Abstract
Kidney stones, a common urological disease, may involve the brain-kidney axis in their formation, though the specific mechanism remains unclear. This study aimed to investigate the effects of blue light on relevant metabolic indicators and oxidative stress status in rats with kidney stones through the brain-kidney axis. A rat model of kidney stones was established by administering 1% ethylene glycol and 2% ammonium chloride. Subsequently, blue light intervention was applied, and the outcomes were compared with those of a control group of normal rats. Our findings revealed that rats with kidney stones receiving blue light intervention exhibited significantly increased levels of antidiuretic hormone, intensified oxidative stress response, and augmented stone formation compared to kidney stone rats without blue light intervention. However, in normal rats, blue light intervention did not cause significant changes in these indicators. In summary, this study indicates that under pathological conditions, blue light may promote the secretion of antidiuretic hormone in serum and enhance oxidative stress response in renal tissues by affecting the brain-kidney axis, thereby accelerating the formation of kidney stones in rats.
Similar content being viewed by others
Introduction
Kidney stones, as a common urinary system disease, have shown an increasing incidence globally, posing a significant threat to public health1,2,3. Currently, multiple studies have revealed that the formation of kidney stones is a complex process influenced by various factors, including living environment, dietary habits, and metabolic diseases4,5,6. Among them, the brain-kidney axis plays a crucial role in kidney diseases and is an important physiological network that regulates kidney function. Abnormalities in this axis may induce the formation of kidney stones by affecting metabolic pathways7,8.
Recent studies have found that, apart from traditional environmental factors, light, especially blue light, may have potential effects on human physiological functions9,10. Blue light is closely related to the human biological clock and metabolic regulation mechanisms. It can influence the brain through the mediation of retinal ganglion cells (RGCs) and further affect relevant physiological functions11,12. Therefore, we hypothesize that blue light may exert an impact on the kidneys through the brain-kidney axis by regulating brain function. Currently, there is limited research on the relationship between the brain-kidney axis and kidney stone formation. Our study used a rat model induced by 1% ethylene glycol and 2% ammonium chloride gavage to simulate human hyperoxaluric and hypercalciuric conditions. This study aims to investigate how blue light influences kidney stone formation in rats through its effects on the brain-kidney axis and to discuss the underlying mechanisms involved.
Materials and methods
Laboratory animals
All work was approved and implemented by the Animal Ethics Committee of Shanghai Jiao Tong University School of Medicine (2024SQ013) in accordance with the national and regional guidelines. All authors complied with the ARRIVE guidelines. To minimize the influence of rat gender, particularly the effects related to hormone levels and estrous cycles in female rats, this study utilized forty Specific Pathogen Free (SPF) grade Sprague-Dawley (SD) male rats, aged 10 weeks, with an average weight of (210 ± 15) g. These rats were provided by the Animal Center of Songjiang Research Institute Affiliated to Shanghai Jiao Tong University School of Medicine. These rats were housed in the laboratory of the Animal Experiment Center and underwent adaptive feeding for 7 days at a temperature range of 22 to 25 °C and a relative humidity of 50–60%. This study was approved by the Animal Ethics Committee of Songjiang hospital affiliated to Shanghai jiaotong university school of medicine (2024SQ013).
Materials and instruments
Malonaldehyde (MDA) assay kit (provided by Hefei Lair Biotechnology Co., Ltd.), superoxide dismutase (SOD) assay kit (also from Hefei Lair Biotechnology Co., Ltd.), oxalic acid (Oxa) detection kit (supplied by Shanghai Youxuan Biotechnology Co., Ltd.), a blue light laser device (from Shanghai Xilong Optoelectronic Technology Co., Ltd.), and a multi-functional full-wavelength microplate reader.
Grouping and modeling of rats
Forty SPF grade SD male rats aged 10 weeks were randomly divided into four groups (n = 10 per group): Group A, Group B, Group C, and Group D. To induce kidney stones, rats in Group C and Group D were administered 1% ethylene glycol + 2% ammonium chloride via gavage (2 mL per rat) daily for four weeks. Ethylene glycol serves as a precursor for oxalate synthesis, leading to hyperoxaluria, while ammonium chloride acidifies the urine, promoting the formation of calcium oxalate crystals. Rats in Group A and Group B received an equal volume of 0.9% saline solution via gavage as controls. The irradiation standard was based on previous methods used in animal experiments related to blue light, typically ranging from 1 to 2 h. To ensure the success rate of model establishment, a 2-hour irradiation duration was chosen. The intensity of blue light irradiation was set at 1300 lx, as referenced in prior literature, while the intensity of fluorescent light irradiation was 3000 lx13. Rats in Group A and Group C received blue light exposure twice daily (1 h each time) starting from the second day after gavage as an intervention, while rats in Group B and Group D were exposed to daylight (using the same method as described). After four weeks of continuous intervention, 24-hour urine samples were collected from the rats for urine-related indicator detection. After anesthetizing the rats with an intraperitoneal injection of 4% pentobarbital sodium, blood and kidney tissues were collected for subsequent analysis of various indicators. Euthanasia was performed on the rats using the carbon dioxide asphyxiation method.
The pathological changes of renal tissue were observed using Von Kossa staining
The left kidney of the rat was excised and processed with normal saline, followed by fixation with 4% paraformaldehyde. Subsequently, the standard Von Kossa staining procedure was followed for staining. The pathological changes of renal tissue were observed in detail under an optical microscope, and the severity of these pathological changes was evaluated based on the quantitative score of calcium salt crystallization. Specifically, the extent of CaOx crystal deposition was semi-quantitatively graded from “no crystal deposition” (grade 0) to “massive crystal deposition” (grade 3)14.
Detection of serum antidiuretic hormone (ADH) and urinary Ca2+ and oxa levels
A 2 ml sample of blood was collected from the abdominal aorta, centrifuged at 3500 r/min for 15 min, and sent to the clinical laboratory of our hospital for the detection of serum ADH. Urine was collected and centrifuged at 3500 r/min for 20 min, with urine Ca2+ content detected using an automatic biochemical analyzer. The determination of urinary Oxa was conducted strictly according to the kit instructions.
MDA and SOD levels were detected using the enzyme-linked immunosorbent assay (ELISA) method
The right kidney was homogenized to make a 10% homogenate, which was then centrifuged at 3500 r/min for 15 min. The supernatant was collected and processed according to the kit instructions. The wavelength of the microplate reader was set to read the optical density values, and the levels of MDA and SOD were detected.
Statistical analysis
SPSS 22.0 software was utilized for data analysis. All measurement data were expressed as mean ± standard deviation. For group comparisons, one-way ANOVA was employed, with the T-test used for pairwise comparisons. The Kruskal-Wallis test was applied when variances were inconsistent. Statistical significance was considered at P < 0.05.
Experimental flow chart

Results
Von Kossa staining was used to observe pathological changes in renal tissue
There were no significant pathological changes in the kidney tissues of rats in groups A and B, and no calcium salt crystals were observed. Compared to groups A and B, groups C and D exhibited varying degrees of renal tubule dilation, severe renal tubule damage and atrophy, inflammatory cell infiltration in the surrounding renal tubule tissue, and the formation of calcium oxalate crystals within the renal interstitium, which appeared brown due to calcium binding (as indicated by the black arrow in Fig. 1), and the calcium salt crystal scores were higher (P < 0.05). Compared with group D, the calcium salt crystal score in the kidney tissue of group C was higher (P < 0.05) (Figs. 1 and 2).
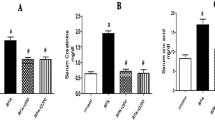
Comparison of serum ADH and urinary Ca2+ and oxa levels in four groups of rats
There were no significant differences in serum ADH and urinary Ca2+, Oxa levels between groups A and B. Compared with groups A and B, groups C and D had higher serum ADH and urinary Ca2+, Oxa levels (P < 0.05). Compared with group D, group C had higher serum ADH and urinary Ca2+, Oxa levels (P < 0.05) (Table 1).
Comparison of MDA and SOD levels in kidney tissues of four groups of rats
There were no significant differences in the levels of MDA and SOD in kidney tissues between groups A and B. Compared with groups A and B, groups C and D had lower SOD levels and higher MDA levels in kidney tissues (P < 0.05). Compared with group D, group C had lower SOD levels and higher MDA levels in kidney tissues (P < 0.05) (Fig. 3).
Discussion
Kidney stones are one of the most prevalent diseases in urology, potentially accompanied by abnormal kidney function, urinary tract infections, and other complications2. While the pathogenesis remains incompletely understood, the theory of multifactorial interaction, encompassing metabolic abnormalities, lifestyle habits, and physiological states, has been widely accepted4. With the deepening of related research, it has been found that the brain-kidney axis plays a role in kidney diseases8,15. The brain-kidney axis involves the interaction between the central nervous system and the kidneys, primarily mediated through the hypothalamus-pituitary gland and the renal sympathetic nervous system. The hypothalamus regulates the secretion of related hormones through the pituitary gland, which subsequently influences various kidney functions, such as blood flow, filtration rate, and sodium excretion. The renal sympathetic nervous system regulates renal blood vessels, thereby affecting blood flow and urine production15. The paraventricular nucleus (PVN) within the hypothalamus serves as a pivotal node for information output from the hypothalamus16, utilizing neuroendocrine neurons to modulate both the endocrine and autonomic nervous systems, thereby influencing metabolism. Both the supraoptic nucleus (SON) and PVN play crucial roles in renal water-salt balance, neuroregulation, and endocrine function. The large neurosecretory cells in the SON and PVN synthesize ADH, which is released in response to elevated plasma osmotic pressure, acts on the renal collecting ducts, and regulates fluid metabolic balance17. This suggests that under pathological conditions, blue light can stimulate increased production of ADH by affecting the brain-kidney axis, thereby promoting the formation of kidney stones.
Previously, it was believed that the central nervous system possessed immune privileges due to the blood-brain barrier; however, recent studies have revealed its significant interactions with the immune system18. Various cells within the nervous system, including microglia and astrocytes, are capable of releasing cytokines and chemokines, thereby stimulating inflammatory responses and oxidative stress. In studies using rat models of kidney injury, significant changes in neurotransmitter levels have been observed, which exert profound effects on multiple aspects of the body. Meanwhile, Toll-like receptors (TLRs) in innate immunity, particularly TLR2 and TLR4, are activated upon kidney damage, exerting important influences on the interaction between the brain and kidney. These receptors are closely related to local inflammation, neuronal damage, and the regulation of cytokine expression19. The results of this study indicate that, compared to group D, group C exhibited lower levels of SOD and higher levels of MDA. Therefore, blue light may play a significant role in the pathogenesis of kidney stones by inducing oxidative stress via the brain-kidney axis. Current research has shown that optical regulation plays a crucial role in the function of the brain-kidney axis10,20. The effects of light are not limited to visual guidance but also involve a series of non-visual effects. These non-visual effects are partially mediated by the recently discovered RGCs that are highly sensitive to blue light11. Their influence extends to a wide range of physiological processes, including hormone secretion, heart rate, sleep propensity, body temperature, and gene expression21. Upon further investigation, a novel type of photoreceptive cell, the intrinsically photosensitive retinal ganglion cell (ipRGC), was discovered. Although these special cells constitute only a small fraction of RGCs, they play a crucial role in multiple non-visual functions such as diurnal rhythms, sleep, and emotions22. Notably, research by Zhang et al.23 further indicates that stimulating ipRGCs can influence the secretion of neurotransmitters in the hypothalamus. Additionally, further studies have found that under the same light intensity, short-wavelength light with a wavelength of approximately 460 nm (i.e., blue light) has a particularly significant stimulatory effect on brain nerves12.
This study found that the calcium salt crystallization scores in kidney tissue, serum ADH, and urinary Ca2+ and Oxa levels were higher in groups C and D than in groups A and B. Furthermore, the calcium salt crystallization scores in kidney tissue, serum ADH, and urinary Ca2+ and Oxa levels were higher in group C than in group D. Compared with groups A and B, the SOD levels in kidney tissue were lower and MDA levels were higher in groups C and D (P < 0.05). Compared with group D, the SOD levels in kidney tissue were lower and MDA levels were higher in group C, while there were no significant differences in the relevant data between groups A and B. These findings suggest that under pathological conditions, blue light may affect the level of ADH in the body through the brain-kidney axis and promote oxidative stress, thereby facilitating the formation of kidney stones. This observation is consistent with previous research conclusions regarding the role of ADH and oxidative stress in the formation of kidney stones14,24,25. Despite the absence of significant differences in the relevant data between Group A and Group B, the elevated levels of serum ADH, urine Ca2+, Oxa, and renal MDA observed in Group B compared to Group A suggest that blue light irradiation may not directly induce stone formation under physiological conditions but could potentially act as a promoting factor for stone growth. This finding offers a novel perspective on understanding the impact of blue light on kidney stone formation, highlighting the need to consider potential risks associated with blue light exposure, especially given the rapid technological advancements and widespread exposure to blue light in daily life.
While this study preliminarily reveals the potential role of the brain-kidney axis in the formation of kidney stones, it still has certain limitations. Firstly, the sample size of the experiment is relatively insufficient. Secondly, this study does not delve deeply into the specific mechanisms of the brain-kidney axis in the pathogenesis of kidney stones, as well as how blue light exerts its effects through the brain-kidney axis. In summary, this study preliminarily confirms the potential role of the brain-kidney axis in the formation of kidney stones, laying a foundation for further research on its mechanisms.
Conclusions
This study established a rat model of kidney stones to investigate the impact of blue light on kidney stone formation via the brain-kidney axis, offering a novel perspective for understanding the complex mechanisms underlying this process. The results demonstrated that, compared to groups A, B, and D, group C exhibited a higher calcium salt crystallization score in kidney tissue, elevated serum ADH, urinary Ca²⁺, and Oxa levels, as well as increased MDA levels in kidney tissue, while SOD levels were significantly decreased. These findings suggest that blue light may expedite kidney stone formation by modulating the brain-kidney axis, stimulating ADH secretion, and enhancing oxidative stress responses. This research not only provides preliminary insights into the potential role of blue light in kidney stone formation but also lays an experimental foundation for further exploration of the brain-kidney axis in kidney diseases. Future studies should expand the sample size and delve deeper into the specific mechanisms through which blue light affects the brain-kidney axis, thereby facilitating the development of novel preventive and therapeutic strategies for kidney stones.
Data availability
Since this research is funded by the Shanghai Songjiang District Science and Technology Research Project, the datasets generated and/or analyzed during the current study are not publicly available until the completion of this project. But are available from the corresponding author on reasonable request.
Abbreviations
- SPF:
-
Specific pathogen free
- SD:
-
Sprague-Dawley
- RGCs:
-
Retinal ganglion cells
- MDA:
-
Malonaldehyde
- SOD:
-
Superoxide dismutase
- Oxa:
-
Oxalic acid
- ADH:
-
Antidiuretic hormone
- TLRs:
-
Toll-like receptors
- IpRGC:
-
Intrinsically photosensitive retinal ganglion cell
- ELISA:
-
Enzyme-Linked Immunosorbent Assay
References
Zeng, G. et al. Prevalence of kidney stones in China: an ultrasonography based cross-sectional study. BJU Int. 120, 109–116 (2017).
Sorokin, I. & Pearle, M. S. Medical therapy for nephrolithiasis: state of the art. Asian J. Urol. 5, 243–255 (2018).
Singh, P. et al. The genetics of kidney stone disease and nephrocalcinosis. Nat. Rev. Nephrol. 18, 224–240 (2022).
Wagner, C. A. Etiopathogenic factors of urolithiasis. Arch. Esp. Urol. 74, 16–23 (2021).
Wang, Z. et al. Recent advances on the mechanisms of kidney stone formation (review). Int. J. Mol. Med. 48. (2021).
Hocker, S. E. Ren. Disease Neurol. Continuum (Minneap Minn) 23: 722–743. (2017).
Yang, T. et al. The gut microbiota and the brain-gut-kidney axis in hypertension and chronic kidney disease. Nat. Rev. Nephrol. 14, 442–456 (2018).
Ghoshal, S. Renal and Electrolyte disorders and the nervous system. Continuum (Minneap Minn). 29, 797–825 (2023).
Johns, E. J., Kopp, U. C. & DiBona, G. F. Neural Control Ren. Function Compr. Physiol. 1: 731–767. (2011).
Osborn, J. W., Tyshynsky, R. & Vulchanova, L. Function of renal nerves in kidney physiology and pathophysiology. Annu. Rev. Physiol. 83, 429–450 (2021).
Vandewalle, G., Maquet, P. & Dijk, D. J. Light as a modulator of cognitive brain function. Trends Cogn. Sci. 13, 429–438 (2009).
Antemie, R. G., Samoila, O. C. & Clichici, S. V. Blue light-ocular and systemic Damaging effects: a narrative review. Int. J. Mol. Sci. 24. (2023).
Bilu, C. et al. Red white and blue - bright light effects in a diurnal rodent model for seasonal affective disorder. Chronobiol Int. 36, 919–926 (2019).
Huang, H. & Ma, M. High Sodium-Induced oxidative stress and poor anticrystallization defense aggravate calcium oxalate crystal formation in rat hyperoxaluric kidneys. PLoS One. 10, e0134764 (2015).
Ariton, D. M. et al. Diabetes, Albuminuria and the kidney-brain Axis. J. Clin. Med. 10. (2021).
Wen, S. et al. An overview of energy and metabolic regulation. Sci. China Life Sci. 62, 771–790 (2019).
Uvnas-Moberg, K. et al. The Yin and Yang of the oxytocin and stress systems: opposites, yet interdependent and intertwined determinants of lifelong health trajectories. Front. Endocrinol. (Lausanne). 15, 1272270 (2024).
Salvador, A. & Kipnis, J. Immune response after central nervous system injury. Semin Immunol. 59, 101629 (2022).
Liu, C. C. et al. Tau and apolipoprotein E modulate cerebrovascular tight junction integrity independent of cerebral amyloid angiopathy in Alzheimer’s disease. Alzheimers Dement. 16, 1372–1383 (2020).
Bedrosian, T. A. & Nelson, R. J. Timing of light exposure affects mood and brain circuits. Transl Psychiatry. 7, e1017 (2017).
Gronfier, C. [Circadian clock and non-visual functions: the role of light in humans]. Biol. Aujourdhui. 208, 261–267 (2014).
Mure, L. S. Intrinsically photosensitive retinal ganglion cells of the human retina. Front. Neurol. 12, 636330 (2021).
Zhang, Z. et al. Superior Colliculus GABAergic neurons are essential for Acute Dark induction of Wakefulness in mice. Curr. Biol. 29, 637–644e3 (2019).
Kavouras, S. A. et al. Urine osmolality predicts calcium-oxalate crystallization risk in patients with recurrent urolithiasis. Urolithiasis 49, 399–405 (2021).
Wang, Z. et al. Carboxymethylated Rhizoma Alismatis polysaccharides reduces the risk of calcium oxalate stone formation by reducing cellular inflammation and oxidative stress. Urolithiasis 52, 63 (2024).
Funding
This work was supported by the Shanghai Songjiang District Science and Technology Research Project (No. 2023SJKJGG84).
Author information
Authors and Affiliations
Contributions
Dao-Cheng Fang, Yuan-Yuan Hu, and Chao Wang contributed equally to the work and wrote the main manuscript text; Jie Fan* and Hui Wen* are directors. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Statement of animal rights
This study was approved by the Animal Ethics Committee of Songjiang hospital affiliated to Shanghai jiaotong university school of medicine.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Fang, DC., Hu, YY., Wang, C. et al. A study on the effect of blue light on kidney stone formation in rats via the brain-kidney axis. Sci Rep 15, 3825 (2025). https://doi.org/10.1038/s41598-025-85586-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-85586-y