pharmacology. These compounds were labeled from CS01 to CS18, in descending order of area. Detailed information on these compounds can be found in Supplementary Table (ST) 1, and the chemical formulas of the 18 compounds are shown in SF.2.
Targeted network Pharmacology results
Screening of PRR components and active targets
Based on the UHPLC-QE-MS/MS analysis results, the 18 active compounds identified were screened on the SwissTargetPrediction website. All 18 compounds were found to have targets with a “probability > 0”, indicating that these compounds may contribute to the herb’s therapeutic effects. By consolidating all targets associated with these compounds, a total of 515 potential therapeutic targets were obtained. The relationship between the 18 compounds and their 515 targets was visualized using Cytoscape, as shown in Fig. 1A.
Core Target Screening for PRR in the Treatment of Hyperlipidemia. (A) The network topology diagram illustrating 18 medicinal compounds and 515 target components. The purple square represents the medicinal herb Paeoniae Radix Rubra, pink triangles denote the 18 chemical constituents, blue ellipses indicate the 515 target components, and black lines reflect the interactions between these nodes. (B) The volcano plot derived from the GSE15653 microarray dataset, with red dots on the right representing 991 upregulated genes, and blue dots on the left indicating 1,300 downregulated genes. (C) The volcano plot from the GSE111412 microarray dataset, where red dots on the right signify 787 upregulated genes, and blue dots on the left correspond to 467 downregulated genes. (D) The intersection between 515 component targets and 4,716 disease targets, illustrated by an Upset plot on the left and a Venn diagram on the right, with the intersecting targets constituting 4% of the total. (E) The protein-protein interaction (PPI) network of 217 intersecting targets, where three protein targets—PANK3, QTRT1, and DYRK1B—are shown as isolated, with no interactions with other protein targets. (F) Visualization of 214 targets obtained from the PPI analysis imported into Cytoscape. Each node symbolizes a target, represented by a circle, while the edges depict the interactions between targets. Targets with higher degree values are represented by larger areas and warmer colors; stronger interactions are indicated by thicker and cooler-colored edges. (G) Identification of 14 core targets selected through the CytoNCA, MCODE, and CytoHubba modules.
Screening of hyperlipidemia disease targets
To identify disease targets associated with hyperlipidemia, we searched databases such as OMIM, DisGeNET, GeneCards, and TTD using keywords like “Hyperlipidemia” and “Dyslipidemia”. We have found 896 disease targets from the OMIM database, 471 from the DisGeNET database, 1378 from the GeneCards database, and 10 from the TTD database were obtained. Additionally, microarray datasets related to hyperlipidemia were retrieved from the GEO database. From the GSE15653 dataset, which included liver tissue samples from 5 normal individuals and 4 hyperlipidemia patients, 2291 differentially expressed genes (DEGs) were identified through limma analysis, as shown in Fig. 1B. From the GSE111412 dataset, which included adipose tissue samples from 3 normal mice and 3 high-fat diet (HFD) mice, 1254 DEGs were identified, as shown in Fig. 1C. After consolidating and removing duplicates from all disease database targets, a total of 4716 hyperlipidemia-related targets were identified.
PPI network analysis and core target screening for PRR in treating hyperlipidemia
The 515 therapeutic targets of PRR were intersected with the 4716 hyperlipidemia-related targets, resulting in 217 common targets, as shown in Fig. 1D. Next, network topology analysis were performed on these common targets using the STRING 12.0 database, with a comprehensive score threshold set to > 0.7. The network analysis calculated three topological parameters for common targets: “degree”, “betweenness centrality”, and “closeness centrality”, which were used as scoring criteria. The definitions of the topological features are as follows: “degree” refers to the number of interactions a node has, “betweenness centrality” indicates the number of shortest paths that pass through the node and “closeness centrality” represents the sum of distances from a node to all other nodes, reflecting the time required for information to propagate sequentially from the node to all others. The larger these parameters, the more important the node is within the network.
Before screening, the network consisted of 214 nodes and 2757 edges. Three protein targets (PANK3, QTRT1, and DYRK1B) were isolated and had no interactions with other protein targets, as shown in Fig. 1E. Therefore, these three were removed from the 217 common targets. Then the CytoNCA, MCODE, and CytoHubba modules in Cytoscape were used to screen for core targets. The final network consisted of 14 nodes and 75 edges, representing 14 core targets: TNF, TP53, AKT1, PPARG, HMGCR, CASP3, ESR1, HSP90AA1, MMP9, PPARA, ACE, SREBF2, PPARD, and SERPINE1. These core targets were identified as key therapeutic targets for PRR in the treatment of hyperlipidemia. The interaction network was visualized using Cytoscape software, which can be observed in Fig. 1F and G.
Enrichment analysis results of PRR for hyperlipidemia in the metascape database
After identifying the core targets for PRR in treating hyperlipidemia, we sought to understand how the herb mediates these targets to exert its lipid-lowering effects. Therefore, we utilized the Metascape database to conduct enrichment analyses on the 14 identified core targets. which were represented in chord diagrams, as shown in Fig. 2A, B and C. The enrichment degree and significance of the three GO categories were visualized using bar charts and bubble plots, as shown in Fig. 2D and E.
GO Enrichment and KEGG Pathway Enrichment Analysis of Core Targets for PRR in the Treatment of Hyperlipidemia. A. A chord diagram visualizing the top 10 Biological Process (BP) terms from the GO enrichment analysis, illustrating the relationships between different biological processes and core targets. B., C. Chord diagrams representing the top 10 Cellular Component (CC) and Molecular Function (MF) terms from the GO enrichment analysis, respectively, showcasing the connections between cellular components, molecular functions, and core targets. D. A bar chart summarizing the GO enrichment analysis results for the core targets in the treatment of hyperlipidemia. The chart integrates BP, CC, and MF categories, distinguished by different colors—pink for BP, dark blue for CC, and light blue for MF. The y-axis, labeled as -Log10(p), reflects the significance level of each term. E. A bubble chart representing the enrichment analysis for BP, CC, and MF categories, where the size and color of the bubbles display multidimensional data. The y-axis indicates the enriched biological processes, cellular components, and molecular functions, while the x-axis represents the Gene Ratio (the ratio of genes enriched in a given term to the total genes used in the analysis). The bubble size corresponds to the number of targets enriched in each category, and the color indicates the significance level, with pink signifying higher significance. F. A Sankey-bubble chart visualizing the pathway enrichment analysis results for hyperlipidemia treatment using Paeoniae Radix Rubra. The left side features a Sankey diagram showing the genes involved in each pathway, while the right side displays a bubble chart. The bubbles are positioned according to the Gene Ratio, sized according to the number of genes enriched in each pathway, and colored according to the p-value, with red indicating higher significance.
For KEGG enrichment analysis, the pathways enriched by each target are listed in ST 2, and the results were visualized using a Sankey-bubble diagram, which can be found in Fig. 2F. The analysis revealed that PRR might involve 17 biological processes in treating hyperlipidemia. The top 10 enriched processes were: regulation of miRNA transcription, response to decreased oxygen levels, response to hormone, cellular response to lipid, rhythmic process, glucose metabolic process, response to lipopolysaccharide, regulation of mitochondrion organization, female pregnancy, and positive regulation of miRNA transcription. Additionally, PRR may exert its lipid-lowering effects by regulating molecular functions such as nitric-oxide synthase regulator activity, nuclear receptor activity, protease binding, and endopeptidase activity. These functions may act on cellular components including the collagen-containing extracellular matrix, neuronal cell body, transcription regulator complex, and cilium. The potential mechanisms of PRR in treating hyperlipidemia might be associated with pathways like Lipid and Atherosclerosis, Proteoglycans in Cancer, IL-17 Signaling Pathway, Chagas Disease, and Diabetic Cardiomyopathy.
Molecular Docking results
To further explore the potential mechanism of PRR in treating hyperlipidemia, the researchers performed molecular docking to elucidate the lipid-lowering effects of the herb at the molecular level. It revealed that compounds CS14 and CS15 exhibited lower binding energies with several core targets, indicating stronger binding affinities. Among the core targets, P53 (TP53), HMGCR, and SREBP2 (SREBF2) displayed favorable docking results with multiple compounds, particularly with CS14 and CS15, which showed strong binding activity, as shown in ST 3.
The docking results between compounds CS14, CS15, and the three key targets (P53, HMGCR, and SREBP2) were visualized. The 2D and 3D docking diagrams, along with the protein surface electrostatic energy maps, are illustrated in Fig. 3 (showing only the lowest binding energy sites). These electrostatic energy maps were generated using the MOE software by applying electrostatic properties to color the protein surface, forming molecular docking pockets. The oxygen atom of the carbonyl group in the five-membered ring of CS14 acted as a receptor, forming hydrogen bonds with the hydrogen atoms of Lys101 on the P53 target and Lys549 on the HMGCR target. When docked with SREBP2, the oxygen atom of the acetoxy carbonyl group in CS14 formed hydrogen bonds with the hydrogen atom of Try63 on the SREBP2 target. For CS15, during docking with P53, the oxygen atom of the carbonyl group on the acetoxy chain formed hydrogen bonds with the hydrogen atom of Ser95 on the P53 target. When docking with HMGCR and SREBP2, the hydroxyl hydrogen atom on the carbon chain of CS15 acted as a donor, forming hydrogen bonds with the oxygen atoms of Glu505 on HMGCR and Glu29 on SREBP2, respectively. The docking results are summarized in ST 4, with additional docking details provided in the supplementary figures.
Molecular Docking Visualization of CS14, CS15 with Core Targets P53, HMGCR, and SREBP2. A., B. Display the docking interactions between CS14, CS15, and P53. C., D. Display the docking interactions of CS14, CS15 with HMGCR. E., F. Display the docking interactions between CS14, CS15, and SREBP2. G. Serves as the 2D docking diagram legend, including types of binding bonds and the solvent environment of amino acids. In the Enlarge view (2D), the 2D structure of the small molecules and their docking with amino acids are presented. Specifically, CS14 and CS15 dock with Lys101 and Ser95 of P53, Lys549 and Glu505 of HMGCR, and Try63 and Glu29 of SREBP2. In the Enlarge view (3D), the small molecule ligands are shown in green, docking amino acid residues are in blue-pink, both represented in ball-and-stick models. The macromolecular receptors are shown in pale pink, with the docking atoms of the residues and binding bonds marked in orange boxes. The protein surface electrostatic potential map is depicted in blue (high energy) and red (low energy), with binding energies indicated in the bottom right corner.
Based on the above results, the components CS14 and CS15 from PRR may exert lipid-lowering effects. CS14, identified as Gelomulide N ([(1 S,3R,8R,10 S,11R,12 S,14 S,16R)−12-acetyloxy-5,11,15,15-tetramethyl-6-oxo-2,7-dioxapentacyclo[8.8.0.01,3.04,8.011,16]octadec-4-en-14-yl] acetate), is a diterpenoid compound composed of three six-membered rings and a furan ring. It is derived from Gelonium aequoreum, a plant endemic to Taiwan and belonging to the Euphorbiaceae family20. CS15, identified as (e)−5-[(1s,4ar,8ar)−2-formyl-5,5,8a-trimethyl-1,4,4a,6,7,8-hexahydronaphthalen-1-yl]−3-(acetyloxymethyl)pent-2-enoic acid, is another diterpenoid compound composed of two six-membered rings and a pentadiene structure. It was first discovered in the aerial parts of acritopappus confertus21.
The lipid-lowering mechanisms of these compounds may be related to P53, HMGCR, and SREBP2. P53 is a well-known tumor suppressor that has been shown to influence lipid metabolism, with a strong connection between the two22. HMGCR and SREBP2 are both involved in the mevalonate pathway. Targeted network pharmacology enrichment analysis indicated that the most significant pathway enrichment was in “Lipid and Atherosclerosis”, leading us to hypothesize that Paeoniae Radix Rubra’s treatment of hyperlipidemia is closely related to lipid metabolism. To further validate our hypothesis, we plan to conduct animal experiments to verify these findings.
Effects of PRR on body weight, food intake, liver index, and fat content in High-Fat diet mice
During the first 8 weeks of high-fat diet feeding to establish a hyperlipidemia mouse model, the changes in body weight for each group were recorded. The NC group (normal control) showed a steady increase in body weight, while the HFD group (high-fat diet) exhibited a faster weight gain after one week of high-fat feeding compared to the NC group, as shown in Fig. 4A. At the end of the 8th week, serum total cholesterol (TC) and triglyceride (TG) levels were measured using biochemical assay kits. The results demonstrated that serum TC and TG levels were significantly elevated in the HFD group compared to the NC group (P < 0.05, P < 0.01), indicating the establishment of the hyperlipidemia model, as shown in Fig. 4B and C.
The Effects of PRR on Body Weight, Food Intake, Liver Index, and Fat Content in Mice Fed a High-Fat Diet. A. Shows the changes in body weight over 8 weeks of feeding in the NC (normal control) group and the HFD (high-fat diet) group. By the 8th week, the average body weight of mice in the NC group was 41.08 g, while in the HFD group, it was 50.05 g. B., C. Represent the levels of total cholesterol (TC) and triglycerides (TG) in the HFD group at the 8th week. D. Illustrates the changes in body weight across different groups during the 5-week treatment period. E. Shows the changes in food intake across all groups during the 13 weeks of feeding. F., G. Display the liver index and fat content across the groups. H. Provides imaging of fat content in mice across the different groups. Significance levels are indicated as follows: compared to the NC group, #P<0.05, ##P<0.01; compared to the HFD group, *P<0.05, **P<0.01.
During the administration period, the body weight of the NC group fluctuated between 40 ± 2.0 g, while the HFD group’s weight continued to increase. The body weights of the treatment groups were lower than those of the HFD group, suggesting that PRR had an inhibitory effect on the weight gain caused by the high-fat diet, as illustrated in Fig. 4D. But there was no significant difference in food intake between the treatment groups and the HFD group, as shown in Fig. 4E, indicating that PRR likely does not exert its effects by influencing appetite or dietary behavior.
The liver index were significantly elevated in the HFD group compared to the NC group (P < 0.01), while the liver indices of the treatment groups were significantly lower than that of the HFD group (P < 0.01), suggesting that PRR may reduce lipid accumulation in liver tissue, as shown in Fig. 4F. And the fat content of the mice was quantified using the X-ray in vivo imaging system. As shown in Fig. 4G and H, the fat content in the HFD group was significantly higher than that in the NC group (P < 0.01), and the body size of the HFD group was also larger. After drug intervention, the fat content in the treatment groups was significantly lower than in the model group (P < 0.05, P < 0.01), and their body sizes were reduced to varying degrees. These results demonstrate that PRR has the potential to reduce lipid accumulation caused by a high-fat diet.
Effects of PRR on serum biochemical indicators in High-Fat diet mice
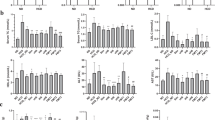
As shown in Fig. 5A and D, compared with the NC group, the HFD group exhibited significantly elevated levels of TC, TG, and LDL-C (P < 0.05, P < 0.01) and significantly reduced levels of HDL-C (P < 0.01). After drug intervention, the serum concentrations of TC, TG, and LDL-C in both the SIM group and the PRR extract groups were significantly lower than the HFD group (P < 0.05, P < 0.01), while HDL-C levels were significantly higher (P < 0.01).
The Effects of PRR on Biochemical Indicators and Histopathology in Mice Fed a High-Fat Diet. A-H Represent the levels of various biochemical indicators in the serum of mice across different groups: total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), alanine aminotransferase (ALT), aspartate aminotransferase (AST), superoxide dismutase (SOD), and malondialdehyde (MDA). I. Displays the hematoxylin and eosin (HE) staining of liver tissues across groups. J. Displays the Oil Red O staining of liver tissues across groups, indicating lipid accumulation. K. Quantifies the positive Oil Red O stained area in liver tissues. L. Displays the HE staining of perirenal adipose tissues across groups. The scale bar for images in I, J, and L is 100 μm.Significance levels are indicated as follows: compared to the NC group, #P<0.05, ##P<0.01; compared to the HFD group, *P<0.05, **P<0.01.
As shown in Fig. 5E and H, the HFD group exhibited significantly elevated levels of ALT, AST, and MDA (P < 0.01) and significantly reduced levels of SOD (P < 0.05) compared to the NC group. Following therapeutic intervention, both SIM group and the PRR extract groups demonstrated significantly lower serum levels of ALT, AST, and MDA compared to the HFD group (P < 0.05, P < 0.01), while SOD levels were significantly higher (P < 0.05). Elevated serum levels of ALT and AST typically indicate impaired liver function, which may be due to hepatocyte injury or hepatitis. Oxidative stress caused by a high-fat diet is also noteworthy. The decrease in SOD levels and the increase in MDA levels suggest oxidative stress damage during the high-fat diet. The results indicated that PRR could protect the liver of high-fat diet mice, restore liver function, and significantly reduce oxidative stress damage caused by the high-fat diet.
Effects of PRR on pathological changes in liver and adipose tissue of High-Fat diet mice
As shown in Fig. 5I, the liver tissues of the NC group exhibited intact structure, with neatly arranged hepatocytes, round nuclei located centrally within the cells, and no signs of fat degeneration. The cytoplasm was abundant and evenly stained, appearing pink. The liver tissues of the HFD group displayed extensive fat degeneration (black arrows), with small round vacuoles visible in the cytoplasm, many ballooning hepatocytes (red arrows), cell enlargement, and loose cytoplasm. Congestion was observed in the veins and surrounding hepatic sinusoids (yellow arrows), indicating lipid accumulation and inflammatory damage in the liver of the HFD group. After drug intervention, the treatment groups showed a significantly reduction in the size and number of vacuoles, and the severity of pathological changes was alleviated. Some mild hydropic degeneration was still present (blue arrows), and a small amount of hepatocyte necrosis was observed in the CSM group (green arrows). These results suggest that PRR has the potential to alleviate lipid accumulation and hepatic inflammation caused by a high-fat diet.
As shown in Fig. 5J, lipid droplets appeared orange-red to bright red, and the nuclei were stained blue. The NC group had a small number of lipid droplets, while the HFD group exhibited the highest degree of red staining in hepatocyte lipid droplets, indicating the most severe fat degeneration. The degree of red staining in the hepatocyte lipid droplets decreased to varying extents in the treatment groups. Further quantitative analysis indicated a substantially greater positive area ratio in the HFD group compared to the NC group (P < 0.01), as shown in Fig. 5K. In contrast, the SIM and PRR extract groups exhibited notably lower positive area ratios than the HFD group. (P < 0.01).
As shown in Fig. 5L, the adipocytes in the NC group were tightly packed, polygonal, or irregularly round in shape, relatively small in size, and exhibited clear and intact morphology. In contrast, adipocytes in the HFD group were significantly larger, with fewer cells visible in the same field of view. The cytoplasm formed a thin layer surrounding the lipid droplets, and the nuclei appeared flattened and oval. After drug intervention, the size of adipocytes in all treatment groups decreased to varying degrees.
The liver is particularly susceptible to lipid metabolic disorders induced by a high-fat diet, which leads to excessive fatty acid uptake by adipocytes and abnormal lipid accumulation in the liver. This, in turn, can result in enlarged adipocytes, liver damage, impaired liver function, abnormal blood lipid levels, and the onset of hyperlipidemia23. In this study, PRR effectively restored lipid metabolism in high-fat diet mice, significantly alleviating abnormal lipid accumulation and cellular damage in the liver.
Effects of PRR on gut microbiota in High-Fat diet mice
Based on our previous studies, 16 S rRNA high-throughput sequencing technology were use to analyze changes in gut microbiota among the NC group, HFD group, SIM group, and CSH group.
Alpha diversity reflects biodiversity within a single sample, including species richness and diversity. The ACE and Chao1 indices primarily assess species richness, whereas the Shannon and Simpson indices focus on assessing species diversity. As shown in Fig. 6A and D, Alpha diversity analysis revealed that, compared with the NC group, the HFD group had significantly lower ACE, Chao1, Shannon, and Simpson indices (P < 0.01), suggesting that a high-fat diet leads to a reduction in both the richness and diversity of gut microbiota in mice. Following drug treatment, a significant rise (P < 0.01) was observed in the four indices within the SIM and CSH groups, suggesting that simvastatin and PRR extract could enhance the richness and diversity of gut microbiota in hyperlipidemic mice. The Venn plot in Fig. 6E vividly displays the shared and unique operational taxonomic units (OTUs) among the samples, visually presenting their overlap. In this experiment, a total of 6244 OTUs representative sequences were identified, suggesting that after drug intervention, the gut microbiota composition in the treatment groups became more similar to that of the NC group.
The Effects of PRR on the Gut Microbiota Composition of Mice Fed a High-Fat Diet. A-D Represent the changes in four α-diversity indices (ACE, Chao1, Shannon, Simpson) across the four groups, reflecting the diversity within each group. E. Displays the Venn diagram of OTU intersections among the four groups, showing the shared and unique OTUs. F., G. Represent the β-diversity analysis results, including Principal Coordinates Analysis (PCoA) and Non-metric Multidimensional Scaling (NMDS), which illustrate differences in microbial community composition among the groups. H. Shows the composition of gut microbiota at the phylum level across the groups. I. Indicates the changes in the Firmicutes/Bacteroidota (F/B) ratio across the groups. J. Displays the gut microbiota composition at the genus level across the groups. K. Presents the results of the LEfSe (Linear discriminant analysis Effect Size) analysis, highlighting significant differences in specific bacteria between the groups. L. Shows the changes in the phyla Firmicutes, Bacteroidota, and Proteobacteria across the groups. M. Illustrates the changes in specific genera, including Escherichia_Shigella, Allobaculum, unclassified_Muribaculaceae, Parabacteroides, Lactobacillus, Bacteroides, Alloprevotella, Bacillus, and Ligilactobacillus, across the groups. Significance levels are indicated as follows: compared to the NC group, #P<0.05, ##P<0.01; compared to the HFD group, *P<0.05, **P<0.01.
Beta diversity is used to evaluate the differences in microbial communities between samples. As shown in Fig. 6F and G, both analyses revealed that the elliptical areas of the NC and HFD groups did not overlap, indicating significant differences in gut microbiota between the two groups (P < 0.01). After drug intervention, the elliptical areas of the SIM and CSH groups partially overlapped with those of the NC group, indicating that the composition of gut microbiota in the treatment groups progressively aligned with that of the NC group.
The top 10 most abundant phyla were listed: Firmicutes, Bacteroidota, Proteobacteria, Actinobacteriota, Desulfobacterota, Campylobacterota, Deferribacterota, Patescibacteria, Verrucomicrobiota, and Cyanobacteria (Fig. 6H). Among these, Firmicutes and Bacteroidota were the dominant phyla, which are also the main components of the gut microbiota in mice24. Firmicutes are primarily involved in energy absorption, while Bacteroidota are responsible for degrading various substances, such as polysaccharides, bile acids, and steroids. The Firmicutes to Bacteroidota (F/B) ratio is frequently employed to describe ecological imbalance, with an elevated F/B ratio typically associated with obesity and metabolic disorders, often accompanied by increased energy intake, lipogenesis, and fat deposition25. As shown in Fig. 6I, a prolonged high-fat diet was observed to elevate the relative abundance of Firmicutes and Proteobacteria in mice, with proportions of 42.67% and 33.95%, respectively, while the relative abundance of Bacteroidota decreased to only 15.66%. The F/B ratio was as high as 272.48%, showing a significant difference compared to the NC group (P < 0.01). After treatment with simvastatin and PRR extract, this phenomenon was significantly improved (P < 0.01), with the F/B ratio in the treatment groups reduced to below 200%, trending toward the NC group.
As shown in Fig. 6J, the top 10 most abundant genera were listed: Escherichia_Shigella, Allobaculum, unclassified_Muribaculaceae, Parabacteroides, Lactobacillus, Bacteroides, Alloprevotella, Dubosiella, Bacillus, and Ligilactobacillus. It was evident that the genus-level structure of the gut microbiota in hyperlipidemic mice fed a high-fat diet had changed. Compared to the NC group, the HFD group showed an increase in the relative abundance of Escherichia_Shigella, while the relative abundances of Allobaculum, unclassified_Muribaculaceae, Parabacteroides, Lactobacillus, Bacteroides, and Alloprevotella decreased. After the administration of the drug, the levels of Escherichia_Shigella were reduced in the treatment groups, with only 0.03% remaining in the CSH group. The relative level of the other genera increased, suggesting that after intervention with PRR, the genus-level abundance of gut microbiota in hyperlipidemic mice shifted toward the NC group.
LEfSe analysis aims to identify microbial taxa that exhibit significant differences between groups. As shown in Fig. 6K, three distinct phyla differentiated the blank group from the model group, with Bacteroidota being the predominant phylum in the blank group, while Firmicutes and Proteobacteria were dominant in the model group. The Fig. 6L illustrated the relative abundance changes of Firmicutes, Bacteroidota, and Proteobacteria at the phylum level across different groups. Notably, the relative level of Firmicutes and Proteobacteria in the HFD group showed a substantial increase compared to the NC group (P < 0.01), and both decreased significantly following treatment (P < 0.01).
At the genus level, the groups exhibited significant enrichment effects on 21 different species. By analyzing the trends in relative abundance and statistical significance (P < 0.05), nine species that were markedly regulated by PRR in the gut microbiota of mice were identified on a high-fat diet. The Fig. 6M displayed the changes in relative abundance of these nine dominant genera across groups. It was evident that Escherichia_Shigella, the sole significantly dominant genus in the HFD group, showed a substantial increase in relative abundance compared with the NC group (P < 0.01). Conversely, the relative abundance of the remaining eight dominant genera in the HDL group was significantly lower (P < 0.01), a trend that was notably reversed following drug intervention (P < 0.01). In particular, three genera were significantly enriched in the NC group: unclassified_Muribaculaceae, Bacteroides, and Alloprevotella. The SIM group, after simvastatin treatment, showed a significant increase in the relative abundance of Lactobacillus, Ligilactobacillus, and Bacillus (P < 0.01), which also became the dominant genera in the SIM group. Similarly, the CSH group exhibited a significant elevation in the relative abundance of Allobaculum and Parabacteroides (P < 0.01), establishing these as the dominant genera in the CSH group. These findings are consistent with the compositional analyses conducted at both the phylum and genus levels.
The PICRUSt2 functional prediction analysis method were employed to predict the functions of the gut microbiota. The Fig. 7A illustrated the KEGG functional classification analysis, highlighting the top 10 functions with significant differences in relative levels between the two groups (P < 0.05). As shown in Fig. 7B and D, the most enriched functional category across all groups was metabolic pathways, accounting for 16%, followed by the biosynthesis of secondary metabolites, which comprises approximately 7%. Both of these functions play crucial roles in regulating the body’s metabolic processes. Metabolic pathways encompassed carbohydrate metabolism, lipid metabolism, and amino acid metabolism, while the biosynthesis of secondary metabolites includes the acetyl-CoA pathway, mevalonate pathway, shikimate pathway, and amino acid pathways. These pathways may be the primary mechanisms through which a prolonged high-fat diet induced hyperlipidemia and were also the key pathways by which PRR exerted its lipid-lowering effects. The Fig. 7E presented the COG functional classification analysis, similarly highlighting the top 10 functions with significant differences in relative abundance between the two groups (P < 0.05). The most predominant COG category was general function prediction only, accounting for 11–12%, followed by amino acid transport and metabolism, and carbohydrate transport and metabolism, each comprising 8–9%, as shown in Fig. 7F and H. These functional predictions align were closely with the results of the KEGG enrichment analysis, suggesting that the therapeutic effect of PRR on hyperlipidemia may be related to the regulation of functional pathways and energy metabolism, particularly in the transport and metabolism of carbohydrates and amino acids.
The Effects of PRR on the Enrichment Analysis of Gut Microbiota in Mice Fed a High-Fat Diet. A-D Show the differential abundance analysis of KEGG functional classifications, which from KEGG (https://www.kegg.jp/)19. A. Displays the top 10 functional categories with the most significant differences (P<0.05) between the two groups, focusing on functions with the highest relative abundance. B-D Present the top 10 functional categories with significant differences in abundance between the HFD group and the NC group, the SIM group and the HFD group, and the CSH group and the HFD group, respectively. On the left, “Mean Proportion” indicates the relative abundance of different functions in the two sample groups, while the right shows the difference in functional abundance proportions within the 95% confidence interval. E-H Illustrate the differential abundance analysis of COG functional classifications. E. Similarly filters the top 10 functional categories with the most significant differences (P<0.05) between the two groups based on relative abundance. F-H Correspond to the same comparisons as in B-D, highlighting the top 10 COG functional categories with significant differences in abundance between the groups. The left side shows “Mean Proportion”, and the right side displays the difference in functional abundance proportions within the 95% confidence interval.
The effect of PRR on P53, HMGCR, and SREBP2 protein levels in the liver of mice on a High-Fat diet
In previous studies, P53, HMGCR, and SREBP2 were identified as potential key targets for PRR in the treatment of hyperlipidemia through targeted network pharmacology and molecular docking techniques. Among these, SREBP2 and HMGCR are closely related to the mevalonate pathway, which is essential for cholesterol synthesis. During the establishment of the hyperlipidemia model, PPR significantly ameliorated lipid abnormalities in mice fed a high-fat diet. Also, gut microbiota analysis revealed the herb’s potential to influence glucose and lipid metabolism, including the mevalonate pathway. These results suggested that PRR may reduce cholesterol synthesis by regulating proteins such as HMGCR and SREBP2, thereby inhibiting the mevalonate pathway and ultimately lowering blood lipid levels. Therefore, western blotting and immunohistochemistry techniques were used for experimental validation.
As shown in Fig. 8A and B, compared to the NC group, the HFD group exhibited a significant decrease in P53 protein expression levels in liver tissue (P < 0.01), while the expression levels of HMGCR and mature SREBP2 protein (SREBP2(M), molecular weight 73 kDa) were significantly elevated (P < 0.01). The expression levels of SREBP2 (M) were higher than those of the precursor SREBP2 protein (SREBP2(P), molecular weight 123 kDa) in all groups. After drug intervention, the SIM group and the PRR extract treatment group showed a significant increase in P53 protein expression levels in liver tissue compared to the model group (P < 0.05, P < 0.01), while the expression levels of HMGCR and SREBP2(M) were significantly reduced (P < 0.05, P < 0.01). Notably, the CSL group did not exhibit a significant effect on HMGCR protein expression levels (P > 0.05), as depicted in Fig. 8C and E. It suggested that PRR may exert its lipid-lowering effects by regulating P53, HMGCR, and SREBP2. P53, a well-known tumor suppressor, has been shown to influence lipid metabolism, with a close relationship between the two. Previous studies have demonstrated that the downregulation of P53 can enhance SREBP2 maturation, stabilize SREBP2 transcription, and interact with intermediates of the mevalonate pathway to evade ubiquitin-mediated degradation26.
The Effects of PRR on Mevalonate Pathway-Related Proteins in the Livers of Mice Fed a High-Fat Diet. A., B. Show the results of immunoblotting analysis for the proteins P53, HMGCR, SREBP2, ABCA1, MVD, and MVK in liver tissues of the different groups of mice. C-H Quantify the immunoblotting results for each of the above proteins across the different groups. I. Represents the ACAT enzyme levels in the liver tissues of each group. J-M Show the immunohistochemical analysis of HMGCR and SREBP2 proteins in the liver tissues. J., K. Quantify the positive staining for HMGCR and SREBP2 proteins. L., M. Display the immunohistochemical staining images, where blue indicates the cell nucleus, and brownish-yellow granules indicate positive protein staining. The scale bar represents 100 μm. Significance levels are indicated as follows: compared to the NC group, #P<0.05, ##P<0.01; compared to the HFD group, *P<0.05, **P<0.01.
To further validate the expression of HMGCR and SREBP2 in liver tissues, immunohistochemical analysis was performed on liver sections from each group of mice, as shown in Fig. 8J-M. In these images, cell nuclei were stained blue, with HMGCR protein predominantly localized in the cytoplasm, while SREBP2 protein is detected both inside and outside the nucleus. Under the same field of view, HMGCR and nuclear SREBP2 proteins were strongly positive expression in the liver tissues of HFD group mice, with an increase in the number of brown-stained cells. The positive rate was significantly higher than that in the NC group (P < 0.01). In contrast, the SIM group and the PRR extract treatment group exhibited weak positive expression of HMGCR and nuclear SREBP2 proteins, significantly lower than that in the HFD group (P < 0.01). In the liver, HMGCR, or HMG-CoA reductase, is a rate-limiting enzyme that mediates the mevalonate pathway, thus influencing cholesterol biosynthesis. SREBP2, as a key regulatory element of the mevalonate pathway, is intrinsically linked to cholesterol synthesis. When cholesterol is depleted in the endoplasmic reticulum, SREBP2 (P) is transported to the Golgi apparatus, where it is cleaved into its active mature form, SREBP2 (M). This mature form then translocates to the nucleus, where it binds to sterol regulatory elements (SREs) in promoters, thereby activating the transcription of mevalonate pathway genes27.
ACAT is an endoplasmic reticulum-resident enzyme that esterifies free cholesterol into cholesterol esters. Its activity is typically determined by the availability of cholesterol within the endoplasmic reticulum membrane. To assess the cholesterol content in the endoplasmic reticulum, we analyzed ACAT activity in liver cells28. As shown in Fig. 8I, compared to the NC group, the ACAT levels in liver cells of the HFD group were decreased significantly (P < 0.01). After administration of simvastatin and PRR extract, the ACAT levels in the liver cells of the treatment groups were significantly elevated (P < 0.05, P < 0.01). Notably, the CSL group exhibited a trend toward reduced ACAT levels, but the effect was not statistically significant (P > 0.05).
The effect of PRR on ABCA1 protein and mevalonate pathway proteins in the liver of mice on a High-Fat diet
To further investigate how P53 regulates SREBP2 and how PRR influences downstream proteins in the mevalonate pathway, the changes in ABCA1 protein and key proteins such as MVD and MVK in the mevalonate pathway were detected, during the lipid-lowering process induced by PRR. As shown in Fig. 8A and B, and Fig. 8F and H, compared to the NC group, the expression level of ABCA1 protein in the liver tissue of the HFD group was significantly reduced (P < 0.01), while the expression levels of MVD and MVK proteins were significantly elevated (P < 0.01). Following drug administration, the SIM group and the PRR extract treatment group showed a significant increase in ABCA1 protein expression levels in liver tissue compared to the HFD group (P < 0.05, P < 0.01), while the expression levels of MVD and MVK proteins were significantly reduced (P < 0.05, P < 0.01). However, the CSL group did not exhibit a marked effect on ABCA1 protein expression levels (P > 0.05). These findings suggested that the mevalonate pathway played an indispensable role in the lipid-lowering effects of PRR.
Discussion
Hyperlipidemia is a disorder characterized by abnormal lipid metabolism, including increased absorption of dietary lipids or dysregulation of endogenous lipid metabolism. When cholesterol levels in the body are abnormal, such as during prolonged periods of a high-fat diet, hyperlipidemia usually results. The assessment of lipid profiles is usually conducted by monitoring serum total cholesterol (TC) and triglyceride (TG) levels29. Moreover, total cholesterol comprises both low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C), with a reduction in the former or a rise in the latter is strongly linked to a heightened likelihood of developing hyperlipidemia. As such, regulating serum cholesterol levels is considered an effective strategy for preventing the progression of hyperlipidemia30,31. Our research has demonstrated that PRR can ameliorate the rise in LDL-C and the reduction in HDL-C induced by a high-fat diet, facilitating the transport of TC and TG from the bloodstream to the liver. This results in decreased blood levels of TC and TG, which helps to reduce lipid build up and contributes to lipid-lowering benefits.
The metabolism of a high-fat diet is inseparable from the gastrointestinal system, which is the most complex microecosystem in the human body. The gut microbiota, composed of numerous species, is even considered a “metabolic organ” due to its critical role in regulating lipid metabolism, maintaining energy balance, and protecting the integrity of the mucosal barrier32. In this research, mice fed a high-fat diet showed a significant increase in the abundance of Firmicutes, Proteobacteria, and the genus Escherichia-Shigella, while the abundance of Bacteroidetes significantly decreased. The ratio of Firmicutes to Bacteroidetes (F/B ratio) is one indicator of metabolic imbalance33,34. Bacteria from the phylum Proteobacteria are all Gram-negative, with cell walls containing lipopolysaccharides (LPS). When the integrity of the intestinal barrier is disrupted, LPS can enter the bloodstream, leading to systemic inflammation and a host of related diseases35. The genus Escherichia-Shigella, a member of the class γ-Proteobacteria, is notably elevated in hyperlipidemic mice and has been linked to the severity of non-alcoholic fatty liver disease (NAFLD). This genus can induce hepatic steatosis and hepatocyte ballooning, accompanied by some fibrosis and inflammation36. Treatment with PRR significantly increased the relative abundance of the genera Allobaculum, unclassified_Muribaculaceae, Parabacteroides, Lactobacillus, Bacteroides, and Alloprevotella.
Allobaculum and Alloprevotella are recognized for generating short-chain fatty acids (SCFAs), which are critical for promoting epithelial cell proliferation, reducing gut-derived endotoxins in the bloodstream, and improving intestinal barrier integrity. These bacteria have the potential to inhibit LPS release and exhibit anti-obesity activity37. The genus unclassified_Muribaculaceae belongs to the family Muribaculaceae, which was previously classified as part of the Bacteroidaceae family. Members of this family can utilize mucin monosaccharides, making them important for the production of intestinal mucus. It is speculated that the unclassified_Muribaculaceae genus may have similar functions, protecting intestinal mucosal tissues and maintaining the integrity of the intestinal barrier38. Parabacteroides is one of the core microbial genera. Studies have demonstrated that its presence can significantly alleviate symptoms caused by a high-fat diet, such as obesity, lipid metabolism disorders, and NAFLD39. Correlation analysis reveals a significant negative association between its abundance and disease states like obesity and NAFLD, suggesting that Parabacteroides might contribute positively to the regulation of glucose and lipid metabolism. Lactobacillus, a well-known probiotic, can inhibit the growth of harmful bacteria, maintain gut microbiota balance, and prevent intestinal infections and inflammation. Both Lactobacillus and Bacteroides can lower serum cholesterol levels, a function related to bile salt hydrolase (BSH). BSH, found in Lactobacillus and Bacteroides, accelerates the conversion of cholesterol into bile acids40,41. These bacterial genera are also associated with glucose and lipid metabolism, as indicated by KEGG and COG functional prediction analyses, involving pathways such as the mevalonate pathway, cholesterol biosynthesis, and the metabolism of carbohydrates and amino acids. In regulating lipid metabolism, SCFAs produced by fermentation of dietary fiber by intestinal flora inhibit gene expression of cholesterol synthase in the liver, increase the conversion of cholesterol to bile acids, and regulate intestinal cholesterol absorption-related transporter proteins to lower cholesterol41.
The mevalonate pathway is a crucial cellular route for the biosynthesis of cholesterol and other isoprenoid compounds, utilizing acetyl-CoA as its primary substrate to synthesize isopentenyl pyrophosphate (IPP). A key enzyme in this pathway is 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR), which catalyzes the conversion of HMG-CoA to mevalonate, a rate-limiting step in cholesterol biosynthesis42,43. Sterol regulatory element-binding protein 2 (SREBP2) also plays a pivotal role in initiating the mevalonate pathway. SREBP2 exists as both an inactive precursor and an active, mature form, which differ in molecular weight. The inactive precursor is embedded in the endoplasmic reticulum (ER) membrane44,45.
Acyl coenzyme A-cholesterol acyltransferase (ACAT) is an enzyme that resides in the ER and is responsible for monitoring cholesterol levels in the ER. When cholesterol is depleted in the ER, the SREBP2 precursor is transported to the Golgi apparatus, where its NH2-terminal transcription activation ___domain is released from the precursor protein via proteolytic cleavage. This activated form of SREBP2, now in its mature state, is translocated to the nucleus. There, it binds to sterol regulatory elements (SREs) within promoter regions, upregulating the expression of HMGCR and activating the transcription of genes involved in the mevalonate pathway27,46,47. The mechanistic process is shown in SF. 17.
In this study, we found that PRR significantly enhanced ACAT activity in hepatocytes of high-fat diet mice, while inhibiting the translocation of the SREBP2(P). Further Western blot and immunohistochemistry results revealed that both SREBP2(M) and HMGCR were highly expressed in the HFD group, indicating that a high-fat diet promotes the transport of SREBP2(P) to the Golgi, where it is proteolytically cleaved into the active SREBP2(M). This mature SREBP2 then translocates to the nucleus, binds to SREs, and increases the expression of HMGCR, thereby further activating the mevalonate pathway. Treatment with PRR inhibited the high-fat diet-induced elevation of SREBP2(M) and HMGCR protein expression, subsequently suppressing the expression of the mevalonate pathway and reducing the production of mevalonate.
In the cholesterol synthesis pathway, mevalonate (MVA) generated by the HMG-CoA reductase (HMGCR)-mediated mevalonate pathway undergoes a series of enzymatic reactions, such as decarboxylation and phosphorylation, to synthesize isopentenyl pyrophosphate (IPP). IPP then further forms farnesyl pyrophosphate (FPP), and the condensation of two FPP molecules leads to the formation of squalene, which is oxidized by squalene epoxidase (SQLE) into 2,3-oxidosqualene, followed by cyclization into lanosterol, and ultimately converted into cholesterol42,48. IPP is a critical precursor in the synthesis of isoprenoids and cholesterol, and enzymes such as MVD (mevalonate diphosphate decarboxylase) and MVK (mevalonate kinase) serve as rate-limiting enzymes in the decarboxylation and phosphorylation processes of mevalonate to form IPP, playing pivotal roles in cholesterol biosynthesis. MVD catalyzes the ATP-dependent decarboxylation of (R)−5-diphosphomevalonate into IPP49, while MVK catalyzes the phosphorylation of mevalonate into 5-phosphomevalonate, which is further synthesized into IPP50.
In our study, we found that a high-fat diet increases the expression of MVD and MVK, thereby accelerating the decarboxylation and phosphorylation of mevalonate to generate IPP, leading to elevated cholesterol levels and dyslipidemia. PRR extract significantly downregulated the expression levels of MVD and MVK, suggesting that it exerted its lipid-lowering effects by inhibiting the activity of these two key rate-limiting enzymes, reducing the production of the cholesterol precursor IPP, and consequently suppressing cholesterol synthesis in the body.
Through targeted network pharmacology and molecular docking analysis, Gelomulide N and (E)−5-[(1 S,4aR,8aR)−2-formyl-5,5,8a-trimethyl-1,4,4a,6,7,8-hexahydronaphthalen-1-yl]−3-(acetyloxymethyl)pent-2-enoic acid, both of which are diterpenoids, were identified as active compounds in PRR that may be potentially responsible for its lipid-lowering effects. However, the specific pharmacological activities of these two compounds remain largely unexplored, particularly in the context of lipid reduction.
Meanwhile, our predictions suggest that P53 played a crucial role in the lipid-lowering effects of PRR. P53 can influence the mevalonate pathway, with cancer-derived P53 missense mutations being associated with increased gene expression in this pathway51. The loss of P53 function, along with the gain-of-function activities of mutant P53, is key factors in regulating the mevalonate pathway27. Most research indicates that, in mouse models subjected to a high-fat diet, the expression levels of P53 are typically either increased or remain stable, this study revealed that P53 expression was suppressed in the hyperlipidemia mouse model. However, this suppression was improved following treatment with PRR extract, a result that appears paradoxical and warrants further investigation. Moreover, ABCA1, a membrane-associated protein, is a member of the ATP-binding cassette (ABC) transporter superfamily, which facilitates the translocation of various molecules across cell membranes. ABCA1 specifically functions as a cholesterol efflux pump in the cellular lipid clearance pathway52. P53 can inhibit cholesterol synthesis by inducing ABCA1 expression in the liver and suppressing the activation of SREBP227. Therefore, ABCA1 may also play a role in the lipid-lowering effects of PRR. Our analysis of ABCA1 protein levels across different groups revealed a significant reduction in ABCA1 expression in the HFD group mice. In contrast, PRR extract significantly upregulated ABCA1 protein levels, suggesting that it enhanced cholesterol efflux by increasing ABCA1 expression, thereby lowering cholesterol levels.
A prolonged high-fat diet induces chronic, persistent inflammatory responses, leading to the depletion of the body’s antioxidant system, resulting in oxidative stress and tissue damage53,54. External signals such as endoplasmic reticulum stress, low pH, and inflammatory mediators can stimulate the migration of mature SREBP2 into the nucleus, where it becomes activated42. Our findings suggested that PRR mitigated oxidative stress damage caused by a high-fat diet by reducing the expression of mature SREBP2, thereby inhibiting the mevalonate pathway and decreasing cholesterol synthesis, ultimately exerting a lipid-lowering effect. Moreover, PRR played a significant role in modulating the gut microbiota disrupted by a high-fat diet, thereby regulating glucose and lipid metabolism and contributing to its cholesterol-lowering properties.However, there are limitations in this study. Questions remain regarding the actions of the two screened components, as well as the deeper mechanisms by which PRR regulates gut microbiota to influence cholesterol levels. These areas warrant further experimental investigation.
Conclusions and perspective
This study demonstrates the lipid-lowering potential of PRR in high-fat diet-induced hyperlipidemia in mice, highlighting its regulatory effects on both lipid metabolism and gut microbiota. Our results from network pharmacology and molecular docking suggest that key compounds in PRR, particularly Gelomulide N and (E)−5-[(1 S,4aR,8aR)−2-formyl-5,5,8a-trimethyl-1,4,4a,6,7,8-hexahydronaphthalen-1-yl]−3-(acetyloxymethyl)pent-2-enoic acid, target critical proteins like P53, HMGCR, and SREBP2. These interactions appear to modulate the mevalonate pathway, thereby reducing cholesterol synthesis and ameliorating hyperlipidemia. In vivo experiments further validate the efficacy of PRR, showing significant reductions in serum lipid levels, improvement in liver histopathology, and modulation of the gut microbiota structure. The gut microbiota analysis revealed that PRR treatment shifted the gut microbial composition towards a more beneficial profile, characterized by increased microbial diversity and a favorable Firmicutes/Bacteroidota ratio, which are associated with improved lipid metabolism. Overall, these findings suggest that PRR could serve as a promising alternative or complementary therapy for hyperlipidemia.
Data availability
The sources of the datasets analyzed in this study are provided in the text, and specific datasets are available upon request from the corresponding author.
References
Worldwide trends in underweight and obesity from 1990 to 2022: a pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet 403, 1027–1050 (2024). https://doi.org/10.1016/s0140-6736(23)02750-2
Zhang, Y., Wang, Z., Jin, G., Yang, X. & Zhou, H. Regulating dyslipidemia effect of polysaccharides from pleurotus ostreatus on fat-emulsion-induced hyperlipidemia rats. Int. J. Biol. Macromol. 101, 107–116. https://doi.org/10.1016/j.ijbiomac.2017.03.084 (2017).
Svegliati-Baroni, G. et al. Glucagon-like peptide-1 receptor activation stimulates hepatic lipid oxidation and restores hepatic signalling alteration induced by a high-fat diet in nonalcoholic steatohepatitis. Liver Int. 31, 1285–1297. https://doi.org/10.1111/j.1478-3231.2011.02462.x (2011).
Jia, X. et al. Impact of gut microbiota and microbiota-Related metabolites on hyperlipidemia. Front. Cell. Infect. Microbiol. 11, 634780. https://doi.org/10.3389/fcimb.2021.634780 (2021).
Tan, Y. Q., Chen, H. W., Li, J., Wu, Q. J. & Efficacy Chemical constituents, and Pharmacological actions of Radix paeoniae rubra and Radix paeoniae Alba. Front. Pharmacol. 11, 1054. https://doi.org/10.3389/fphar.2020.01054 (2020).
Fan, Z. et al. Paeoniae Radix rubra: A review of ethnopharmacology, phytochemistry, Pharmacological activities, therapeutic mechanism for blood stasis syndrome, and quality control. Chem. Biodivers. 21, e202401119. https://doi.org/10.1002/cbdv.202401119 (2024).
Ngan, L. T., Moon, J. K., Kim, J. H., Shibamoto, T. & Ahn, Y. J. Growth-inhibiting effects of Paeonia lactiflora root steam distillate constituents and structurally related compounds on human intestinal bacteria. World J. Microbiol. Biotechnol. 28, 1575–1583. https://doi.org/10.1007/s11274-011-0961-6 (2012).
Ngan, L. T., Moon, J. K., Shibamoto, T. & Ahn, Y. J. Growth-inhibiting, bactericidal, and urease inhibitory effects of Paeonia lactiflora root constituents and related compounds on antibiotic-susceptible and -resistant strains of Helicobacter pylori. J. Agric. Food Chem. 60, 9062–9073. https://doi.org/10.1021/jf3035034 (2012).
Smith, C. A., Want, E. J., O’Maille, G., Abagyan, R. & Siuzdak, G. XCMS: processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal. Chem. 78, 779–787. https://doi.org/10.1021/ac051437y (2006).
Daina, A., Michielin, O. & Zoete, V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 7, 42717. https://doi.org/10.1038/srep42717 (2017).
Lipinski, C. A., Lombardo, F., Dominy, B. W. & Feeney, P. J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv Rev. 46, 3–26. https://doi.org/10.1016/s0169-409x(00)00129-0 (2001).
Daina, A., Michielin, O. & Zoete, V. SwissTargetPrediction: updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 47, W357–w364. https://doi.org/10.1093/nar/gkz382 (2019).
Li, X. et al. Network Pharmacology prediction and molecular docking-based strategy to explore the potential mechanism of Huanglian Jiedu Decoction against sepsis. Comput. Biol. Med. 144, 105389. https://doi.org/10.1016/j.compbiomed.2022.105389 (2022).
Tang, Y., Li, M., Wang, J., Pan, Y. & Wu, F. X. CytoNCA: a cytoscape plugin for centrality analysis and evaluation of protein interaction networks. BioSystems 127, 67–72. https://doi.org/10.1016/j.biosystems.2014.11.005 (2015).
Shannon, P. et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504. https://doi.org/10.1101/gr.1239303 (2003).
Chin, C. H. et al. CytoHubba: identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 8 (Suppl 4). https://doi.org/10.1186/1752-0509-8-s4-s11 (2014).
Hsin, K. Y., Ghosh, S. & Kitano, H. Combining machine learning systems and multiple Docking simulation packages to improve Docking prediction reliability for network Pharmacology. PLoS One. 8, e83922. https://doi.org/10.1371/journal.pone.0083922 (2013).
Rudrapal, M. et al. Identification of bioactive molecules from Triphala (Ayurvedic herbal formulation) as potential inhibitors of SARS-CoV-2 main protease (Mpro) through computational investigations. J. King Saud Univ. Sci. 34, 101826. https://doi.org/10.1016/j.jksus.2022.101826 (2022).
Kanehisa, M., Furumichi, M., Sato, Y., Matsuura, Y. & Ishiguro-Watanabe, M. KEGG: biological systems database as a model of the real world. Nucleic Acids Res. 53 (D672-d677). https://doi.org/10.1093/nar/gkae909 (2025).
Lee, C. L. et al. Cytotoxic ent-abietane diterpenes from gelonium aequoreum. Phytochemistry 69, 276–287. https://doi.org/10.1016/j.phytochem.2007.07.005 (2008).
Bohlmann, F., Zdero, C., Jakupovic, J., King, R. M. & Robinson, H. Diterpenes from Acritopappus confertus. Phytochemistry 22, 2243–2252. https://doi.org/10.1016/S0031-9422(00)80155-7 (1983).
Yahagi, N. et al. p53 activation in adipocytes of obese mice. J. Biol. Chem. 278, 25395–25400. https://doi.org/10.1074/jbc.M302364200 (2003).
Torruellas, C., French, S. W. & Medici, V. Diagnosis of alcoholic liver disease. World J. Gastroenterol. 20, 11684–11699. https://doi.org/10.3748/wjg.v20.i33.11684 (2014).
Lin, G. et al. Role of green macroalgae Enteromorpha Prolifera polyphenols in the modulation of gene expression and intestinal microflora profiles in type 2 diabetic mice. Int. J. Mol. Sci. 20 https://doi.org/10.3390/ijms20010025 (2018).
Paik, D. et al. Human gut bacteria produce Τ(Η)17-modulating bile acid metabolites. Nature 603, 907–912. https://doi.org/10.1038/s41586-022-04480-z (2022).
Loughran, R. M. & Emerling, B. M. Mechanistic roles of mutant p53 governing lipid metabolism. Adv. Biol. Regul. 83, 100839. https://doi.org/10.1016/j.jbior.2021.100839 (2022).
Moon, S. H. et al. p53 represses the mevalonate pathway to mediate tumor suppression. Cell 176, 564–580e519. https://doi.org/10.1016/j.cell.2018.11.011 (2019).
Suckling, K. E. & Stange, E. F. Role of acyl-CoA: cholesterol acyltransferase in cellular cholesterol metabolism. J. Lipid Res. 26, 647–671 (1985).
Guo, W. L. et al. Preparation of a novel Grifola frondosa polysaccharide-chromium (III) complex and its hypoglycemic and hypolipidemic activities in high fat diet and streptozotocin-induced diabetic mice. Int. J. Biol. Macromol. 131, 81–88. https://doi.org/10.1016/j.ijbiomac.2019.03.042 (2019).
Chapman, M. J. et al. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. Eur. Heart J. 32, 1345–1361. https://doi.org/10.1093/eurheartj/ehr112 (2011).
Hao, W. et al. Wild melon seed oil reduces plasma cholesterol and modulates gut microbiota in hypercholesterolemic hamsters. J. Agric. Food Chem. 68, 2071–2081. https://doi.org/10.1021/acs.jafc.9b07302 (2020).
Bäckhed, F. et al. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. U S A. 101, 15718–15723. https://doi.org/10.1073/pnas.0407076101 (2004).
Ley, R. E. et al. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. U.S.A. 102, 11070–11075 (2005). https://doi.org/10.1073/pnas.0504978102
Ley, R. E., Turnbaugh, P. J., Klein, S. & Gordon, J. I. Microbial ecology: human gut microbes associated with obesity. Nat 444, 1022–1023. https://doi.org/10.1038/4441022a (2006).
Yin, X. et al. Structural changes of gut microbiota in a rat non-alcoholic fatty liver disease model treated with a Chinese herbal formula. Syst. Appl. Microbiol. 36, 188–196. https://doi.org/10.1016/j.syapm.2012.12.009 (2013).
Xin, F. Z. et al. Escherichia fergusonii promotes Nonobese nonalcoholic fatty liver disease by interfering with host hepatic lipid metabolism through its own MsRNA 23487. Cell. Mol. Gastroenterol. Hepatol. 13, 827–841. https://doi.org/10.1016/j.jcmgh.2021.12.003 (2022).
Mu, H. N. et al. Caffeic acid prevents non-alcoholic fatty liver disease induced by a high-fat diet through gut microbiota modulation in mice. Food Res. Int. 143, 110240. https://doi.org/10.1016/j.foodres.2021.110240 (2021).
Pereira, F. C. et al. Rational design of a microbial consortium of mucosal sugar utilizers reduces clostridiodes difficile colonization. Nat. Commun. 11, 5104. https://doi.org/10.1038/s41467-020-18928-1 (2020).
Wang, K. et al. Parabacteroides distasonis alleviates obesity and metabolic dysfunctions via production of succinate and secondary bile acids. Cell. Rep. 26, 222–235. https://doi.org/10.1016/j.celrep.2018.12.028 (2019). e225.
Horáčková, Š., Plocková, M. & Demnerová, K. Importance of microbial defence systems to bile salts and mechanisms of serum cholesterol reduction. Biotechnol. Adv. 36, 682–690. https://doi.org/10.1016/j.biotechadv.2017.12.005 (2018).
Jia, B., Zou, Y., Han, X., Bae, J. W. & Jeon, C. O. Gut microbiome-mediated mechanisms for reducing cholesterol levels: implications for ameliorating cardiovascular disease. Trends Microbiol. 31, 76–91. https://doi.org/10.1016/j.tim.2022.08.003 (2023).
Huang, B., Song, B. L. & Xu, C. Cholesterol metabolism in cancer: mechanisms and therapeutic opportunities. Nat. Metab. 2, 132–141. https://doi.org/10.1038/s42255-020-0174-0 (2020).
Tang, C. et al. Natural polysaccharides protect against diet-induced obesity by improving lipid metabolism and regulating the immune system. Food Res. Int. 172, 113192. https://doi.org/10.1016/j.foodres.2023.113192 (2023).
Brown, M. S. & Goldstein, J. L. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell 89, 331–340. https://doi.org/10.1016/s0092-8674(00)80213-5 (1997).
Horton, J. D., Goldstein, J. L. & Brown, M. S. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Invest. 109, 1125–1131. https://doi.org/10.1172/jci15593 (2002).
Durst, R. et al. The discrete and combined effect of SREBP-2 and SCAP isoforms in the control of plasma lipids among Familial hypercholesterolaemia patients. Atherosclerosis 189, 443–450. https://doi.org/10.1016/j.atherosclerosis.2006.01.001 (2006).
Friedlander, Y. et al. SREBP-2 and SCAP isoforms and risk of early onset myocardial infarction. Atherosclerosis 196, 896–904. https://doi.org/10.1016/j.atherosclerosis.2007.02.006 (2008).
Göbel, A., Rauner, M., Hofbauer, L. C. & Rachner, T. D. Cholesterol and beyond - The role of the mevalonate pathway in cancer biology. Biochim. Biophys. Acta Rev. Cancer. 1873, 188351. https://doi.org/10.1016/j.bbcan.2020.188351 (2020).
Hinson, D. D., Chambliss, K. L., Toth, M. J., Tanaka, R. D. & Gibson, K. M. Post-translational regulation of mevalonate kinase by intermediates of the cholesterol and nonsterol isoprene biosynthetic pathways. J. Lipid Res. 38, 2216–2223 (1997).
Cho, Y. K., Ríos, S. E., Kim, J. J. & Miziorko, H. M. Investigation of invariant serine/threonine residues in mevalonate kinase. Tests of the functional significance of a proposed substrate binding motif and a site implicated in human inherited disease. J. Biol. Chem. 276, 12573–12578. https://doi.org/10.1074/jbc.M011478200 (2001).
Freed-Pastor, W. A. et al. Mutant p53 disrupts mammary tissue architecture via the mevalonate pathway. Cell 148, 244–258. https://doi.org/10.1016/j.cell.2011.12.017 (2012).
Segrest, J. P. et al. ABCA1 is an extracellular phospholipid translocase. Nat. Commun. 13, 4812. https://doi.org/10.1038/s41467-022-32437-3 (2022).
Medina-Urrutia, A. et al. Role of adiponectin and free fatty acids on the association between abdominal visceral fat and insulin resistance. Cardiovasc. Diabetol. 14, 20. https://doi.org/10.1186/s12933-015-0184-5 (2015).
Reilly, S. M. & Saltiel, A. R. Adapting to obesity with adipose tissue inflammation. Nat. Rev. Endocrinol. 13, 633–643. https://doi.org/10.1038/nrendo.2017.90 (2017).
Acknowledgements
This study was funded by the Jilin Province Science and Technology Development Program (Grant No. 20230204006YY). We acknowledge databases like GEO, UniProt, and GeneCards for their platforms and the contributors for uploading valuable datasets.
Funding
This work was supported by the Jilin Province Science and Technology Development Program (Grant No. 20230204006YY).
Author information
Authors and Affiliations
Contributions
Yuchen Wang, Guangfu Lv, He Lin, and Weichen Qi played a significant role in the conception and design of the manuscript. Zhi Lin was instrumental in drafting the manuscript and collecting the data. Haiqing Luo took primary responsibility for creating the graphs and figures. Shuang Pan, Yvmo Li, Xinrui Zhou and Jiarui Zhao were primarily responsible for analyzing and organizing the data, and Xinyi Xu and Xiaowen Li managed the software operation. The authors collaborated on drafting the manuscript, with Zhe Lin, He Lin, Weichen Qi providing critical revisions and overall supervision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All animal experiments followed ARRIVE guidelines and the NIH Guide for the Care and Use of Laboratory Animals (NIH Publication No. 8023, revised 1978) and the Experimental Animal Ethics Committee of Changchun University of Chinese Medicine (Ethics Approval No.: 2020262).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lin, Z., Luo, H., Pan, S. et al. Effects of Paeoniae Radix Rubra on lowering lipid via bioinformatics and gut microbiome. Sci Rep 15, 21117 (2025). https://doi.org/10.1038/s41598-025-08486-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-08486-1