Abstract
Periodontitis is a chronic gum disease characterised by inflammation and the loss of bone. We have explored the potential prophylactic effects of lysates from four Lactobacillus strains against the toxic effects of three periodontal pathogens (Porphyromonas gingivalis, Fusobacterium nucleatum, and Aggregatibacter actinomycetemcomitans). TR146 oral epithelial cells were pre-treated with Lactobacillus lysates (L. rhamnosus - GG, L. rhamnosus - SD11, L. reuteri and L. plantarum) and then challenged with pathogenic material (live cells, lysates, or supernatants). Cytokine analysis was performed on supernatants of cells treated with probiotic lysates from 1.5 h to 24 h. Effects of probiotic lysates on re-epithelialisation were determined using keratinocyte scratch assays, monitoring both migration and proliferation. Epithelial barrier function was observed after lysate addition by trans-epithelial electrical resistance (TEER) and by quantifying claudin-1 expression. Treatment of host cells with Lactobacillus lysates before pathogen exposure conferred significant protection against viability loss. Although extended pre-treatment did not generally increase protection, against live Aggregatibacter actinomycetemcomitans, significant increases in viability were seen after 24 h of pre-treatment for GG, SD11 and L. plantarum. Pro-inflammatory cytokines TNF-α, IP-10, IL-6, and IL-8 increased significantly with extended probiotic treatment, while IL-1β and IL-1α secretion significantly increased but remained constant over time. Secretion of the growth-promoting cytokine TGF-β increased after 3 h of treatment, however no increases in the regulatory cytokine IL-10 were recorded. Only exposure to SD11 significantly enhanced re-epithelialisation, TEER and claudin-1 expression while GG increased TEER but decreased claudin-1 expression. L. plantarum significantly inhibited re-epithelialisation but did not impact TEER or claudin-1 expression. All lysates significantly improved TEER in the presence of pathogenic material, demonstrating a protective effect on barrier function.
Similar content being viewed by others
Introduction
The World Health Organisation (WHO) estimates that 3.5 billion people were affected by oral diseases worldwide in 2022, with periodontitis accounting for 19% of these cases1. Periodontitis is a chronic gum disease associated with host-mediated inflammation and the loss of key structural oral elements, including bone2. It is often characterised by the increased abundance of common periodontal pathogens. In the UK, NHS dental care is currently facing significant challenges, contributing to deteriorating oral health3. As access to healthcare for many, especially those in the developing world, is challenging; paired with an increased incidence of periodontitis1, there is a pressing need for new sustainable treatments to combat oral disease.
The term probiotic refers to live microorganisms administered to exert positive effects on a host4,5. Recent studies have considered the enteral and topical application of probiotics in skin care to enhance barrier integrity and treat conditions such as eczema6,7. Additionally, probiotics have been investigated for their ability to support the maintenance of oral health through microbiome regulation and protection against pathogens such as Streptococcus mutans8,9. However, limited research exists regarding the therapeutic use of postbiotics, which refers to the intra- and extracellular components of probiotics.
Previous work by Moman et al.10 reported that Lactobacillus rhamnosus (GG), L. reuteri, and Streptococcus salivarius pro- and post-biotics offered significant protection against periodontal pathogens in vitro. Furthermore, the protective effects of GG lysate and supernatant have been observed against Staphylococcus aureus, with postbiotic treated cells demonstrating improved cell viability11. GG has also been shown to enhance re-epithelialisation in wound healing models12. Notably, Prince et al.13 observed that live and lysed L. reuteri offers protection against S. aureus induced cell death in a time dependent manner, with longer probiotic pre-treatment resulting in improved cell viability. However, it was also reported that probiotic treatment post pathogenic infection did not reduce host cell death.
The use of L. plantarum to protect against oral pathogens has not been extensively studied, however, its positive influence on oral14 and gut15 barrier function has been documented. The effects of other Lactobacilli, and related non-viable formulations, on gut barrier function have also been thoroughly investigated, with numerous studies reporting increases in tight-junction (TJ) protein expression and enhanced barrier integrity after treatment16,17,18 as well as modulatory effects in the presence of pathogens19. TJ proteins are essential for regulating the passage of substances, including microorganisms, through the epithelial layer20. Periodontal pathogens compromise barrier integrity by reducing TJ expression21, hindering effective disease management22. Therefore, treatments that can increase TJ expression and enhance barrier integrity are of particular interest.
Postbiotics provide a logistical advantage over live cells by overcoming issues associated with viability. The most common way of storing probiotics is freeze drying23. This, as well as other formulation solutions, can reduce the viability of the bacterial cells24. Cheng et al.25 reported that the survival rate of L. plantarum after freeze drying was 6.57%, and complex formulations were required to maintain a high level of viability.
In the current study, we have examined the effects of four Lactobacillus lysates on oral keratinocytes, observing their prophylactic effects against three periodontal pathogens: Porphyromonas gingivalis (PG), Fusobacterium nucleatum (FN) and Aggregatibacter actinomycetemcomitans(AA)26. We studied the ability of Lactobacillus lysates to maintain cell viability against intra- and extracellular pathogenic material in addition to live bacteria. Furthermore, we analysed a diverse range of cytokines and assessed the effects of Lactobacillus lysates in a wound healing model. Finally, we studied the impact of our lysates on barrier integrity and TJ protein expression.
Methods
Cell culture
TR146 cells (buccal carcinoma cell line: ECACC 10032305; obtained from Sigma-Aldrich, Dorset, UK), passage 8–12, were used in all in vitro work. Cells were cultured using T225 culture flasks in Hams F-12 nutrient mix (Sigma-Aldrich, Germany) supplemented with 10% foetal bovine serum (FBS; ThermoFisher Scientific, UK), 1% Penicillin-Streptomycin (Sigma-Aldrich) and 1% L-glutamine (ThermoFisher Scientific). Flasks were incubated at 37 °C and 5% CO2 until at least 80% confluency was reached. Trypsin-EDTA (Sigma-Aldrich) was used to detach cells between passages. Cells were frozen in liquid nitrogen vapour in 90% FBS and 10% DMSO (Sigma-Aldrich).
Bacterial culture, lysate and supernatant preparation
Pathogens: Porphyromonas gingivalis ATCC 33277 (PG), Fusobacterium nucleatum ATCC 10953 (FN) and Aggregatibacter actinomycetemcomitans ATCC 33384 (AA); and probiotics: Lactobacillus rhamnosus ATCC 53103 (GG), Lactobacillus rhamnosus SD11 (SD)27,28, Lactobacillus reuteri ATCC 55730 (LR) and Lactobacillus plantarum ATCC 10241 (LP), were cultured in Wilkins-Chalgren anaerobe broth (WCB; ThermoFisher Scientific) and incubated in an anaerobic cabinet (Don Whitley, UK) supplied with an anaerobe gas mix (5% CO2, 5% H2, 90% N2), at 37 °C for 48 h.
Cultures were centrifuged at 1520 xg for 10 min to pellet bacterial cells. Supernatants were collected and filtered using a 0.22 μm syringe filter and stored at −20 °C. Bacterial pellets were washed in Dulbecco’s Phosphate Buffered Saline (DPBS; Sigma-Aldrich) and resuspended in 20mL DPBS. The cells were lysed by sonication using a Sonoplus HD4100 Sonicator (BANDELIN electronic GmbH & Co., Germany) on ice and at 100% amplitude, with a pulse-on for 30 s, pulse-off for 10 s; 30 kJ were applied to each sample. Lysates were filtered using a 0.22 μm syringe filter and concentrated using 3 kDa protein concentrators (centrifuged at 3000xg for 90 min at 4 °C). Protein concentration was determined by a Qubit Protein Assay (ThermoFisher Scientific). The relationship between colony forming units (CFU/mL), optical density (OD) and protein concentration of the supernatants and lysates has been included in Supplemental Fig. 1.
Both supernatants and lysates were tested for sterility by plating on Wilkins-Chalgren agar (WCA) and the pH of treatments and media were determined before use.
Cell culture assays
Concentration associated effects
TR146 cells were seeded into 48-well cell culture plates (1.2 × 105 cells/mL), using Hams F-12 (Sigma-Aldrich) supplemented with 1% L-glutamine: Hams + L. Cultures were incubated at 37 °C and 5% CO2 until 80% confluency was reached. Bacterial lysates or supernatants were added to each well at a range of concentrations (250 µg/mL – 5 µg/mL). For the control (0 µg/mL), treatment was substituted with DPBS for lysates, or DPBS and WCB (1:1) for supernatants. After 24 h, the plates were centrifuged at 200xg for 3 min to pellet dead cells. Cells were then harvested using Accutase (ThermoFisher Scientific) and viability was determined using trypan blue exclusion as previously described29.
Pre-treatment with probiotic lysates
TR146 cells were seeded into 48-well cell culture plates (1.2 × 105 cells/mL), using Hams + L. Cultures were incubated at 37 °C and 5% CO2 until 80% confluency was reached. Cells were pre-treated with probiotic lysate (250 µg/mL) for either 0 h (baseline), 2 h, 6 h, 12 h, or 24 h. Pathogen (live, supernatant or lysate) was added to the probiotic-treated cells at the relevant time points for 24 h. Pathogenic lysates and supernatants were added at 250 µg/mL protein concentration. Live bacteria were added at 1 × 104 CFU/mL, as determined by plate counting. The pathogen-only control cells received pre-treatment with DPBS and were challenged with pathogenic material. Control cells were treated and ‘challenged’ with DPBS (lysates and live pathogen) or treated with DPBS and ‘challenged’ with DPBS and WCB 1:1 (supernatants).
After 24 h incubation in the presence of pathogen, the plates were centrifuged at 200xg for 3 min to pellet dead cells. Cells were then harvested using Accutase (ThermoFisher Scientific). Viability was determined using trypan blue exclusion29, and the mean viability of the pathogen-only control was deducted from each sample viability value to give the difference from the pathogen control. A diagram depicting the controls used in these experiments can be found in the supplemental material (Supplemental Fig. 2).
Cytokine analysis
TR146 cells were seeded into 48-well cell culture plates (1.2 × 105 cells/mL) using Hams + L and incubated at 37 °C and 5% CO2 until 80% confluency was reached. Cells were treated with probiotic lysates (250 µg/mL) and supernatants were collected at 1.5, 3, 6, 12, and 24 h and stored at −20 °C. Cytokine analysis was performed on the Bio-Plex 200 magnetic bead system (Bio-Rad, UK) using the Human XL Cytokine Panel (BioTechne, R&D Systems).
Re-epithelialisation
TR146 cells were seeded into 24-well cell culture plates (3 × 105 cells/mL) using Hams + L. Cultures were incubated at 37 °C and 5% CO2 until 80–100% confluency was reached. Scratches were made in the centre of the well through the keratinocyte monolayer using a p200 pipette tip. Media was removed and wells were washed twice with DPBS to remove cellular debris. Hams + L, supplemented with 1% FBS, was added along with lysates (250 µg/mL). For the positive control, 1% human keratinocyte growth supplement (HKGS: Thermofisher Scientific) was used in place of treatment, while DPBS was used for the control. Images were then taken at 0 (baseline), 6, 12, 18 and 24 h at 5x magnification (Leica, Germany). Scratch size was measured using Image-Pro version 10 (Media Cybernetics Inc, Rockville, MD).
Trans-epithelial electrical resistance (TEER)
TR146 cells were seeded at a density of 2 × 105 cells/mL into 12 mm, 0.4 μm pore transwells using Hams + L and incubated at 37 °C and 5% CO2 until 80% confluency was reached. Media was removed and replaced with EP + S: EpiLife medium containing 60 µM calcium (ThermoFisher Scientific), supplemented with an additional 1.44mM calcium (ThermoFisher Scientific) and 1% HKGS. Cultures were incubated for 24 h before TEER measurements (day 1) were taken using a World Precision Instruments Evometer fitted with Chopstick electrodes (WPI, UK). Apical media was removed and replaced with 400µL EP + S and 100µL of probiotic lysate or a combination of 50µL of probiotic lysate and 50µL of PG supernatant (final apical protein concentrations 100 µg/mL). Following a further 24 h incubation, TEER measurements were repeated (day 2). Cultures were then maintained for a further 12 days with TEER measurements as well as apical media and treatment changes, performed every 2–3 days. Basal media changes were performed every 4–6 days.
Claudin-1 expression
TR146 cells were seeded into 24-well cell culture plates (2 × 105 cells/mL) using Hams + L and incubated at 37 °C and 5% CO2 until 80% confluency was reached. Cells were treated with pathogenic or probiotic lysates (250 µg/mL) for 24 h. RIPA buffer (Thermofisher Scientific) was subsequently used to lyse cells and preserve protein. Protein concentration was determined using a Bradford assay and normalised for Western blot.
Samples containing 40 µg of protein were loaded into 4–12% agarose precast gels (Thermofisher Scientific) and run at 100 V for 75 min. Proteins were transferred to a nitrocellulose membrane using the trans-blot turbo blotting system (Bio-Rad). The membrane was blocked with skimmed milk (1 h) before being incubated with a primary claudin-1 or β-actin antibody (Thermofisher Scientific or abcam respectively) for 1 h. An HRP-conjugated secondary antibody (Biolegend, UK) was subsequently applied for 45 min, followed by the addition of a chemiluminescent reagent for 1 min. Wash steps were performed in between incubations using 1x Tween 20. Protein bands were visualized using a chemiluminescent reader (Azure Biosystems, US) and relative abundance of claudin-1 was determined by calculating the band intensity (ImageJ software), determining the ratio to the control, and normalizing to β-actin expression.
Statistical analyses
All statistical analyses were performed using GraphPad Prism 10.0.2 (GraphPad Software, US). Two-way ANOVA with a Fishers LSD test was used for viability and re-epithelialisation data. After determining normal distribution, Two-way ANOVA with a Dunnett’s posthoc test was used for statistical analysis of the cytokine dataset. One-way ANOVA with a Fishers LSD test was used for statistical analysis of the TEER data and a two-tailed unpaired t-test was used to calculate statistical significance of the western blot data. For cytokine data, in-text p-values have been summarized; all significant p-values are shown in full in Supplemental Table 1.
Results
Concentration associated effects
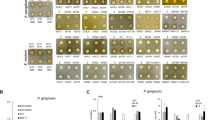
To observe the toxicity of postbiotics and to determine a lethal pathogen concentration, TR146 cells were treated with various concentrations (250 µg/mL – 5 µg/mL) of lysates and supernatants. Cell viability after 24 h treatment with bacterial lysate or supernatant is shown in Fig. 1. Increased pathogenic lysate concentration is strongly associated with decreased cell viability. When the control is compared to treatment with 250 µg/mL, PG and FN significantly reduce viability (p = 0.0003 and p < 0.0001 respectively), whereas AA demonstrates trends towards reduced viability (p = 0.0708). Conversely, no relationship was found between increased probiotic lysate concentration and cell viability (p > 0.05). Similarly to the lysates, the pathogens display a significant relationship between increased supernatant concentration and decreased cell viability. At the highest concentration of treatment, PG, FN and AA significantly reduce cell viability (p = 0.0002, p = 0.0135 and p = 0.0046). No difference was found between increased probiotic supernatant concentration and decreased viability (p > 0.05) and are therefore non-toxic at high concentrations.
Viability of TR146 cells treated with varying concentrations (250 µg/mL – 5 µg/mL) of pathogenic (a & b) or probiotic (c & d) lysates (a & b) or supernatants (c & d). Porphyromonas gingivalis (PG), Fusobacterium nucleatum (FN), Aggregatibacter actinomycetemcomitans (AA), Lactobacillus rhamnosus (SD), Lactobacillus plantarum (LP), Lactobacillus reuteri (LR) n = 3. Lactobacillus rhamnosus (GG) n = 6. Control (0 µg/mL) represents TR146 cells where DPBS was added in place of treatment (Lysates) or DPBS and WCB (supernatants). Bars represent the mean+/- SD. Two-way ANOVA A=(p < 0.05), B=(p < 0.01), C=(p < 0.001), D=(p < 0.0001).
This data demonstrates the toxic effects of pathogenic material at 250 µg/mL and therefore this concentration was selected as a significant challenge to the keratinocytes. Furthermore, postbiotics at this concentration demonstrated non-toxic effects.
Pre-treatment analysis: probiotic lysate vs. pathogenic lysate
Intracellular components of pathogens are toxic to cells (Fig. 1), therefore, to assess whether probiotic lysates could protect against intracellular pathogenic material, and if longer pre-treatment enhanced protection, TR146 cells were treated with probiotic lysates for 0, 2, 6, 12, and 24 h and challenged with pathogenic lysates for 24 h; results are shown as difference in percent viability to the pathogen-only control (Fig. 2). On average, all probiotic treatment conditions (60/60) led to an observed improvement in cell viability compared to the pathogen only control, 15% (9/60) of which were statistically significant (p < 0.05). No significant difference was found between pre-treatment timepoints or between individual probiotic performance (p > 0.05).
Differences in cell viability compared to the pathogen only control (sample cell viability minus the mean viability of the pathogen-only control). TR146 cells were pre-treated with probiotic lysates (250 µg/mL) for 0, 2, 6, 12 and 24 h. Pathogenic lysates (250 µg/mL) were then added for 24 h after pre-treatment. Porphyromonas gingivalis (PG), Fusobacterium nucleatum (FN), Aggregatibacter actinomycetemcomitans (AA), Lactobacillus rhamnosus (SD), Lactobacillus plantarum (LP), Lactobacillus reuteri (LR) n = 3. Lactobacillus rhamnosus (GG) n = 6. Control represents cells which were treated with DPBS and were not challenged with pathogenic lysate. Bars represent the mean+/- SD. Statistics are compared to the pathogen-only control: Two-way ANOVA: A=(p < 0.05), B=(p < 0.01), C=(p < 0.001), D=(p < 0.0001).
The average effects of 24 h pre-treatment with probiotics is shown in Supplemental Fig. 3A. Cell viability was improved against FN by 22.2% (p = 0.0113), with non-significant trends towards improved cell viability being observed against PG (13.6%, p = 0.1227) and AA (14.87%, p = 0.0845).
Pre-treatment analysis: probiotic lysate vs. pathogenic supernatant
As shown in Fig. 1, the secreted components of pathogens can be toxic to cells. To determine the prophylactic effects of probiotic lysates against secreted pathogenic material, TR146 cells were treated with probiotic lysates for 0, 2, 6, 12, and 24 h and subsequently challenged with pathogenic supernatants for 24 h (Fig. 3). Results are shown as difference in cell viability compared to the pathogen only control.
Differences in cell viability compared to the pathogen only control (sample cell viability minus the mean viability of the pathogen-only control). TR146 cells were pre-treated with probiotic lysates (250 µg/mL) for 0, 2, 6, 12 and 24 h. Pathogenic supernatants (250 µg/mL) were then added for 24 h after pre-treatment. Porphyromonas gingivalis (PG), Fusobacterium nucleatum (FN), Aggregatibacter actinomycetemcomitans (AA), Lactobacillus rhamnosus (SD), Lactobacillus plantarum (LP), Lactobacillus reuteri (LR) n = 3. Lactobacillus rhamnosus (GG) n = 6. Control represents cells which were treated with DPBS and WCB and were not challenged with pathogenic supernatant. Bars represent the mean+/- SD. Statistics are compared to the pathogen-only control: Two-way ANOVA: A=(p < 0.05), B=(p < 0.01), C=(p < 0.001), D=(p < 0.0001).
In almost all cases (54/60), treatment with probiotic lysates lead to an observed improvement in cell viability, 5% of which (3/60) were found to be significant (p < 0.05). No significant difference was found between pre-treatment timepoints or between probiotics (p > 0.05). As shown in Supplemental Fig. 3B, after 24 h pre-treatment, probiotic lysates significantly improved cell viability against FN (16.3%: p = 0.0251). Furthermore, trends towards improved cell viability against PG was shown by an 11.8% improvement in viability compared to the positive control (p = 0.1019).
Pre-treatment analysis: probiotic lysate vs. live pathogen
After observing the prophylactic effects of Lactobacillus lysates against non-viable pathogenic material we aimed to simulate a live pathogenic infection. TR146 cells were treated with probiotic lysates for 0, 2, 6, 12, and 24 h followed by a 24 h challenge with live pathogen (104 CFU/mL) (Fig. 4). Results are shown as difference to the pathogen only control. As before, almost all treatments led to an observed improvement in viability compared to the pathogen-only control (54/60), none of which were significant for PG (0/20) and two of which were significant against FN (2/20). However, against AA, 35% (7/20) of treatments significantly improved cell viability compared to the pathogenic control (p < 0.01). No correlation was found between longer pre-treatment and improved viability against PG or FN (p > 0.05), whereas against live AA, longer pre-treatment led to improved cell viability, with GG, SD, and LP significantly improving cell viability after 24 h pre-treatment (p < 0.001).
Differences in cell viability compared to the pathogen only control (sample cell viability minus the mean viability of the pathogen-only control). TR146 cells were pre-treated with probiotic lysates (250 µg/mL) for 0, 2, 6, 12 and 24 h. Live pathogen (104) was then added for 24 h after pre-treatment. Porphyromonas gingivalis (PG), Fusobacterium nucleatum (FN), Aggregatibacter actinomycetemcomitans (AA), Lactobacillus rhamnosus (SD), Lactobacillus plantarum (LP), Lactobacillus reuteri (LR) n = 3. Lactobacillus rhamnosus (GG) n = 6. Control represents cells which were treated with DPBS and were not challenged with pathogen. Bars represent the mean+/- SD. Statistics are compared to the pathogen-only control: Two-way ANOVA: A=(p < 0.05), B=(p < 0.01), C=(p < 0.001), D=(p < 0.0001).
On average, pre-treatment with postbiotics for 24 h also showed trends towards improved viability against PG (13.9%, p = 0.1162) but limited effects against FN (p = 0.5043) (Supplemental Fig. 3C).
Cytokine analysis
The impact of postbiotics on keratinocyte cytokine expression was assessed to determine any time-dependent effects. Figure 5 (IL-8, IL-6, IP-10, IL-1α) and Supplemental Fig. 4 (TNF-α, IL-10, IL-1β, TGF-β) show results for eight cytokines after treatment with 250 µg/mL of probiotic lysates for 1.5, 3, 6, 12 and 24 h. In-text p-values have been summarized, individual values are shown in Supplemental Table 1. Increases in IL-8, IL-6 and TNF-α concentration were recorded after 6 h of postbiotic treatment and became significant after 12 h (p < 0.05); all probiotics showed a significant increase in these cytokines after 24 h of treatment (p < 0.05). IP-10 concentration also increased with time however significance was only recorded for GG and LR after 24 h (p < 0.05). For all probiotics, IL-1β concentration increased significantly after 1.5 h pre-treatment (p < 0.05) and decreased over time to levels like the control by 24 h. Similarly, IL-1α increased significantly after 1.5 h pre-treatment (p < 0.05) for all probiotics however it maintained levels higher than the control for 24 h (p < 0.05). Finally, TGF-β concentration significantly increased 3 h after treatment with all probiotics (p < 0.05) and decreased over time to levels like the control after 24 h.
Cytokine expression of TR146 cells treated with probiotic lysates (250 µg/mL) for 1.5, 3, 6, 12 and 24 h. Lactobacillus rhamnosus (GG) n = 12, Lactobacillus rhamnosus (SD), Lactobacillus plantarum (LP), Lactobacillus reuteri (LR) n = 6. Control refers to cells where DPBS were added in place of treatment (n = 4). Bars represent the mean+/- SD.
Between probiotics, some significant differences were recorded. Generally, treatment with LR led to the greatest cytokine response, demonstrating significantly higher IL-8, TGF-β, IL-6, IL-10 and TNF-α expression than SD and GG at various timepoints (p < 0.05). LP also demonstrated an increase in IL-8 compared with SD after 12 h of treatment (p < 0.01), as well as an increase in IL-6 compared to GG after 6 h of treatment (p < 0.05).
IFN-γ and IL-17 A were also considered in this panel however neither were produced at a high enough concentration to be detected.
Re-epithelialisation
Pathogen associated damage can compromise epithelial barrier integrity. To determine if postbiotics can improve healing, re-epithelialisation of TR146 cells after exposure to pathogenic and probiotic lysates was observed (Fig. 6). Representative scratch images are shown in Supplemental Fig. 5. When compared to the negative control, treatment with SD significantly improved re-epithelialisation at 24 h (p = 0.0229), with trends towards improvements at earlier timepoints (p = 0.0606, 18 h). This suggests SD lysates could have beneficial properties in restoring compromised barrier integrity. GG and LR had no significant impact on re-epithelialisation and demonstrated similar results to the control (p > 0.05). Interestingly, LP significantly inhibited re-epithelialisation (p < 0.0001) and showed comparable results to pathogens FN and PG.
Difference in scratch size compared to 0 h (baseline) after treatment with lysates (250 µg/mL). Pathogens (A) Porphyromonas gingivalis (PG), Fusobacterium nucleatum (FN), Aggregatibacter actinomycetemcomitans (AA), and probiotics (B) Lactobacillus rhamnosus (SD), Lactobacillus plantarum (LP), Lactobacillus reuteri (LR) n = 3, Lactobacillus rhamnosus (GG) n = 6. Control represents cells treated with DPBS in place of lysate. Positive represents cells treated with 1% HKGS. Bars represent the mean+/- SD. Two-way ANOVA *(p < 0.05) relative to the control at 24 h.
Trans-epithelial Electrical Resistance (TEER)
The maintenance of epidermal barrier integrity is essential for the management of periodontal pathogens. TEER was used to determine if probiotic lysates could enhance or maintain the epithelial barrier (Fig. 7: A) or moderate the effects subsequent exposure to PG supernatant (Fig. 7: B). Results obtained after treatments have been combined into boxplots in a non-time dependent manner.
Trans-epithelial electrical resistance (TEER). TR146 cells treated with 100 µg/mL of pathogenic or probiotic lysate (A) or a combination of 50 µg/mL of probiotic lysate and 50 µg/mL PG supernatant (B). PBS represents cells where treatment was replaced by DPBS. PG_sn (B) represents cells treated with 50 µg/mL PG supernatant and DPBS in place of probiotics. Porphyromonas gingivalis (PG), Fusobacterium nucleatum (FN), Aggregibacter actinomycetemcomitans (AA), Lactobacillus rhamnosus (SD), Lactobacillus plantarum (LP), Lactobacillus reuteri (LR). (n = 3) Lactobacillus rhamnosus (GG) n = 6 (A) n = 3 (B). Time-course data from after treatment has been added to boxplots in a non-time dependent manner. One-way ANOVA *(p < 0.05), **(p < 0.01), ***(p < 0.001), ****(p < 0.0001).
On average, over the 12-days of treatment, GG and SD significantly increased TEER by 11.3 Ω/cm2 (p = 0.0165) and 18.0 Ω/cm2 (p = 0.0003) respectively. Probiotics LR and LP showed a comparable TEER to the control, suggesting they have minimal effect on the expression of TJ proteins (p > 0.05). Pathogenic lysates significantly reduced TEER compared to the control (p < 0.0001), however TEER of cells exposed to AA began to increase after day seven.
Addition of PG supernatant without probiotic treatment led to a significant reduction in TEER (17.5 Ω/cm2: p < 0.0001). However, in the presence of all probiotics, TEER was significantly increased when compared to the pathogen-only control, demonstrating a prophylactic effect on barrier integrity. SD conveyed the best protection (12.5 Ω/cm2 increase in TEER: p < 0.0001) followed by LP (11 Ω/cm2 increase: p = 0.0053), LR (9 Ω/cm2 increase: p = 0.0155) and GG (8.5 Ω/cm2 increase: p = 0.0047).
Claudin-1 expression
To determine if increased TEER correlated with claudin-1 expression, western blot analysis was performed (Fig. 8). Treatment with SD led to a 22% increase in claudin-1 (p = 0.0174), with LP and LR demonstrating an observed increase in claudin-1 by 20% (p = 0.1513) and 11% (p = 0.1283) respectively. Interestingly, treatment with GG significantly reduced claudin-1 concentration (p = 0.0337) suggesting increases in TEER were mediated by other factors.
(A) Representative western blot. TR146 cells treated with 250 µg/mL of probiotic lysate. Control represents cells where treatment was replaced by DPBS. Lactobacillus rhamnosus (GG), Lactobacillus rhamnosus (SD), Lactobacillus plantarum (LP), Lactobacillus reuteri (LR). (B) Relative abundance of claudin-1 quantified by western blot as in A (n = 3). Bars represent mean+/- SD. Unpaired t-test *(p < 0.05).
Discussion
We have determined a high concentration at which pathogens significantly reduce cell viability, and postbiotics are non-toxic. Many studies do not consider protein concentration in lysate preparation7,30,31 despite numerous investigations demonstrating that proteins from Lactobacillus species, particularly membrane components, can have positive effects on host cells32,33,34. Our data indicates that increased protein concentration can lead to greater changes in cell viability and have demonstrated a significant relationship between the protein concentration of a lysate and its effects on oral epithelial cells. Additionally, this study presents data linking CFU/mL and OD, to intra- and extra-cellular protein concentration (Supplemental Fig. 1). Interestingly, we observed that AA and LR had the lowest protein concentrations in their lysates but high levels in their supernatants. This was especially notable for AA, which showed strong extracellular protein expression despite having a low CFU/mL count, demonstrating its tendency to form biofilms35. Additionally, probiotics GG and LP exhibited the highest lysate protein concentrations; however, while GG showed low supernatant protein levels, LP had one of the highest.
Our study utilised trypan blue staining to assess various effects on cell viability. This is a commonly used method, particularly suited to co-culture models where metabolic assays like MTT can be unsuitable due to interference from microbial metabolism. However, it is important to note that trypan blue staining may not detect cells with impaired or failing metabolism that retain an intact membrane, potentially overlooking a specific subset of non-viable cells36,37.
Orally-relevant protective effects of probiotics have been reported in numerous studies using live formulations. Morales et al.38 for example, reported that L. rhamnosus can control chronic periodontitis in a clinical setting. Similarly, Ince et al.39 reported modulatory effects in volunteers with chronic periodontitis when administered lozenges containing L. reuteri. This has also been observed in some studies in the context of postbiotics10,11,13 however, to our knowledge previous investigations have not considered the pathogens included in this study.
Significant prophylactic effects were seen against live AA after 24 h pre-treatment with GG, SD and LP; LR also demonstrated a non-significant protective effect. AA can cause aggressive periodontitis and bone resorption35; it has been detected in 90% of localised periodontitis infections and is therefore characterised as one of the leading causes of periodontitis40. Future studies could consider why keratinocytes are protected from AA in the presence of Lactobacillus lysates.
Lactobacilli are known to alter cytokine production in host cells by the expression of peptidoglycans in the cell wall. Binding of Lactobacilli peptidoglycans to toll-like receptors (which are highly expressed on keratinocytes) activates the transcription factor NF-κB41, which has a role in regulating cytokine production, as well as genes relating to differentiation and proliferation42. In our study, longer pre-treatment led to greater increases in pro-inflammatory cytokines. The release of inflammatory molecules in response to Lactobacillus strains has previously been reported. For example, Mohammedsaeed et al.11, reported that the expression of 14 molecules relating to the inflammatory response were increased by GG lysate, with TNF-α and IP-10 both being upregulated. In addition, Lee et al.43, showed that live L. rhamnosus increased TNF-α and IFN-γ expression in skin keratinocytes, while Jiang et al.44 that live L. acidophilus significantly upregulated the expression of IL-1α and IL-1β in Caco-2 cells.
Despite the association of periodontitis with inflammation, the release of inflammatory cytokines in response to postbiotics could be a key element of their protective effects. TNF-α is associated with antimicrobial host response; recruiting immune cells and increasing vascular permeability to allow access of immune cells45. IL-6 has been associated with aggressive periodontitis and inflammation, with high concentrations being found in the saliva of those affected with periodontal disease46, but it is also a potent stimulator of cell differentiation, boosting keratinocyte growth and survival, as well as enhancing the immune response47. Irwin and Myrillas48 suggest IL-6 may play both an immunopathogenic pathogenic and protective role in oral disease. Similarly, higher levels of IL-8 are often found in infected periodontal tissue and are associated with periodontitis49. Despite this, IL-8 is also an essential part of the immune response and a key element in fighting infection50.
IL-1α and IL-1β concentration increased significantly 1.5 h after treatment (although IL-1β secretion was relatively low). While both cytokines are associated with severe periodontitis due to their inflammatory nature and promotion of bone reabsorption, this appears to be a time-dependent effect. It has been noted that low-level increases of IL-1 can be beneficial to host health and lack of IL-1 secretion in response to infection can result in a reduced ability to control infection51. It is therefore important to quantify changes demonstrated in the presence of probiotics in this study. Sanchez et al.52 demonstrated that salivary IL-1β concentration in volunteers with moderate periodontitis was 500% higher than healthy participants, this is a far more considerable increase than shown in our in vitro study. Furthermore, Kim et al.53 showed the mean salivary levels of IL-1α in those with periodontal disease was 590.78 pg/mL, and healthy volunteers were 343.60 pg/mL. As before, those with periodontal disease demonstrate a much higher IL-1α concentration than shown above. Therefore, although levels of the IL-1 family show an increase, this could be an essential mechanism of action of postbiotics.
TGF-β decreased over time for all samples including the control, although expression was relatively low. For all probiotics, expression was significantly higher when compared to the control, and the time it took for these levels to decrease was maintained for longer. TGF-β plays a key role in the management of periodontitis as a powerful stimulator of tissue regeneration and barrier enhancement54 therefore, significant increases demonstrated by our probiotic lysates could be an essential aspect in the management of periodontitis.
IP-10 demonstrated a time-dependent increase, with longer pre-treatment resulting in higher expression. IP-10 is a chemoattractant essential for recruiting immune cells to infected tissue55. Gemmell et al.56 found that IP-10 levels decreased with increasing inflammation in periodontal disease. However, it has been noted that IP-10 concentration generally increases in infected periodontal tissue55. Notably, high levels of IP-10 has been shown to inhibit wound healing in mice57.
IL-10 levels remained comparable to the control for most probiotics, with non-significant increases shown for LR. IL-10 is an important regulatory cytokine, and can reduce inflammation in periodontal disease; however, it has been suggested that this can lead to a lack of immune cell recruitment contributing to the persistence of bacterial presence in infected tissue58.
Overall, the cytokine data presented in this document and the wider literature is complex. Studies have reported conflicting arguments for almost all cytokines, for example, Kim et al.53 present no significant difference in salivary TNF-α and IL-8 between individuals with and without periodontitis. Furthermore, as discussed for the IL-1 family, the degree of concentration change is relevant. Increased inflammatory cytokine production results in immune cell recruitment, it is only when cytokines are persistently overproduced for long periods that their effects can become immunopathogenic. Moreover, Zhang et al.45 found a relationship between the short (14 days) and long term (35 days) effects of TNFα and IL-1 secretion, noting that blocking the activity of these cytokines in non-human primates with lab-induced periodontitis, improved management of the disease in the short term, but led to disease progression over the long term. The results discussed in this document do not consider cytokine release for an extended treatment period. Finally, despite the increases in inflammatory markers following treatment with postbiotics, the cell viability data presented in this study demonstrates the protective effects of postbiotic lysates, suggesting that alterations to cytokine secretion do not cause any adverse effect and may contribute to the mechanism of action ‘priming’ the immune system to facilitate bacterial clearance.
The ability for L. rhamnosus to improve re-epithelialisation has been previously reported. Mohammedsaeed et al.12 found re-epithelialisation of epidermal keratinocytes was significantly enhanced in the presence of L. rhamnosus lysates. Given the association of periodontitis with barrier breakdown59, an important characteristic of a periodontitis treatment could be regulating barrier function. Probiotic lysates may contribute to improved wound healing by upregulating cytokines such as IL-1α, IL-1β, TGF- β, IL-6, and TNF-α, all known to enhance wound healing, cell migration, and proliferation60. Notably, the ability of L. rhamnosus lysates to accelerate re-epithelialisation, highlights the potential of postbiotics in facilitating immediate wound healing and closure. Interestingly, LP significantly inhibited wound closure when compared to the control, contradicting previous findings61,62 and warranting further investigation.
Increases in TEER after treatment with Lactobacillus probiotics have been previously reported in gut models15,63. Sultana et al.14 demonstrated that GG lysate significantly increases TEER of human keratinocytes, with higher doses (108 CFU/mL) being increasingly effective, which the authors suggest could be facilitated by interaction with toll-like receptors on the keratinocyte surface. Given that disruption of the epithelial barrier and intrusion by periodontal pathogens leads to inflammation and chronic infection59, improving barrier integrity could be one way in which Lactobacillus lysates exert their prophylactic effects64.
In contrast to our findings, claudin-1 expression has been shown to increase after exposure to L. rhamnosus lysate14. Furthermore, live L. plantarum can increased the expression of claudin-1, as well as occludin and ZO-1 in the guts of IL-10 knockout mice65. Yang et al.63 has previously explored the effects of L. reuteri on tight-junction expression pig intestine models, demonstrating that LR can increase claudin-1 expression even in a lipopolysaccharide challenged environment. Claudin-1 is a major component of keratinocyte TJ’s, with downregulation in the skin barrier being linked water-loss conditions such as dermatitis66. It has also been shown that lipopolysaccharides can reduce TJ expression63 suggesting that periodontal pathogens may be able to supress TJ’s67 leading to a compromised barrier. Decreases in claudin expression after exposure to GG has not been previously reported. We suggest the increase in TEER could be driven by another member of the TJ family that has not been investigated here; occludin and ZO-1 have all been shown to increase after treatment with GG14.
The mechanism by which Lactobacillus probiotics enhance the epithelial barrier is not fully understood. However, changes in cytokine expression could be one mechanism of action. IL-6 (which significantly increased for all postbiotics) has been shown to have protective effects on epithelial barriers in gut models68. Furthermore, Stolte et al.69 demonstrated that, although IL-1β often causes barrier compromise in intestinal models, in oral cells its presence significantly increases IL-6 and IL-8 expression, leading to an enhancement in TEER and claudin-1. These findings validate that our lysates could increase TEER by raising IL-6, IL-8 and IL-1β expression.
Conclusion
Lactobacillus lysates exhibited prophylactic effects against high concentrations of intra and extracellular pathogenic material as well as live pathogens. Moreover, treatment with postbiotics significantly altered cytokine expression of TR146 cells. Despite increases in some inflammatory cytokines, the viability, re-epithelialisation, and TJ data demonstrated positive effects after exposure to Lactobacillus lysates. Furthermore, increases in inflammatory cytokines are essential for immune recruitment, access to infected tissue, keratinocyte proliferation and barrier maintenance. We suggest a key mechanism of action of probiotic lysates could be the ability to ‘prime’ the host’s immune system. Furthermore, the fact that probiotic lysates maintain and improve barrier integrity could enhance disease management, representing a potential periodontitis therapeutic. Future work could confirm the mechanisms by which Lactobacillus lysates are able to protect oral keratinocytes from periodontal pathogens. Characterisation and isolation of key proteins within the lysates would be of particular of interest, to examine their effects individually.
Data availability
Data available upon request of the corresponding author.
References
WHO, W.H.O. Oral Health. (2023). Available from: https://www.who.int/news-room/fact-sheets/detail/oral-health
Kwon, T., Lamster, I. B. & Levin, L. Current concepts in the management of Periodontitis. Int. Dent. J. 71 (6), 462–476 (2021).
HealthandSocialCareComitee NHS Dentistry - Report. ; (2023). 20/11/2023] Available from: https://publications.parliament.uk/pa/cm5803/cmselect/cmhealth/964/summary.html
Allaker, R. P. & Stephen, A. S. Use of probiotics and oral health. Curr. Oral Health Rep. 4 (4), 309–318 (2017).
(ISAPP), I.S.A.f.P.a.P. Probiotics. ; (2019). Available from: https://isappscience.org/for-scientists/resources/probiotics/
Yu, J. et al. Application and mechanism of probiotics in skin care: A review. J. Cosmet. Dermatol. 21 (3), 886–894 (2022).
Jung, Y. O. et al. Lysates of a probiotic, Lactobacillus rhamnosus, can improve skin barrier function in a reconstructed human epidermis model. Int. J. Mol. Sci., 20(17). (2019).
Parasuraman, S., Vedam, V. K. V. & Sabesan, G. S. Probiotics: An emerging strategy for oral Health Care, in Probiotics, Prebiotics, Synbiotics, and Postbiotics: Human Microbiome and Human Health, V. Kothari, P. Kumar, and S. Ray, Editors. Springer Nature Singapore: Singapore. 275–306. (2023).
Homayouni Rad, A., Pourjafar, H. & Mirzakhani, E. A comprehensive review of the application of probiotics and postbiotics in oral health. Front. Cell. Infect. Microbiol.,13. (2023).
Moman, R. et al. Mitigation of the toxic effects of Periodontal pathogens by candidate probiotics in oral keratinocytes, and in an invertebrate model. Front. Microbiol.,11. (2020).
Mohammedsaeed, W. et al. Lactobacillus rhamnosus GG inhibits the toxic effects of Staphylococcus aureus on epidermal keratinocytes. Appl. Environ. Microbiol. 80 (18), 5773–5781 (2014).
Mohammedsaeed, W. et al. Lactobacillus rhamnosus GG lysate increases re-epithelialization of keratinocyte scratch assays by promoting Migration. Sci. Rep. 5, 16147 (2015).
Prince, T. & McBain, A. J. O’Neill, Lactobacillus reuteri protects epidermal keratinocytes from Staphylococcus aureus-Induced cell death by competitive exclusion. Appl. Environ. Microbiol. 78 (15), 5119–5126 (2012).
Sultana, R., McBain, A. J. & O’Neill, C. A. Strain-dependent augmentation of tight-Junction barrier function in human primary epidermal keratinocytes by Lactobacillus and Bifidobacterium Lysates. Appl. Environ. Microbiol. 79 (16), 4887–4894 (2013).
Anderson, R. C. et al. Lactobacillus plantarum MB452 enhances the function of the intestinal barrier by increasing the expression levels of genes involved in tight junction formation. BMC Microbiol. 10 (1), 316 (2010).
Patel, R. M. et al. Probiotic bacteria induce maturation of intestinal claudin 3 expression and barrier function. Am. J. Pathol. 180 (2), 626–635 (2012).
Zheng, J. et al. Lactobacillus rhamnosus CY12 enhances intestinal barrier function by regulating tight Junction protein expression, oxidative stress, and inflammation response in Lipopolysaccharide-Induced Caco-2 cells. Int. J. Mol. Sci. 23 (19), 11162 (2022).
Miyauchi, E., Morita, H. & Tanabe, S. Lactobacillus rhamnosus alleviates intestinal barrier dysfunction in part by increasing expression of zonula occludens-1 and myosin light-chain kinase in vivo. J. Dairy Sci. 92 (6), 2400–2408 (2009).
Johnson-Henry, K. C. et al. <i > Lactobacillus rhamnosus strain GG prevents enterohemorrhagic < i > Escherichia coli O157:H7-Induced changes in epithelial barrier Function</i >. Infect. Immun. 76 (4), 1340–1348 (2008).
González-Mariscal, L. et al. Tight junction proteins. Prog. Biophys. Mol. Biol. 81 (1), 1–44 (2003).
Fujita, T. et al. Loss of claudin-1 in lipopolysaccharide-treated periodontal epithelium. J. Periodontal Res. 47 (2), 222–227 (2012).
Vitkov, L. et al. Breaking the gingival barrier in periodontitis. Int. J. Mol. Sci., 24 (5). (2023).
Mishra, S., Rath, S. & Mohanty, N. Probiotics—A complete oral healthcare package. J. Integr. Med. 18 (6), 462–469 (2020).
Kiepś, J. & Dembczyński, R. Current trends in the production of probiotic formulations. Foods 11 (15), 2330 (2022).
Cheng, Z. et al. Effects of freeze drying in complex lyoprotectants on the survival, and membrane fatty acid composition of Lactobacillus plantarum L1 and Lactobacillus fermentum L2. Cryobiology 105, 1–9 (2022).
Aruni, A. W. et al. The Biofilm Community: Rebels with a cause. Curr. Oral Health Rep. 2 (1), 48–56 (2015).
Piwat, S. et al. Lactobacillus species and genotypes associated with dental caries in Thai preschool children. Mol. Oral Microbiol. 25 (2), 157–164 (2010).
Piwat, S. & Teanpaisan, R. 16S rRNA PCR-Denaturing gradient gel electrophoresis of oral lactobacillus casei group and their phenotypic appearances. ISRN Microbiol,2013 342082. (2013).
Strober, W. Trypan Blue Exclusion Test of cell viability. Curr. Protocols Immunol. 21 (1), A3B1–A3B2 (1997).
Kim, M. Y. et al. In vitro anti-inflammatory and Antibiofilm Activities of Bacterial Lysates from Lactobacilli against oral Pathogenic bacteria13p. 12755–12765 (Food & Function, 2022). 24.
Vale, G. C. & Mayer, M. P. A. Effect of probiotic Lactobacillus rhamnosus by-products on gingival epithelial cells challenged with Porphyromonas gingivalis. Arch. Oral Biol. 128, 105174 (2021).
Wang, G. et al. A surface protein from Lactobacillus plantarum increases the adhesion of Lactobacillus strains to human epithelial cells. Front. Microbiol. 9, 2858 (2018).
Muscariello, L., De Siena, B. & Marasco, R. Lactobacillus cell surface proteins involved in Interaction with mucus and Extracellular Matrix Components. Curr. Microbiol. 77 (12), 3831–3841 (2020).
Meng, J., Wang, Y. Y. & Hao, Y. P. Protective function of surface layer protein from Lactobacillus casei fb05 against intestinal pathogens in vitro. Biochem. Biophys. Res. Commun. 546, 15–20 (2021).
Gholizadeh, P. et al. Oral pathogenesis of Aggregatibacter actinomycetemcomitans. Microb. Pathog. 113, 303–311 (2017).
Strober, W. Trypan Blue Exclusion Test of cell viability. Curr. Protocols Immunol. 111 (1), A3B1–A3B3 (2015).
Braissant, O. et al. A review of methods to determine viability, vitality, and metabolic rates in Microbiology. Front. Microbiol. 11, 547458 (2020).
Morales, A. et al. Clinical effects of Lactobacillus rhamnosus in non-surgical treatment of chronic periodontitis: A randomized placebo-controlled trial with 1-Year Follow-Up. J. Periodontol. 87 (8), 944–952 (2016).
İnce, G. et al. Clinical and biochemical evaluation of lozenges containing Lactobacillus reuteri as an adjunct to non-surgical periodontal therapy in chronic periodontitis. J. Periodontol. 86 (6), 746–754 (2015).
Raja, M., Ummer, F. & Dhivakar, C. P. Aggregatibacter actinomycetemcomitans - a tooth killer?. J. Clin. Diagn. Res., 8(8): p. (2014). Ze13-6.
Sun, J. et al. Distinct immune response induced by peptidoglycan derived from Lactobacillus sp. World J. Gastroenterol. 11 (40), 6330–6337 (2005).
Liu, T. et al. NF-κB signaling in inflammation. Signal. Transduct. Target. Therapy. 2 (1), 17023 (2017).
Lee, J. Y. et al. Anti-inflammatory response in TNFα/IFNγ-Induced HaCaT keratinocytes and Probiotic properties of Lacticaseibacillus rhamnosus MG4644, lacticaseibacillus paracasei MG4693, and Lactococcus lactis MG5474. J. Microbiol. Biotechnol. 33 (8), 1039–1049 (2023).
Jiang, Y. et al. Lactobacillus acidophilus induces cytokine and chemokine production via NF-κB and p38 mitogen-activated protein kinase signaling pathways in intestinal epithelial cells. Clin. Vaccine Immunol. 19 (4), 603–608 (2012).
Zhang, X. et al. Short- and Long-Term effects of IL-1 and TNF antagonists on Periodontal Wound Healing1. J. Immunol. 173 (5), 3514–3523 (2004).
Nibali, L. et al. The Role of Interleukin-6 in Oral Diseases. Dimen. Dental Hygeine 11 (1): 28, 30, 32–34 (2013)
Hirano, T., Ishihara, K. & Hibi, M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene 19 (21), 2548–2556 (2000).
Irwin, C. R. & Myrillas, T. T. The role of IL-6 in the pathogenesis of periodontal disease. Oral Dis. 4 (1), 43–47 (1998).
Finoti, L. S. et al. Association between interleukin-8 levels and chronic periodontal disease: a PRISMA-compliant systematic review and meta-analysis. Med. (Baltim). 96 (22), e6932 (2017).
Brennan, K. & Zheng, J. Interleukin 8, in xPharm: The Comprehensive Pharmacology Reference, S.J. Enna and D.B. Bylund, Editors. Elsevier: New York. 1–4. (2007).
Dinarello, C. A. Modalities for reducing interleukin 1 activity in disease. Immunol. Today. 14 (6), 260–264 (1993).
Sánchez, G. A. et al. Salivary IL-1β and PGE2 as biomarkers of periodontal status, before and after periodontal treatment. J. Clin. Periodontol. 40 (12), 1112–1117 (2013).
Kim, J. Y., Kim, K. R. & Kim, H. N. The potential impact of salivary IL-1 on the diagnosis of Periodontal Disease: a pilot study. Healthc. (Basel), 9(6). (2021).
Fan, C. et al. TGF–β induces periodontal ligament stem cell senescence through increase of ROS production. Mol. Med. Rep. 20 (4), 3123–3130 (2019).
Figen Öngöz, D., Şeyma, D. & Bozkurt Chemokines in Periodontal Diseases, in Chemokines Updates (Ş. Murat, Editor., 2023). IntechOpen: Rijeka. p. Ch. 4.
Gemmell, E., Carter, C. L. & Seymour, G. J. Chemokines in human periodontal disease tissues. Clin. Exp. Immunol. 125 (1), 134–141 (2001).
Werner, S. Regulation of Wound Healing by Growth factors and cytokines. Physiol. Rev. 83 (3), 835–870 (2003).
Saremi, L. et al. Evaluation of interleukin 10, interleukin 1-beta, and tumor necrosis factor-alpha gene polymorphisms in patients with periodontitis and healthy controls. Egypt. J. Med. Hum. Genet. 23 (1), 157 (2022).
Takahashi, N. et al. Gingival epithelial barrier: regulation by beneficial and harmful microbes. Tissue Barriers. 7 (3), e1651158 (2019).
Rodrigues, M. et al. Wound healing: A cellular perspective. Physiol. Rev. 99 (1), 665–706 (2019).
Brandi, J. et al. Exploring the wound healing, anti-inflammatory, anti-pathogenic and proteomic effects of lactic acid bacteria on keratinocytes. Sci. Rep. 10 (1), 11572 (2020).
Dubey, A. K. et al. Insight into the beneficial role of lactiplantibacillus plantarum supernatant against bacterial infections, oxidative stress, and wound healing in A549 cells and BALB/c mice. Front. Pharmacol., 12. (2021).
Yang, F. et al. Lactobacillus reuteri I5007 modulates tight junction protein expression in IPEC-J2 cells with LPS stimulation and in newborn piglets under normal conditions. BMC Microbiol. 15 (1), 32 (2015).
Şenel, S. An overview of Physical, Microbiological and Immune barriers of oral mucosa. Int. J. Mol. Sci., 22(15). (2021).
Chen, H. Q. et al. Lactobacillus plantarum ameliorates colonic epithelial barrier dysfunction by modulating the apical junctional complex and PepT1 in IL-10 knockout mice. Am. J. Physiol. Liver Physiol. 299 (6), G1287–G1297 (2010).
Bergmann, S. et al. Claudin-1 decrease impacts epidermal barrier function in atopic dermatitis lesions dose-dependently.Sci. Rep.,10 (1): 2024. (2020).
Fujita, T. et al. Regulation of defensive function on gingival epithelial cells can prevent periodontal disease. Jpn. Dent. Sci. Rev. 54 (2), 66–75 (2018).
Wang, L. et al. Interleukin-6 induces keratin expression in intestinal epithelial cells: Potential role of keratin-8 in interleukin-6-induced barrier function alterations. J. Biol. Chem. 282 (11), 8219–8227 (2007).
Stolte, K. N. et al. IL-1β strengthens the physical barrier in gingival epithelial cells. Tissue Barriers. 8 (3), 1804249 (2020).
Author information
Authors and Affiliations
Contributions
Steven D Mercer: Investigation, Data Curation, Project Administration, Writing—Original Draft, Writing—Review and Editing Preparation. Christopher Doherty: Investigation, Writing—Original Draft, Writing—Review and Editing. Gurdeep Singh: Investigation, Writing—Original Draft, Writing—Review and Editing. Thomas Willmott: Investigation, Writing—Original Draft, Writing—Review and Editing. Tanaporn Cheesapcharoen: Investigation, Writing—Review and Editing. Rawee Teanpaisan: Investigation, Writing—Review and Editing. Catherine O’Neill, Writing—Review and Editing. Ruth G Ledder, Writing—Review and Editing. Andrew J McBain: Conceptualization, funding acquisition, Methodology, Resources, Supervision, Project Administration, Writing—Original Draft, Writing—Review and Editing Preparation. All authors contributed to and read the manuscript.
Corresponding authors
Ethics declarations
Competing interests
This work was funded by SkinBioTherapeutics, a microbiome company. A.J.M. and C.O. hold equity in SkinBiotherapeutics. RT is an inventor of a probiotic product. SDM, CD, GS, TW, TC, RGL, no conflict of interest to declare.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mercer, S.D., Doherty, C., Singh, G. et al. Lactobacillus lysates protect oral epithelial cells from pathogen-associated damage, increase secretion of pro-inflammatory cytokines and enhance barrier integrity. Sci Rep 15, 5894 (2025). https://doi.org/10.1038/s41598-025-86914-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-86914-y
Keywords
This article is cited by
-
In Vivo Evaluation of Lacticaseibacillus reuteri and Lacticaseibacillus rhamnosus Lysates for Oral Wound Healing
Probiotics and Antimicrobial Proteins (2025)