Abstract
Our study aims to evaluate the risk of drug-induced hypertrophic rhinitis and analyze its epidemiological characteristics utilizing real-world data. We employed reporting odds ratios (ROR) to assess the disproportionality in reports of drug-induced hypertrophic rhinitis between January 2004 and September 2024. Single-factor, LASSO, and multi-factor regression analyses were conducted to investigate drugs associated with hypertrophic rhinitis. The Bonferroni correction was implemented to conduct multiple comparisons. 250 drugs were linked to hypertrophic rhinitis, with 85 drugs (case number > 100) identified as independent risk factors for drug-induced hypertrophic rhinitis. Those drugs’ indications were classified as Allergic disease (19/85), Multi-indication (13/85), Cardiovascular disease (12/85), Rheumatoid disease (6/85), Autoimmune disease (6/85), Respiratory disease (5/85), Cancer (4/85), Metabolic disease (4/85), Contrast agent (3/85), Erectile dysfunction (3/85), Infection (3/85), Disease of the nervous system (2/85), Urologic Disease (2/85) Esophageal disease (1/85), Hereditary disorder (1/85), and Ophthalmic disease (1/85). Multiple medications have possible risks of drug-induced hypertrophic rhinitis. Further research is needed to clarify causality and guide clinical decision-making.
Similar content being viewed by others
Introduction
Chronic rhinitis imposes a significant global epidemiological burden, with prevalence rates approximating 30% worldwide1. This condition profoundly impairs patients’ health-related quality of life through multiple pathways: compromised occupational/academic efficiency, disrupted social functioning attributable to symptom burden, and sleep architecture disturbances. The clinical manifestations exhibit a severity spectrum ranging from transient psychosocial impairments (e.g., situational anxiety) to pathophysiological sequelae, including chronic fatigue, resonant breathing disturbances, and sleep-disordered breathing patterns such as obstructive apnea2. It is categorized into the following phenotypes: infectious rhinitis, allergic rhinitis (AR), nonallergic rhinitis (NAR), and mixed rhinitis1. Drug-induced rhinitis is a variant of NAR marked by mucosal edema, vasodilation, and the production of inflammatory mediators3.
Given the multifactorial etiology of drug-induced nasal pathology, understanding the mucosa’s physiological role is critical to deciphering its vulnerability to pharmacological agents. The nasal mucosa functions as a barrier that regulates interactions with the host immune system, fostering tolerance and symbiosis while preventing or mitigating inflammation4. In addition to rhinitis medicamentosa caused by topical nasal decongestants overuse5, rhinitis secondary to systemic medications is also prevalent. Those medications include nonsteroidal anti-inflammatory drugs, psychotropics, α/β-adrenergic receptor modulators, immunomodulators, phosphodiesterase inhibitors, angiotensin converting enzyme inhibitors, hormones, antihypertensives, and others3.
This broad spectrum of causative agents underscores the necessity of systematic pharmacovigilance to map drug-specific risks within heterogeneous patient populations. Pharmacovigilance is the study to find, evaluate, understand, and prevent adverse effects or other problems related to drugs6. Using real-world data in pharmacovigilance databases to analyze and summarize drug-associated risk factors has become essential for assessing drug safety7,8. This study examined the medications most frequently associated with nasal mucosa hypertrophy within the FDA Adverse Event Reporting System (FAERS) database. Additionally, we aimed to enhance the precision of hypertrophic rhinitis risk evaluation by classifying causative drugs according to their applications.
Methods
Database and study subjects
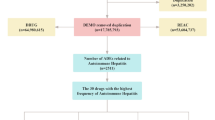
We retrieved all accessible data from the FAERS database between January 2004 and September 2024 and processed it to remove duplicates. During the initial data cleaning phase, medication nomenclature within the FAERS database was harmonized to comply with unified pharmacopeia standards. Non-standard entries were rectified through cross-referencing with authoritative drug registries such as the FDA Drug Database. Subsequently, a deduplication algorithm incorporating patient identifiers and temporal parameters was applied to eliminate redundant case reports, ensuring a singular representation of each adverse event.
Adverse events are classified using the Medical Dictionary for Regulatory Activities (MedDRA) terminology in terms of signs/symptoms, called preferred terms (PTs) in the FAERS database. We use the following 9 PTs to define hypertrophic rhinitis: nasal congestion, rebound nasal congestion, nasal mucosa telangiectasia, nasal mucosal hypertrophy, nasal turbinate hypertrophy, rhinorrhoea, nasal obstruction, nasal oedema, and rhinitis hypertrophic. This study assessed exposure by considering drugs identified as primary suspects.
Data analysis
A four-fold table was constructed (Table S1). The association between hypertrophic rhinitis and suspicious medications was evaluated through disproportionality analysis employing the Reporting Odds Ratio (ROR) and a 95% confidence interval (CI). Risk signals were identified when the ROR and the lower limit of the corresponding 95% CI > 1, and the case number was > 3.
P-adjust refers to the p-value obtained after the application of Fisher’s exact test and the Bonferroni correction.
We conducted the single-factor analysis for those suspicious drugs with a 95% CI lower limit of ROR > 1, a > 100, and p-adjust < 0.01. Drugs with p < 0.01 in the single-factor analysis were selected for the least absolute shrinkage and selection operator (LASSO) regression. Individuals with missing data (e.g., gender or age) were excluded from this analysis. Using 10-fold cross-validation to optimize the penalty parameter (λ), LASSO identified a parsimonious variable subset. A multi-factor logistic regression analysis incorporating age and gender was performed to ascertain risk factors associated with drug-induced hypertrophic rhinitis. Model performance was assessed through the area under the curve (AUC).
Data processing and analysis are predominantly performed using Microsoft Excel 2019, GraphPad Prism 9, and R software (version 4.2.1).
Results
Baseline data description
Starting in 2004, the minimum number of drug-induced hypertrophic rhinitis cases was observed in that year (n = 664), while the maximum occurred in 2022 (n = 9471) (Fig. 1A). The mean age of participants was 54.6 years, with quartiles of 44 (Q1), 58 (Q2), and 68 (Q3) (Fig. 1B). Adverse reaction outcomes associated with drug-induced hypertrophic rhinitis were also analyzed (Fig. 1C).
As shown in Table 1, among the 100,093 reported cases, females (61.8%) experienced a greater impact than males (30.9%). The United States reported the highest incidence at 71.9%, followed by Canada at 12.0%, the United Kingdom at 2.0%, Germany at 1.8%, and Colombia at 0.6%. Pharmacists and physicians submitted 41,610 adverse event reports, representing 40.1% of the total, while consumers submitted an additional 21,662 reports, accounting for 21.7%. Additionally, the mean and median times from drug use to hypertrophic rhinitis occurrence were 245 and 16 days, and 40% of the cases had a time interval of less than one week.
Hypertrophic rhinitis-related drugs
We analyzed the indications of patients with drug-induced hypertrophic rhinitis (cases ≥ 400). The five most commonly reported indications are Rheumatoid Arthritis, Pulmonary Arterial Hypertension, Asthma, Psoriasis, and Multiple Sclerosis (Fig. 2A). The volcano graphs presented in Fig. 2B were constructed to examine the correlation between hypertrophic rhinitis and the suspected drugs. In this graph, a positive x-axis suggests that the adverse effects linked to drug-induced hypertrophic rhinitis were reported with greater frequency than other adverse effects, and a positive y-axis indicates a markedly significant difference. An increase in the intensity of the red hue corresponds to a greater quantity of reports. As a result, drugs in the upper right quadrant of this graph had both differences and significant signal strength.
250 drugs were identified (Table S2). Except multi-indication and others, the categories of drug’ indication included Allergic disease (34/250), Metabolic disease (25/250), Cardiovascular disease (20/250), Respiratory disease (18/250), Cancer (15/250), Infection (14/250), Autoimmune disease (12/250), Contrast agent (9/250), Rheumatoid disease (8/250), Hereditary disorder (8/250), Disease of the nervous system (8/250), Urologic Disease (7/250), Ophthalmic disease (6/250), Hematological disease (3/250), Esophageal disease (3/250), Erectile dysfunction (3/250), Decongestant (3/250) and Mental disorder (2/250).
We conducted a single-factor analysis for drugs with > 100 case numbers, a lower 95% CI limit of the ROR > 1, and p-adjust < 0.01. Then, drugs with p < 0.01 in single-factor analysis were subjected to LASSO regression, followed by multifactorial logistic regression analysis incorporating age and gender (Fig. 3B). The results showed that those 85 drugs were independent drug-induced hypertrophic rhinitis risk factors (Fig. 3A). The ROC-AUC, reflecting the model’s predictive accuracy, was 0.699 (Fig. 4). Those drugs’ indications were classified as Allergic disease (19/85), Multi-indication (13/85), Cardiovascular disease (12/85), Rheumatoid disease (6/85), Autoimmune disease (6/85), Respiratory disease (5/85), Cancer (4/85), Metabolic disease (4/85), Contrast agent (3/85), Erectile dysfunction (3/85), Infection (3/85), Disease of the nervous system (2/85), Urologic Disease (2/85) Esophageal disease (1/85), Hereditary disorder (1/85), and Ophthalmic disease (1/85) (Fig. 3A). And those medications were also classified based on therapeutic mechanisms of action, as outlined in Table S3.
Year, age, and outcome distribution of drug-induced hypertrophic rhinitis reports from the FAERS database (Q1 2004 – Q3 2024). DE: death; OT: other serious; RI: required intervention to prevent permanent impairment/damage; CA: congenital anomaly; DS: disability; HO: hospitalization; LT: life-threatening.
Signal detection. A: The indications of patients with drug-induced hypertrophic rhinitis (cases ≥ 400); B: A positive x-axis suggests that the adverse effects linked to drug-induced hypertrophic rhinitis were reported more frequently than other adverse effects; a positive y-axis indicates a markedly significant difference. Thus, drugs in this graph’s upper right quadrant had differences and significant signal strength.
Single- and multi-factor analysis of drug-induced hypertrophic rhinitis. A: 85 drugs which were independent drug-induced hypertrophic rhinitis risk factors; B: The optimal regularization parameter λ in the Lasso regression model was determined (upper panel), while the evolutionary trajectories of variable coefficients across λ-spectrum were visualized (lower panel).
Discussion
Hypertrophic rhinitis significantly impacts patients’ quality of life. This project involved a thorough characterization, description, and analysis of drug-induced hypertrophic rhinitis utilizing the FAERS database. Our data showed that drug–induced hypertrophic rhinitis cases were predominantly reported among women in Western countries, with a significant rise in reports over time. We identified 85 drugs with positive signals for hypertrophic rhinitis (cases > 100). Those drugs, encompassing diverse mechanisms of action, demonstrated notable differences in their associated risk of inducing hypertrophic rhinitis. The findings present significant real-world data and theoretical support for guiding the appropriate application of medications to mitigate the risk of hypertrophic rhinitis and providing essential insights for future research.
Aetiologically, rhinitis includes infectious rhinitis, vasomotor rhinitis, hormone-induced rhinitis, allergic rhinitis, rhinitis caused by low molecular weight chemicals, structural issues, mixed rhinitis, and other forms9,10. Similar mechanisms are engaged in the response to drug-induced hypertrophic rhinitis. For example, Etanercept, a large molecule that binds to TNFα and alters the immune balance, is reported to have side effects of infections, including severe cases resulting in hospitalization or mortality11. Our analysis indicates that Etanercept is the most frequently implicated drug in inducing hypertrophic rhinitis (n = 6054), as it elevates the risk of upper airway infections.
Vasomotor rhinitis is the most prevalent form of NAR12, while hypertrophic rhinitis resulting from vasodilatory medications is also frequently observed. Among the 12 drugs with indications for cardiovascular disease, endothelin receptor antagonist (Ambrisentan, Macitentan, Bosentan), prostacyclin receptor agonist (Treprostinil, Epoprostenol), angiotensin II receptor blocker (Losartan), soluble guanylate cyclase activator (Riociguat), and angiotensin-converting enzyme inhibitor (Ramipril) have a potential link to hypertrophic rhinitis. Besides, α/β-blockers (for respiratory and urologic disease) and PDE5 inhibitors (for erectile dysfunction) are also considered to be related to the occurrence of hypertrophic rhinitis.
Nervous regulation, encompassing parasympathetic innervation of the nasal mucosa, neurovascular imbalance, and neuropeptides in the upper airways, plays a crucial role in modulating airway tone, mucus secretion, and plasma extravasation13,14. In our study, a dopamine agonist (Apomorphine) and a monoclonal antibody (Eptinezumab) showed strong signals.
The intricate interactions among metabolic, endocrine, and immune responses also constitute a fundamental pathophysiological basis for the onset of rhinitis15,16,17,18. Thus, a significant pharmacovigilance signal exists among various medications for metabolic diseases, hereditary disorders, and hypertrophic rhinitis. Those drugs function in enzyme substitution, metabolic regulation, and the modulation of specific targets through inhibition or activation.
Rhinitis medicamentosa is a specific form of drug-induced rhinitis resulting from the overuse of topical nasal medications, primarily nasal decongestants19,20. The overuse of nasal decongestants can lead to a reduction in vascular sympathetic tone, tachyphylaxis of the vasoconstrictor system, tissue hypoxia due to extended vasoconstriction, and sustained beta-mediated vasodilation of mucosal blood vessels21. Although Xylometazoline (n = 69), combination of Ibuprofen and Pseudoephedrine (n = 19), and Pseudoephedrine (n = 40) were not included in the regression analysis due to their small sample size, the clinical use of nasal decongestants should be approached with caution.
Additionally, our list revealed several other medications potentially linked to the development of hypertrophic rhinitis. For instance, some contrast agents and antiviral drugs are potentially linked to hypertrophic rhinitis. Although some of those medications may induce allergic reactions and alter blood pressure, the underlying mechanism remains ambiguous. Thus, we should pay more attention in the future.
Drug-induced hypertrophic rhinitis involves multifactorial mechanisms beyond pharmacological targets, requiring integrated analysis of pharmacokinetic profiles such as half-life, bioavailability, and tissue accumulation. For instance, among PDE5 inhibitors sharing the same pharmacodynamic target, tadalafil, sildenafil, and vardenafil exhibit distinct risk profiles (ROR: 4.15 (3.84–4.48) vs. 2.44 (2.25–2.64) vs. 7.78 (6.83–8.85)) due to differential drug exposure dynamics (half-life: 17.5 h vs. 3-5 h vs. 4 h; bioavailability: 40% vs. 15% vs. 36%)22. Additionally, avanafil showed no significant risk association, likely due to signal dilution from limited pharmacovigilance data, delayed market entry, low clinical adoption, and potential underreporting of nasal adverse events23.
The nasal lining presents a large area of variable permeability endothelium and a rich vascular supply24. Notably, these unique anatomical and physiological attributes of the nasal cavity — including its extensive vascular network, non-invasive accessibility, and direct nose-to-brain pathways — constitute a key rationale for the growing scientific emphasis on intranasal drug delivery systems as a promising therapeutic avenue25. Based on the findings of this study, drug selection and risk-benefit disclosure should be noted, particularly in individuals with pre-existing rhinologic pathologies such as vasomotor rhinitis, who may exhibit heightened susceptibility to drug-induced nasal mucosal dysregulation.
The primary strength of this study lies in its utilization of large-scale data analysis from publicly accessible databases. However, this research also possesses multiple limitations. Firstly, FAERS generates variability in data quality owing to its heterogeneous sources. Secondly, current understanding of pharmacological mechanisms driving drug-associated hypertrophic rhinitis is speculative and warrants further validation via mechanistic studies. Thirdly, this study exclusively considered age and gender as covariates in the analysis, which may introduce analytical limitations due to unaddressed confounding variables. Crucially, we did not account for potential drug interactions, pre-existing medical conditions, or polypharmacy effects—factors known to influence drug-induced pathologies. Moreover, data shifts arising from missing age/gender records could further compromise the validity of covariate adjustments. Fourth, current understanding of pharmacological mechanisms driving drug-associated hypertrophic rhinitis is speculative and warrants further validation via mechanistic studies. Hence, although our study utilizes real-world data and sophisticated data mining techniques, further study is required to corroborate these findings and investigate the underlying mechanisms.
Conclusion
This pharmacovigilance investigation from the FAERS database was conducted to systematically characterize medication-associated hypertrophic rhinitis. The findings establish a framework for optimizing diagnostic approaches, therapeutic decision-making, and patient management in clinical practice. Future investigations integrating multi-source evidence with mechanistic studies are recommended to enhance medication safety surveillance.
Data availability
The data utilized in this research are publicly available as part of the FAERS database.
7 References
Hellings, P. W. et al. Non-allergic rhinitis: position paper of the European academy of allergy and clinical immunology. Allergy 72 (11), 1657–1665 (2017).
Alromaih S, Alsagaf L, Aloraini N, Alrasheed A, Alroqi A, Aloulah M, Alsaleh S, Alhawassi T. Drug-Induced Rhinitis: Narrative Review. Ear Nose Throat J. 15, 1455613221141214 (2022).
Wise, S. K. et al. International consensus statement on allergy and rhinology: allergic rhinitis – 2023. Int. Forum Allergy Rhinol. 13 (4), 293–859 (2023).
Cabanova, K. et al. Identification of the phase composition of solid microparticles in the nasal mucosa of patients with chronic hypertrophic rhinitis using Raman microspectroscopy. Sci. Rep. 11 (1), 18989 (2021).
Zucker, S. M., Barton, B. M. & McCoul, E. D. Management of rhinitis medicamentosa: A systematic review. Otolaryngol. Head Neck Surg. 160 (3), 429–438 (2019).
Kugener, V. F. et al. Enhancing pharmacovigilance from the US experience: current practices and future opportunities. Drug Saf. 44 (8), 843–852 (2021).
Li, D. et al. Drug-induced QT prolongation and torsade de pointes: a real-world pharmacovigilance study using the FDA adverse event reporting system database. Front. Pharmacol. 14, 1259611 (2023).
Harpaz, R. et al. Performance of pharmacovigilance signal-detection algorithms for the FDA adverse event reporting system. Clin. Pharmacol. Ther. 93 (6), 539–546 (2013).
Baroody, F. M. et al. Nonallergic rhinopathy: A comprehensive review of classification, diagnosis, and treatment. J. Allergy Clin. Immunol. Pract. 12 (6), 1436–1447 (2024).
Avdeeva, K. S. et al. The prevalence of non-allergic rhinitis phenotypes in the general population: A cross-sectional study. Allergy 77 (7), 2163–2174 (2022).
Armaroli, G. et al. Long-term safety and effectiveness of etanercept in JIA: an 18-year experience from the biker registry. Arthritis Res. Ther. 22 (1), 258 (2020).
Liu, Y. et al. Association between migraine and vasomotor rhinitis. Int. Forum Allergy Rhinol. 11 (9), 1378–1380 (2021).
Wu, W. et al. The airway neuro-immune axis as a therapeutic target in allergic airway diseases. Respir Res. 25 (1), 83 (2024).
Kawada, M. et al. Involvement of Galanin and Galanin receptor 2 in a mouse model of allergic rhinitis. Allergol. Int. 71 (1), 83–93 (2022).
Liu QD, Pan GX, Yan YJ, Li JW, Zhang JJ, Liu HL, Li CQ, Meng Y, Liu YX, Ruan Y. Metabolomic profiles in allergic rhinitis: A systematic review and meta-analysis. Ann Allergy Asthma Immunol. 134 (5), 594–602.e2 (2025).
Salib, R. J. et al. Mechanisms and mediators of nasal symptoms in non-allergic rhinitis. Clin. Exp. Allergy. 38 (3), 393–404 (2008).
Lokaj-Berisha, V. & Gacaferri, L. B. Increased thyroxine levels of patients with allergic rhinitis. Sci. Rep. 15 (1), 2667 (2025).
Kuniyoshi Y, Tsujimoto Y, Banno M, Taito S, Ariie T, Kimoto T. Association of obesity or metabolic syndrome with various allergic diseases: An overview of reviews. Obes Rev. 26 (3), e13862 (2025).
Scheire, S. et al. The indispensable nasal decongestant: patients’ views and perspectives on nasal decongestant overuse. J. Allergy Clin. Immunol. Pract. 11 (2), 602–609e1 (2023).
Mokhatrish, M. M. et al. Pharmacists’ attitudes towards Long-Term use of nasal decongestants: A Cross-Sectional study. J. Multidiscip Healthc. 17, 1079–1090 (2024).
Li, W. et al. Long-term treatment outcomes in refractory rhinitis medicamentosa managed with nasal surgery. Int. Forum Allergy Rhinol. 14 (3), 630–638 (2024).
Corona, G. et al. The Italian society of andrology and sexual medicine (SIAMS), along with ten other Italian scientific societies, guidelines on the diagnosis and management of erectile dysfunction. J. Endocrinol. Invest. 46 (6), 1241–1274 (2023).
Shin, Y. E. et al. Safety profile and signal detection of phosphodiesterase type 5 inhibitors for erectile dysfunction: a food and drug administration adverse event reporting system analysis. Sex. Med. 11 (5), qfad059 (2023).
Long, Y. et al. Nose to brain drug delivery - A promising strategy for active components from herbal medicine for treating cerebral ischemia reperfusion. Pharmacol. Res. 159, 104795 (2020).
Ulusoy, S. et al. Mechanisms and solutions for nasal drug delivery - a narrative review. Eur. Rev. Med. Pharmacol. Sci. 26 (2 Suppl), 72–81 (2022).
Acknowledgements
The FAERS dataset is accessible to the general public. The FDA examined and approved the studies involving human subjects and obtained written informed consent from all participants.
Funding
This study was supported by Suzhou Gusu Medical Youth Talent [GSWS2023003].
Author information
Authors and Affiliations
Contributions
Concept and design: Yan He and Xinzhou Yan. Analysis and interpretation of data: Yan He and Long Chen. Drafting of article: Yan He and Zhiqiang Lin.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
He, Y., Yan, X., Chen, L. et al. Pharmacovigilance analysis of drug-induced hypertrophic rhinitis using FAERS data. Sci Rep 15, 23937 (2025). https://doi.org/10.1038/s41598-025-10336-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-10336-z