Abstract
Obesity hypoventilation syndrome (OHS) is a comorbidity in patients with Obstructive sleep apnea (OSA). It has a significant impact on the treatment and outcomes. There is paucity of data on the prevalence of OHS and its associated factors. Our objective was to estimate this for our Indian cohort. This is a retrospective study on consecutive patients who underwent a sleep study for suspicion of OSA from January 2022 to December 2022. Details of these patients were extracted from the electronic medical records, and the sleep studies were analysed. The prevalence of OHS was calculated. Multiple logistic regression analysis was performed to ascertain the independent OHS predictors. In an Indian patient population comprising predominantly southern and eastern Indian residents, 674 who met the specified criteria were included. Their mean age was 51.7 (SD 12.5), 426 (63.2%) were males, and 51.3% were obese. The prevalence of OHS was 5.8%. Only two patients without OSA had OHS. For the BMI ranges 30–40, 40–50, and > 50 kg/m2, the prevalence of OHS was 9%, 13%, and 20%, respectively. Multivariate logistic regression analysis found that female gender (OR = 4.1), BMI ≥ 35 kg/m2 (OR = 3.2), and orthopnoea (OR = 4.8) were independently associated with OHS. Hypothyroidism (OR = 2.2) showed a significant association only in the univariate analysis. In a cohort of Indian patients referred for a sleep study, the prevalence of OHS is low. Increased BMI, female gender and a history of orthopnea were independent predictors of OHS.
Similar content being viewed by others
Introduction
Obesity hypoventilation syndrome (OHS) is a severe form of sleep-disordered breathing and occurs in obese patients, and by definition in those with a BMI of 30 or higher, when they manifest with unexplained daytime hypercarbia (PaCO2 ≥ 45mmHg)1. It often co-exists with obstructive sleep apnea (OSA), which is a more prevalent condition2. The diagnosis of OHS requires arterial blood gas (ABG) results in addition to the sleep study done to diagnose OSA. Around 10% of OHS occurs in the absence of OSA; when it so happens, it is referred to as ‘sleep hypoventilation’3.
While there are no population-based studies to assess the prevalence of OHS, based on certain assumptions, the prevalence in the US has been estimated to be 3.7 per 1000 population4. Most of the available literature on OHS prevalence is from patients referred for a sleep study with suspected OSA or among those with diagnosed OSA. There have been two meta-analyses, one in 2009 by Kaw et al.2. and the other in 2017 by Liu et al.5, which have aggregated the studies of OHS in OSA; at this time, there were no studies from India. The prevalence of OHS in patients with OSA is variable, ranging from 10–38%2. The pooled prevalence estimate by Liu et al.5 was 28%. Subsequently, the only study6 published from India reported an OHS prevalence of 15.8% among those who underwent polysomnography.
The presence of OHS, in addition to OSA, may considerably increase cardiovascular morbidity and mortality2 as well as healthcare expenditures7. The OHS patients are usually treated with bilevel PAP compared to CPAP for OSA. A high index of suspicion, leading to early recognition and initiation of appropriate treatment, would reduce the high burden due to unrecognised and untreated OHS2. Overall, there is a paucity of literature regarding the risk factors of OHS, mainly from India, in those who have OSA.
Therefore, we retrospectively studied the prevalence of OHS among the patients referred for a sleep study and assessed the associated risk factors.
Materials and methods
This retrospective study was performed from January 2022 to December 2022 at the Department of Pulmonary Medicine at Christian Medical College, Vellore, India. The experimental protocol of this study was approved by the Institutional Review Board of the Christian Medical College, Vellore, India (IRB No. 15363; dated 19.04.2023). The list of consecutive patients referred to the sleep laboratory during the study period was obtained from the sleep lab register. The demographic details (age, gender, and occupation), clinical symptoms, STOP-BANG score, height, weight, comorbidities, etc., were extracted from the electronic medical records.
These patients have had limited polygraphy sleep studies (Somnomedics or Somnoscreen plus or SomnoHD), which measured thoracoabdominal movement using respiratory inductance plethysmography (RIP), oxygen saturation, heart rate and oronasal flow.
As per the World Health Organization(WHO) criteria, BMI ≥ 30 kg/m2 is classified as obesity. ABG and serum bicarbonate values were extracted whenever available. Hypercapnia was defined as pCO2 (Partial Pressure of Carbon dioxide) > 45 mm Hg (6 kPa). Lung function was done either to rule out an obstructive airway disease before labelling as OHS in those who had hypercarbia, or in others if they had breathlessness, as part of evaluation, which could have been due to obesity or other causes.
The sleep polygraphy and oximetry data were interpreted and analysed using proprietary software (DOMINO 3.0.0.3). Manual scoring was done on all the studies using the following definitions for adults as per the AASM (American Association of Sleep Medicine) guidelines: Apnoea is the cessation of airflow at the upper airway lasting > 10 s. Hypopnea is a ≥ 30% drop in airflow for ≥ 10 s associated with arousal or a ≥ 3% oxygen desaturation. The apnoea-hypopnea index (AHI) was calculated as the number of apnoea and hypopnea episodes per hour of sleep. Those with AHI > 5 were diagnosed with obstructive sleep apnoea. OSA was classified into three severity categories based on the AHI: severe (≥ 30), moderate (≥ 15 to 30) and mild (5 to < 15). The oxygen desaturation index (ODI) was established by calculating the total number of episodes of significant oxygen desaturation (> 3% fall from baseline) per hour of sleep. AHI, ODI, minimum nocturnal SpO2 (pulse oximeter oxygen saturation), and total recording time with Spo2 < 90% total recording time with SpO2 below 90% (TRT90) were calculated from the device.
Obesity hypoventilation syndrome (OHS) was defined as “the combination of obesity (body mass index (BMI) ⩾ 30 kg/m2), sleep-disordered breathing and daytime hypercapnia (arterial carbon dioxide tension (PaCO2) ⩾ 45 mmHg at sea level) during wakefulness, occurring in the absence of an alternative neuromuscular, mechanical, or metabolic explanation for hypoventilation”8.
Data was analysed using SPSS V 21{License Number (Customer ID): 200699; Vendor: SPSS South Asia Pvt Ltd. Bangalore}. Continuous variables were expressed as mean and standard deviation(SD) or median and interquartile range based on the normality. Categorical variables were expressed as frequencies and percentages. In univariate analysis, the independent t-test or Mann-Whitney U test was used for continuous variables, and the Chi-square test was used for categorical variables. The differences were considered statistically significant at the two-sided p-value < 0.05 level and the odds ratio was calculated to determine the strength of the association. Multiple logistic regression analysis was performed to adjust for the confounding factors, and an adjusted odds ratio was obtained to ascertain the independent predictors of OHS.
Results
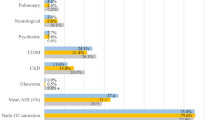
In all, 674 consecutive patients who met inclusion criteria during the study period were included. Their mean age was 51.7 (SD 12.5) years, and 426 (63.2%) were males. Criteria for obesity (BMI ≥ 30 kg/m2) were fulfilled by 346 (51.3%). The clinic-demographic characteristics and sleep-related parameters, stratified by obesity, are presented in Tables 1 and 2. Although nearly two-thirds of the study subjects were males, 50% of the obese subjects were females. Hypertension was the most common comorbidity, affecting 342 (50.7%) participants, followed by Diabetes Mellitus (34.7%). Most (96.1%) reported snoring, and a significant proportion, 287 (42.6), witnessed apnoea. Among comorbidities, hypertension was significantly higher (56.4% vs. 44.8%; p = 0.003) in the obese, and so was hypothyroidism (23.1% vs. 12.5%; p < 0.001). The STOPBANG and Mallampati scores were higher (p < 0.001) among the obese group. Spirometry showed lower FVC (forced vital capacity) and FEV1 (forced expiratory volume in 1 st second) in the obese group, and ABG showed lower PaO2 (85.7 vs. 89.6; p = 0.02). The sleep study of the obese group showed higher AHI (47.2 vs. 30.3; p < 0.001), higher ODI (46.3 vs. 28.4; p < 0.001), lower oxygen nadir (76 vs. 83; p < 0.001) and longer time spent < 90% saturation (6.8% vs. 1.4% of the sleep time; p < 0.001).
The algorithm for the diagnosis of OHS is shown in Fig. 1, and the prevalence of OHS and OSA in the obese and non-obese is tabulated in Table 3. The prevalence of OSAS among all who underwent sleep study was 94.5% (637/674), and among those with obesity, it was higher at 97.4% (337/346). Eighty-seven of 346 obese patients were unwilling to get an arterial blood gas done and hence were excluded from the analysis for ascertaining the prevalence of OHS. Therefore, 587 were included in the assessment of OHS prevalence, which was 5.8% (34/587). Since only two patients had OHS without OSA, the prevalence among those with OSA was the same at 5.8% (32/550).
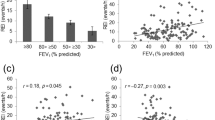
The prevalence of OSA and OHS is depicted per their BMI in Fig. 2. There was a progressive increase in the prevalence of OSA and OHS with increasing BMI. All patients with BMI ≥ 40 kg/m2 had OSA. For the BMI ranges 30–40, 40–50 and > 50, the prevalence of OHS was 9%, 13%, and 20%, respectively.
The analysis of the risk factors of OHS among those with OSA is seen in Table 4. In the univariate analysis (Table 3), female gender (OR = 6.9), orthopnoea (OR = 6.2), BMI ≥ 35 kg/m2 (OR = 5.6), and hypothyroidism (OR = 2.2) showed significant association with OHS. All the sleep study parameters were significantly worse in those with OHS. Likewise, the ABG parameters, pH, PaO2, PaCO2, and HCO3(serum/arterial bicarbonate), were significantly worse. The serum bicarbonate was also significantly higher in the OHS group, although within the normal limit. A multivariate logistic regression analysis (Table 5) was performed adjusting for female gender, BMI (≥ 35 kg/m2), orthopnoea, diabetes mellitus, hypertension, cardiac failure, and hypothyroidism and among these, female gender (OR = 4.1), BMI ≥ 35 kg/m2 (OR = 3.2) and orthopnoea (OR = 4.8) were identified as risk factors for OHS.
Since BMI was one of the major predictors with an exposure-response relationship to OHS, we analysed various cut-offs of BMI and their diagnostic accuracy for OHS. It generated an AUC of 0.83 (95% CI 0.78–0.88). A BMI of 35, 40, 45 and 50 kg/m2 had a sensitivity of 59%, 24%, 15% and 6%, with a specificity of 78%, 93%, 98% and 99%, respectively. This translates to a Youden index of 0.37, 0.17, 0.13 and 0.05, respectively.
Discussion
This is only the second Indian study looking at the prevalence of OHS in a cohort of patients referred for a sleep study, with a sample size of 674, substantially higher than the previous6. The mean age of our population was similar to the Indian study by Patro et al.6 and the meta-analysis by Kaw et al.2. Our population was male-predominant (63%). Still, it was lower than Kaw et al.‘s meta-analysis, which comprised 81% males2. This can be attributed to the gender inequality of obesity in India9, which is skewed towards women. While globally, obesity is higher among men10, in India, it is higher among women9. In our study also, women contributed to half of the obese proportion while being only one-third of the study population. The obese sub-group had a worse sleep study and lung function parameters and as expected, had more comorbidities such as diabetes mellitus, hypertension, and hypothyroidism. While the PaO2 was significantly lower in the obese group (though within the normal range), it was interesting to note that the PaCO2 was not different compared to the non-obese group.
The overall prevalence of OHS was much lower in our cohort, a predominantly southern and eastern Indian study (5.8%), compared to the other global reported series2,5. Patro et al.6 reported a higher prevalence (15.9%) for Indian subjects, but their subjects were from Northern India. Furthermore, only a little over half (51.3%) of our study population were obese, but both in Patro et al.6 and Kaw et al.’s series, there were more obese subjects (60% and 69%, respectively). This could also be a reason for the higher proportion of OHS in these reports. Also, some studies in the meta-analysis by Kaw et al.2 have included only higher AHI cutoffs (AHI of > 10 and > 20/hr). This is likely to have sub-selected more obese individuals in their cohorts who are more likely to have OHS, resulting in a higher prevalence of OHS. In addition, Kaw et al.2 meta-analysis studied the prevalence of hypercarbia and not that of OHS. Since OHS would only be a subset of the patients with hypercarbia, their prevalence is not precisely comparable and is likely to be higher.
A quarter (87/346) of our obese patients had refused an ABG. Although this is likely to be random, there is a possibility that those who opted not to have ABG may have had a higher prevalence of OHS. To see if this would have biased our prevalence, we compared the obese subjects among those who had refused an ABG with those who had performed the test. The former were five years younger (47 vs. 52 years), had lower AHI (41/hr vs. 49/hr), were equal in gender distribution and had lower proportions with morbid obesity (10% vs. 17%). Hence, the lower prevalence of OHS is unlikely due to this. It could well be that the actual prevalence in our patient populations is lower. Genetic factors8 are not well studied and are underreported, which could contribute to differences in prevalences.
Although the prevalence of OHS was relatively low in our study, the large sample size allowed for meaningful analysis of risk factors. Female gender (OR = 4.1), BMI ≥ 35 kg/m2 (OR = 3.2) and orthopnoea (OR = 4.8) were found to be independently associated with OHS, while hypothyroidism also showed association with OHS on univariate logistic regression, it did not stand the test of multivariate logistic regression. Kaw et al.’s2 2009 meta-analysis identified lower FEV1, FVC and TLC(Total Lung Capacity), higher BMI, and AHI to be associated with OHS, but they did not find associations with demographic variables like age and gender. In addition to higher BMI, higher AHI and lower per cent predicted FEV1, Liu et al.’s5 2017 meta-analysis found greater neck circumference associated with OHS. The only Indian study by Patro et al.6 found the nadir SpO2 < 60%, FVC < 74.5% predicted, FVC < 2.33 L for men and < 1.68 L for women and BMI > 30.95 kg/m2, as independent predictors of OHS.
As noted earlier, our study had relatively more females and indeed, the prevalence of obesity in females (172/248 = 69%) was higher than that in males (174/426 = 41%). The prevalence of OHS was also higher in females than males. Females comprised only 36.8% of our study population but accounted for 78% of the diagnosed OHS (25/32), and a female referred for sleep study had 4 times the odds of being diagnosed with OHS. Patro et al. likewise showed a higher prevalence of OHS among their female subjects, but the difference wasn’t statistically significant, presumably due to the lower sample size. Kaw et al.2 meta-analysis included eight studies that evaluated gender as an associated factor, but it did not reject the null hypothesis (OR, 0.96; 95% CI, 0.62 to 1.50; p = 0.9). The other meta-analysis by Liu et al.5 was heavily skewed towards male subjects (93%) and did not look at gender as an association. The apparent reason for identifying female gender as a risk factor for OHS in our study is the higher prevalence of obesity in the women of our cohort, presumably due to the gender inequality of obesity in India, while other reasons need to be explored.
We found BMI (≥ 35 kg/m2) to be an independent risk factor for OHS, with an odds of 3.2. Neither of the meta-analyses, Kaw et al.2 and Liu et al.5, dichotomised the BMI but found a statistically significant difference in BMI of 3.13 and 4.73 kg/m2, respectively, between the groups. We found a progressive increase in the prevalence of OHS with increasing BMI. A BMI above 40 kg/m2 had a 93% specificity for OHS, and this went up to 98% at a 45 kg/m2 cut-off. Obesity-related ventilatory changes are described as contributory to the pathophysiology of OHS8. Reduced lung volumes, notably the functional residual capacity and shrinking of the expiratory reserve volume, may occur as a consequence of excess adipose tissue around the chest and the abdomen. Gas trapping due to premature airway closure produces intrinsic positive end-expiratory pressure and ventilation-perfusion mismatches. There may also be impaired respiratory wall mechanics related to respiratory muscle weakness. All these factors combine to increase the respiratory workload, which needs to be overcome.
Orthopnoea was found to be independently associated with OHS, with an odds ratio of 4.8. All the patients who had cardiac failure had orthopnoea (3/3). However, the association of orthopnoea with OHS was independent of cardiac failure, as shown in Table 5. To the best of our knowledge, this has not been reported before. It is likely due to the abdominal adipose tissue and its splinting effect on the diaphragm, which gets accentuated when lying down. The use of orthopnoea as a screening symptom for OHS in obese subjects who fulfil indications for a sleep study to rule out OSA needs to be investigated further. Hypothyroidism was not proven to be independently associated with OHS in our study, but it had a significant association before adjusting for confounding factors (Table 4). This suggests that the other risk factors, such as female gender and BMI ≥ 35 kg/m2, that we have identified influence OHS more than hypothyroidism. Among the 10 patients with hypothyroidism in the OHS group, 8 (80%) of them were female, and 7 (70%) of them were of BMI ≥ 35 kg/m2. Hypothyroidism has been postulated to cause submucosal infiltration of upper airways11, increasing propensity to OSA and possibly OHS. The data on the association between OHS and hypothyroidism is sparse. An old study from Finland12, which set out to study the relationship between upper airway obstruction and hypothyroidism, reported the association with male gender and obesity and not hypothyroidism. In a series11 of 308 OHS patients, a quarter of them had thyroid dysfunction, although only 18.8% were clinically hypothyroid. However, our findings may not justify routine screening of all the OHS subjects for hypothyroidism.
Our study has a few limitations. Its retrospective nature has inherent shortfalls with data collection and missing data. The essential data was, however, available for the majority. A quarter of obese patients refused to undergo the ABG test. However, this does not appear to be the reason for the low prevalence of OHS that we are reporting.
Conclusion
We have reported herewith the largest series on OHS to date from India. The prevalence of OHS is low in our southern & eastern Indian cohort of patients. Female gender, BMI ≥ 35 kg/m2 and the presence of orthopnoea were found to be independent predictors.
Data availability
The datasets generated and/or analysed during the current study are not publicly available as there are some patient identifiers but are available from the corresponding author on reasonable request.
References
Alzaabi, A., Fizal, S., Moilothkandy, R., Mahboub, B. & Nagelkerke, N. Obesity hypoventilation syndrome in obstructive sleep apnea patients in the united Arab emirates: a retrospective cross-sectional study. JRSM Short. Rep. 4, 2042533313510156 (2013).
Kaw, R., Hernandez, A. V., Walker, E., Aboussouan, L. & Mokhlesi, B. Determinants of hypercapnia in obese patients with obstructive sleep apnea: a systematic review and metaanalysis of cohort studies. Chest 136, 787–796 (2009).
Littleton, S. W. & Mokhlesi, B. The Pickwickian syndrome—Obesity hypoventilation syndrome. Clin. Chest Med. 30, 467–478 (2009).
Mokhlesi, B., Saager, L. & Kaw, R. Q. Should we routinely screen for hypercapnia in sleep apnea patients before elective noncardiac surgery? Cleve Clin. J. Med. 77, 60–61 (2010).
Liu, C., Chen, M. S. & Yu, H. The relationship between obstructive sleep apnea and obesity hypoventilation syndrome: a systematic review and meta-analysis. Oncotarget 8, 93168–93178 (2017).
Patro, M., Gothi, D., Ojha, U. C., Vaidya, S. & Sah, R. B. Predictors of obesity hypoventilation syndrome among patients with sleep-disordered breathing in India. Lung India Off Organ. Indian Chest Soc. 36, 499–505 (2019).
Berg, G., Delaive, K., Manfreda, J., Walld, R. & Kryger, M. H. The use of Health-Care resources in Obesity-Hypoventilation syndrome. Chest 120, 377–383 (2001).
Masa, J. F. et al. Obesity hypoventilation syndrome. Eur Respir Rev 28, (2019). Mar 14;28(151):180097
Alvarez-Saavedra, V., Levasseur, P. & Seetahul, S. The role of gender inequality in the obesity epidemic: A case study from India. J. Dev. Stud. 59, 980–996 (2023).
Muscogiuri, G. et al. Obesity: a gender-view. J. Endocrinol. Invest. 47, 299–306 (2024).
BaHammam, A. S., Aleissi, S., Olaish, A. H., Almeneessier, A. S. & Jammah, A. A. Prevalence of hypothyroidism in a large sample of patients with obesity hypoventilation syndrome. Nat. Sci. Sleep. 12, 649–659 (2020).
Pelttari, L. et al. Upper airway obstruction in hypothyroidism. J. Intern. Med. 236, 177–181 (1994).
Funding
No Funding was received for this research.
Author information
Authors and Affiliations
Contributions
BI- concept, design, analysis, supervision, manuscript writing & approval of the final versionCA- data collection, analysis, manuscript writing & approval of the final versionRJ - data entry, analysis, manuscript editing and approval of the final versionAB- data entry, analysis, manuscript editing and approval of the final versionBT- supervision, manuscript editing & approval of the final versionDJC- concept, design, analysis, supervision, manuscript writing, manuscript editing & approval of the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional review board of the Christian Medical College, Vellore (IRB No. 15363; dated 19.04.2023) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.Informed consent: Since this was a retrospective study, the Institutional Review Board, Christian Medical College, Vellore, India, permitted waiver of informed consent.
Ethics committee approval
Institutional review board (IRB No. 15363; dated 19.04.2023), Christian Medical College, Vellore, India.
Conference presentations
This was presented in part as a poster at the European Respiratory Society Annual Congress in Milan in 2023.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Isaac, B., Alexander, C., John, R. et al. Prevalence and predictors of obesity hypoventilation syndrome in an Indian cohort with obstructive sleep apnea. Sci Rep 15, 23474 (2025). https://doi.org/10.1038/s41598-025-08953-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-08953-9