Abstract
To investigate the association between overt hepatic encephalopathy (OHE) and liver pathology after transjugular intrahepatic portosystemic shunt (TIPS) creation in cirrhotic patients. From July 2015 to April 2024, 73 patients from 4 hospitals in China who received TIPS creation and liver biopsy were retrospectively enrolled in this study. Based on whether OHE occurred within 3 months after TIPS creation, the patients were categorized into OHE (n = 29) and non-OHE (n = 44) groups. The liver pathology was assessed by hematoxylin-eosin (H&E), Sirius red staining, immunohistochemistry, and immunofluorescence. Liver pathology by H&E staining showed typical features of liver cirrhosis (including disordered structure and pseudolobule formation) in all the patients. No marked difference was observed in extracellular matrix (ECM) deposition between the OHE and non-OHE groups. However, the patients in the OHE group had a higher level of liver and systemic inflammation than in the non-OHE group. And there was a strong correction between intrahepatic macrophage infiltration and serum inflammatory indicators. Additionally, the OHE group had more liver neovascularization, which was consistent with liver inflammation. The emergence of OHE after TIPS creation is closely associated with liver pathology, especially in liver inflammation and angiogenesis, but not in ECM deposition.
Similar content being viewed by others
Introduction
Transjugular intrahepatic portosystemic shunt (TIPS) has become an essential method for treating esophagogastric bleeding and refractory ascites in cirrhotic patients, which can significantly relieve symptoms and reduce mortality1,2,3. Overt hepatic encephalopathy (OHE) is one of the main complications after TIPS creation, with an incidence of 30–50%, which increases the risks of death and reduces patients’ quality of life4,5. Previous studies, which investigated the OHE-related risk factors after TIPS creation, mainly focused on laboratory tests and imaging parameters6,7,8,9. However, the association between post-TIPS OHE and liver pathology is rarely reported, possibly because liver biopsy is an invasive procedure.
Liver cirrhosis originates from hepatocyte damage caused by various reasons, followed by hepatic stellate cells (HSCs) activation through the involvement of multiple molecules and cells10,11,12. Activated HSCs synthesize and secrete massive extracellular matrix (ECM) for pathological liver repair. However, excessive ECM deposition can lead to the destruction of liver structure and function, and promote the emergence of cirrhosis and portal hypertension13,14. Meanwhile, the process is frequently accompanied by liver inflammation and angiogenesis15,16,17,18. Theoretically, liver pathology can directly and accurately reflect the degree of liver disease, and is closely related to the prognosis of patients.
This study explored the association between OHE and liver pathology, especially focusing on ECM deposition, liver inflammation and angiogenesis.
Materials and methods
Patients
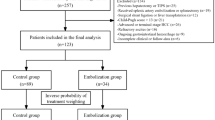
Data from 639 patients, who were diagnosed with liver cirrhosis and received TIPS creation from 4 hospitals (Supplementary Table 1) in China between July 2015 and April 2024, were retrospectively collected. The patients with hepatocellular carcinoma (n = 24), severe infection (n = 3), chronic portal vein occlusion (n = 31), and incomplete medical data (n= 17) were ruled out. Finally, only 73 patients who had undergone liver biopsy were included in this study. As TIPS-related OHE mainly occurred within 3 months after the procedures2,4, we set 3 months as the cut-off and categorized into OHE (n = 29) and non-OHE (n = 44) groups (Fig. 1). Our study was designed based on the Helsinki Declaration and authorized by the Ethics Committee of the First Affiliated Hospital of Henan University of Science and Technology. Written informed consent was achieved from all the patients or their family members.
TIPS procedures
The indication for TIPS creation was esophagogastric bleeding due to portal hypertension. The detailed procedures of TIPS have been described in previous studies19,20. An 8 mm Viatorr (Gore) stent or bare (E-luminexx, Bard) + covered (Fluency, Bard) stent was placed between the hepatic vein and portal vein. Esophagogastric varices were embolized by cyanoacrylate glue and/or coils. Portal pressure gradient (PPG) was calculated in each patient according to previous studies21,22. A patent intrahepatic shunt with satisfied post-TIPS PPG (< 12mmHg or < 50% of pre-PPG) was considered a technical success.
Liver tissue acquisition and histological study
Liver tissues were acquired using a transjugular liver biopsy set (Cook) at the beginning of TIPS procedures. Then, the liver tissues were stained by hematoxylin-eosin (H&E), Sirius red, and antibodies (for immunofluorescence (IF) and immunohistochemistry (IHC)), as previous studies described23,24. ImageJ 1.5.4 was used for semi-quantitative analysis. The antibodies used for IF and IHC included anti-CollagenI (COL1A1, GB124197-100, Servicebio), anti-Alpha Smooth Muscle Actin (αSMA, GB13044-50, Servicebio), anti-CD68 (GB113150-100, Servicebio), and anti-Von Willebrand Factor (VWF, ab6994, Abcam).
Follow-up and endpoint
We obtained the follow-up data from electronic medical records. The clinical outcomes of all the patients were confirmed by telephone communications. OHE was defined according to the West-Heaven criteria25. The endpoint of this study was to explore whether post-TIPS OHE is associated with liver pathology (especially in ECM deposition, liver inflammation and angiogenesis).
Statistical analysis
Quantitative data were expressed as mean ± SD, and their distributions were evaluated by the Shapiro-Wilk W test. The differences between the two groups were calculated by unpaired t-test (normal distribution) or nonparametric test (skew distribution). Qualitative data were expressed as n (%), and the differences were calculated by χ2 test or Fisher exact test. The cumulative incidence of OHE, death, rebleeding, and shunt dysfunction were assessed using the Kaplan-Meier method. The corrections between the two parameters were evaluated by Pearson’s correction analysis. Prognostic factors were identified by multivariate COX analysis, and hazard ratio (HR) and 95% confidence interval (CI) were calculated. GraphPad Prism 10.2.1 (GraphPad Software) was used for data processing and plotting. The statistical difference was ruled as p < 0.05.
Results
Baseline characteristics
Our study enrolled 73 patients, including 29 in the OHE group and 44 in the non-OHE group. Compared with non-OHE patients, OHE patients were older with higher values of total bilirubin, serum ammonia, and Child-Pugh scores, but lower levels of albumin. The other parameters showed no marked differences (Table 1). The follow-up time ranged from 4 to 94 months with a median of 49 months.
Clinical outcomes
All patients obtained technical success with no serious complications due to the operation. After an intrahepatic shunt creation, the PPG decreased from 26.35 ± 6.92mmHg to 10.72 ± 3.09mmHg. Specially, the post-TIPS PPG was less than 12mmHg in 57 patients, and a more than 50% reduction was also achieved in the other 16 patients.
During the follow-up, 33 patients experienced OHE with an average of 2.61 ± 1.49 (range from 1 to 6) episodes. The first episode of OHE occurred within 3 months after TIPS creation in 29 patients, and at 5, 8, 19, and 35 months in the other 4 patients (Fig. 2A). 8 patients died, and the causes included liver failure (n = 5), variceal bleeding (n = 2), and pulmonary infection (n = 1) (Fig. 2B). In addition, variceal rebleeding was observed in 6 patients (Fig. 2C), and shunt dysfunction in 11 patients (Fig. 2D).
Association between OHE and the degree of cirrhosis
H&E staining showed disordered structure and pseudolobule formation in all the patients. Massive ECM deposition was also observed by Sirius red staining, but with no marked difference between OHE and non-OHE groups (Fig. 3A). Additionally, we examined the expression of COL1A1 (the main component of ECM) and αSMA (the marker of HSCs activation) by IF analysis, which both showed an apparent increase in the fibrotic area. Consistent with Sirius red staining, the positive areas of the two proteins were comparable between the two groups (Fig. 3B).
Association between OHE and liver inflammation and angiogenesis
Compared with the non-OHE group, the OHE group had more macrophage infiltration (marked by CD68), suggesting a higher level of liver inflammation (Fig. 4A). Furthermore, the expression of CD68 was positively corrected with serum inflammatory indicators, including procalcitonin, C-relative protein (CRP), and IL-6 (Fig. 4B-D). And the levels of these parameters in the OHE group were markedly higher than those in the non-OHE group (Table 2). Multivariate analysis revealed that total bilirubin (HR 1.028; 95% CI 1.019–1.037; p < 0.001), procalcitonin (HR 3.418; 95% CI 1.372–8.152; p = 0.029), and IL-6 (HR 1.651; 95% CI 1.302–2.107; p < 0.001) were prognostic factors for OHE (Supplementary Table 2). Liver angiogenesis was assessed by IF analysis of VWF. The results showed that the patients in the OHE group had more liver neovascularization (Fig. 5A). And the levels of VWF were closely corrected with CD68 (Fig. 5B).
Discussion
In this study, we observed a strong association between OHE and liver pathology, in which the patients with OHE have a higher level of liver inflammation and angiogenesis. However, the occurrence of OHE appears to be independent of ECM deposition. These results may provide a new perspective for preventing and treating OHE after TIPS creation.
OHE induced by TIPS creation mainly occurred within 3 months after the procedures, which was closely associated with hyperammonemia and impaired liver function26,27. The decrease in the incidence of OHE over time can be accounted for improved nutrition and reduced risk of bleeding28,29. Especially, the improvement of sarcopenia is conducive to enhancing the muscle’s ability to clear serum ammonia21. In our study, 4 patients with the first episode of OHE more than 3 months after TIPS creation were assigned to the non-OHE group, as the emergence of OHE was considered to be caused by liver disease progression rather than TIPS.
Liver cirrhosis features disordered structure, ECM deposition, and pseudolobule formation30. Theoretically, the incidence of OHE is positively correlated with the degree of cirrhosis. Nevertheless, our study found no marked difference in ECM deposition and HSC activation between the OHE and non-OHE groups. The potential reasons may be as follows: the level of ECM deposition has reached an upper limit that the liver can bear in the decompensated stage13, and the limitations were similar in different patients. Compared with ECM deposition, the occurrence of OHE is closer to the reserved liver function, which depends on the numbers and status of hepatocytes. Thus, the OHE group had worse liver function than the non-OHE group, but ECM deposition was similar in this study.
Liver inflammation is the initiating factor of liver cirrhosis and exists throughout the process15,16. When the liver is damaged, the dead hepatocytes trigger an inflammatory response and induce HSC activation. In turn, activated HSCs can release various cytokines which promote liver inflammation. Enhanced inflammation can cause poor liver function and increase the risk of OHE31. In this study, a higher level of liver inflammation was found in the OHE group than in the non-OHE group. Additionally, similar results were observed in systemic inflammation, which showed a strong association with liver inflammation. Previous studies have reported that inflammatory factors can destroy the blood-brain barrier and further induce OHE by enhancing the neurotoxicity of blood ammonia32,33. Taken together, we speculated that inhibiting liver and systemic inflammation may contribute to the treatment of OHE after TIPS creation.
Liver angiogenesis is another important feature of cirrhosis and is frequently accompanied by liver inflammation17,18. On the one hand, liver inflammation stimulates angiogenesis; on the other hand, neovascularization facilitates the progression of inflammation by transporting more inflammatory factors and cells34,35. The positive feedback relationship between liver inflammation and angiogenesis can cause more hepatocyte death and worse liver function, which promotes the occurrence of OHE. Consistently, the results of our study revealed a positive correlation between liver angiogenesis and inflammation. And the liver neovascularization was markedly higher in the OHE group than in the non-OHE group. Mejias et al.36 reported that tyrosine Kinase Inhibitor sorafenib can ameliorate liver inflammation and fibrosis by blocking liver angiogenesis in animal models. Thus, inhibiting liver angiogenesis may be a therapeutic method for OHE.
However, we still need to state the shortcomings of this study. First, this study was retrospectively designed, with unavoidable selective bias. Second, liver biopsy is not routinely conducted in cirrhotic patients due to an invasive procedure, so it is difficult to obtain liver tissues from large numbers of patients. Finally, as cirrhosis heterogeneous exists in different liver regions, the pathology of liver biopsy may not precisely reflect the status of the whole liver37.
In conclusion, our study demonstrated that the the occurrence of OHE after TIPS creation is closely associated with liver inflammation and angiogenesis, but not with ECM deposition. Therefore, targeting liver inflammation and angiogenesis may be a promising strategy for preventing and treating OHE after TIPS creation.
Data availability
All data in this study are available through contacting the corresponding author.
References
Larrue, H. et al. TIPS prevents further decompensation and improves survival in patients with cirrhosis and portal hypertension in an individual patient data meta-analysis. J. Hepatol. 79(3), 692–703 (2023).
Zhao, L. et al. TIPSS plus extrahepatic collateral embolisation may decrease variceal rebleeding and post-TIPSS hepatic encephalopathy. Gut 73(7), 1224–1226 (2024).
Garcia-Pagan, J. C. et al. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl. J. Med. 362(25), 2370–2379 (2010).
Nardelli, S. et al. Episodic overt hepatic encephalopathy after transjugular intrahepatic portosystemic shunt does not increase mortality in patients with cirrhosis. J. Hepatol. 80(4), 596–602 (2024).
Wang, X. et al. Small-diameter Transjugular Intrahepatic Portosystemic Shunt versus Endoscopic Variceal Ligation Plus Propranolol for Variceal rebleeding in Advanced cirrhosis. Radiology 308(2), e223201 (2023).
Ehrenbauer, A. F. et al. Predicting overt hepatic encephalopathy after TIPS: value of three minimal hepatic encephalopathy tests. JHEP Rep. 5(9), 100829 (2023).
Chen, X. et al. Hepatic-associated vascular morphological assessment to predict overt hepatic encephalopathy before TIPS: a multicenter study. Hepatol. Int. 18(4), 1238–1248 (2024).
Yang, C. et al. Development and Validation of Prognostic models to Estimate the risk of overt hepatic Encephalopathy after TIPS Creation: a Multicenter Study. Clin. Transl Gastroenterol. 13(3), e00461 (2022).
Rudler, M. et al. Hepatic encephalopathy is not a contraindication to pre-emptive TIPS in high-risk patients with cirrhosis with variceal bleeding. Gut 72(4), 749–758 (2023).
Kisseleva, T. & Brenner, D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat. Rev. Gastroenterol. Hepatol. 18(3), 151–166 (2021).
Horn, P. & Tacke, F. Metabolic reprogramming in liver fibrosis. Cell. Metab. 36(7), 1439–1455 (2024).
Pistorio, V., Housset, C. & Gautheron, J. Multiple functions of MLKL in liver fibrosis, from necroptosis to hepatic stellate cell activation. Theranostics 12(13), 5820–5823 (2022).
Gines, P. et al. Liver cirrhosis. Lancet 398(10308), 1359–1376 (2021).
Friedman, S. L. & Pinzani, M. Hepatic fibrosis 2022: unmet needs and a blueprint for the future. Hepatology 75(2), 473–488 (2022).
Hammerich, L. & Tacke, F. Hepatic inflammatory responses in liver fibrosis. Nat. Rev. Gastroenterol. Hepatol. 20(10), 633–646 (2023).
Bai, Y. et al. Inflammation-responsive cell membrane-camouflaged nanoparticles against liver fibrosis via regulating endoplasmic reticulum stress and oxidative stress. Adv. Mater. 36(19), e2310443 (2024).
Thomas, H. Liver: delineating the role of angiogenesis in liver fibrosis. Nat. Rev. Gastroenterol. Hepatol. 15(1), 6 (2018).
Liu, L. et al. Mechanotransduction-modulated fibrotic microniches reveal the contribution of angiogenesis in liver fibrosis. Nat. Mater. 16(12), 1252–1261 (2017).
Liu, J. et al. Impact of TIPS on splenic volume and Thrombocytopenia. AJR Am. J. Roentgenol. 216(3), 698–703 (2021).
Xiong, B. et al. The added value of Sarcopenia on existing risk scores to Predict Mortality after TIPS Placement: a Multicenter Study. Acad. Radiol. 30(Suppl 1), S246–S256 (2023).
Liu, J. et al. Sarcopenia in patients with cirrhosis after Transjugular Intrahepatic Portosystemic Shunt Placement. Radiology 303(3), 711–719 (2022).
Yang, C. et al. Effect of splenectomy on the outcomes in patients with cirrhosis receiving transjugular intrahepatic portosystemic shunt. J. Gastroenterol. Hepatol. 36(10), 2893–2902 (2021).
Wang, C. et al. Reversion of liver cirrhosis after endovascular treatment in Chinese patients with Budd-Chiari syndrome. Hepatol. Res. 53(12), 1198–1212 (2023).
Wang, C. et al. Ginkgetin exhibits antifibrotic effects by inducing hepatic stellate cell apoptosis via STAT1 activation. Phytother Res. 38(3), 1367–1380 (2024).
EASL Clinical Practice. Guidelines on the management of hepatic encephalopathy. J. Hepatol. 77(3), 807–824 (2022).
Wang, C. et al. Dynamic changes in liver function after transjugular intrahepatic portosystemic shunt in patients with cirrhosis. J. Interv Med. 5(4), 207–212 (2022).
Senzolo, M. et al. Predictive value of induced hyperammonaemia and neuropsychiatric profiling in relation to the occurrence of post-TIPS hepatic encephalopathy. Metab. Brain Dis. 34(6), 1803–1812 (2019).
Gioia, S. et al. The improvement in body composition including subcutaneous and visceral fat reduces ammonia and hepatic encephalopathy after transjugular intrahepatic portosystemic shunt. Liver Int. 41(12), 2965–2973 (2021).
Jahangiri, Y. et al. Muscle gain after Transjugular Intrahepatic Portosystemic Shunt Creation: Time Course and Prognostic implications for Survival in cirrhosis. J. Vasc Interv Radiol. 30(6), 866–872e4 (2019).
Engelmann, C. et al. Pathophysiology of decompensated cirrhosis: portal hypertension, circulatory dysfunction, inflammation, metabolism and mitochondrial dysfunction. J. Hepatol. 75(Suppl 1), S49–S66 (2021).
Kawashima, K. et al. Priming and maintenance of adaptive immunity in the liver. Annu. Rev. Immunol. 42(1), 375–399 (2024).
Wijdicks, E. F. & Encephalopathy, H. N Engl. J. Med., 375(17), 1660–1670. (2016).
Rose, C. F. et al. Hepatic encephalopathy: novel insights into classification, pathophysiology and therapy. J. Hepatol. 73(6), 1526–1547 (2020).
Affo, S. & Sancho-Bru, P. CCL2: a link between hepatic inflammation, fibrosis and angiogenesis? Gut 63(12), 1834–1835 (2014).
Poisson, J. et al. Liver sinusoidal endothelial cells: physiology and role in liver diseases. J. Hepatol. 66(1), 212–227 (2017).
Mejias, M. et al. Beneficial effects of sorafenib on splanchnic, intrahepatic, and portocollateral circulations in portal hypertensive and cirrhotic rats. Hepatology 49(4), 1245–1256 (2009).
Neuberger, J. et al. Guidelines on the use of liver biopsy in clinical practice from the British Society of Gastroenterology, the Royal College of Radiologists and the Royal College of Pathology. Gut 69(8), 1382–1403 (2020).
Funding
The innovation project for middle-age and young talents majoring in health of henan province (JQRC2023002).
Author information
Authors and Affiliations
Contributions
Chaoyang Wang: writing, investigation, and methodology. Yuyang Gu: data collection and analysis. Guofeng Zhou, Pengfei Chen, and Guorui Zhao: liver tissue acquisition and histopathological study. Jianzhuang Ren: statistical analysis. Wenguang Zhang: funding acquisition and data analysis. Huanzhang Niu: conceptualization and writing review. The final version of this manuscript was approved by all the authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, C., Gu, Y., Zhou, G. et al. Association between overt hepatic encephalopathy and liver pathology after transjugular intrahepatic portosystemic shunt creation in cirrhotic patients. Sci Rep 15, 1548 (2025). https://doi.org/10.1038/s41598-025-86176-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-86176-8