Abstract
Previous studies have suggested that the presence of human epididymal protein 4 (HE4) in pleural fluid can be used to diagnose malignant pleural effusion (MPE) with moderate accuracy. However, the factors that affect the diagnostic accuracy of HE4 remain unknown. This study aimed to examine how age and sex influence the diagnostic accuracy of HE4. Participants with undiagnosed pleural effusion were prospectively enrolled in two cohorts (Hohhot cohort and Changshu cohort), and the presence of HE4 in their pleural fluid upon admission was determined by an electrochemiluminescence immunoassay. A receiver operating characteristic (ROC) curve with its area under the curve (AUC) was utilized to assess the diagnostic value of HE4 for MPE. Additionally, we conducted subgroup analyses and used a resampling method with different upper age limits to investigate the impacts of age and sex on the diagnostic accuracy of HE4 for MPE. The Hohhot cohort included 86 patients with benign pleural effusions (BPEs) and 66 patients with MPE, whereas the Changshu cohort included 26 patients with MPE and 32 patients with BPE. The diagnostic accuracy of HE4 decreased as age increased in both cohorts. The diagnostic accuracy of HE4 in males did not differ significantly from that in females. Therefore, we conclude that age should be considered when using HE4 in pleural fluid to diagnose MPE.
Similar content being viewed by others
Introduction
Pleural effusion is the abnormal accumulation of fluid within the pleural space. It can be broadly categorized as malignant pleural effusion (MPE) and benign (BPE)1,2. MPE is defined as pleural effusion owing to the metastasis of malignancies into the pleural space or primary pleural tumours. Lung adenocarcinoma is the most common tumour that causes MPE, followed by breast and ovarian cancers1,3. The median survival period of MPE patients is less than one year4,5. MPE must be carefully diagnosed because a misdiagnosis can cause a heavy financial burden as well as psychological distress for patients. The reference standards for diagnosing MPE are cytological analysis, pleural biopsy, and thoracoscopy6,7,8,9. However, these diagnostic tools have certain limitations. Cytological analysis has a specificity of 1.00; however, approximately half of MPE patients may be missed10,11. Thoracoscopy or pleural biopsy can also confirm MPE; however, the invasive nature of this approach limits its clinical application in certain patients (e.g., those with coagulation disorders)12. In addition, the diagnostic accuracy of cytological analysis, pleural biopsy, and thoracoscopy may vary among diagnosticians.
Pleural fluid tumour markers are potential tools for diagnosing MPE owing to their advantages of easy access, low invasiveness, rapidity, and objectiveness2. Our previous study revealed that pleural fluid human epididymal protein 4 (HE4) can assist in diagnosing MPE, with an AUC of 0.7513. However, the factors that can affect its diagnostic accuracy remain unknown. Notably, previous studies reported that serum HE4 levels are affected by age14,15 and sex15. Based on the aforementioned evidence, we hypothesize that age and sex confound the diagnostic value of HE4.
In this post hoc study, we investigated the impacts of sex and age on the diagnostic value of HE4. We prepared this work following the reporting guidelines of diagnostic test accuracy studies16.
Methods
Participants
This was a post hoc study in which the participants were enrolled in our previous study13. The participant recruitment inclusion and exclusion criteria were reported in our earlier studies17,18. Briefly, we recruited patients with pleural effusions with undetermined causes who were admitted to Affiliated Changshu Hospital of Nantong University (June 2020 to July 2021; Changshu cohort) and the Affiliated Hospital of Inner Mongolia Medical University (September 2018 to July 2021; Hohhot cohort). The inclusion criteria were as follows: (i) pleural effusion patients whose underlying aetiology was unknown and (ii) patients who received thoracentesis. Ultrasound, chest X-ray, or CT were used to confirm the presence of pleural effusion. The following participants were excluded: (i) patients who had a pleural effusion history during the last three months and whose aetiology was clear; (ii) patients under 18 years old; (iii) pregnant women; (iv) patients without sufficient effusion for research purposes; (v) patients whose pleural effusion developed during hospitalization; and (vi) patients whose pleural effusion was caused by trauma or operation.
The Ethics Committees of the Affiliated Changshu Hospital of Nantong University (No. 2020-KY-009) and the Affiliated Hospital of Inner Mongolia Medical University (No. 2018011) approved this study. All the participants signed an informed consent. We conducted the study in accordance with the Declaration of Helsinki.
Diagnostic criteria
The reference standards for differentiating pleural effusion have been described previously13. Briefly, the reference standards for MPE include a cytological analysis and pleural histology. In patients with negative cytology results who were unwilling or unable to undergo pleural biopsy or thoracoscopy, MPE was defined as evidence of a primary tumour, clinical picture, and exclusions of BPE. Tuberculous pleural effusion (TPE) was diagnosed by pleural fluid Ziehl-Neelsen staining, Mycobacterium tuberculosis (Mtb) culture, or the presence of granulomas confirmed by pleural biopsy. In patients with no microbiologic evidence and who were unwilling or unable to undergo pleural biopsy, TPE was defined based on the antituberculosis treatment response and the exclusion of other pleural effusions. Parapneumonic pleural effusion (PPE) was defined based on the history, presence of pus cells in the effusion, bacterial culture, chest imaging characteristics, pleural biopsy (with evidence of neutrophilic infiltration), and the response to antibiotic therapy19. Congestive heart failure (CHF)-induced pleural effusion was diagnosed based on Light’s criteria, ultrasound and chest imaging characteristics (bilateral pleural effusion), serum chemistry (e.g., natriuretic peptides), echocardiographic features (e.g., enlarged cardiac chambers, decreased left ventricular ejection fraction), and treatment response to diuretics. Two senior clinicians (Zhi-De Hu and Li Yan) interpreted the medical records of participants and independently made the final diagnosis; disagreements were resolved by consensus. The clinicians who diagnosed all the patients were unaware of the levels of HE4 in the pleural fluid.
Pleural fluid HE4 assay
After admission, a 10-mL effusion sample was collected into a tube without any anticoagulant. After centrifugation, the supernatant was collected, aliquoted, and stored between − 80 °C and − 70 °C. The ARCHITECT i2000SR analyzer was used to measure HE4 levels in both cohorts. According to the internal quality control, the intra-assay coefficient of variation (CV) was 4.65% at 148 pmol/L. The upper limit of HE4 detection was 1500 pmol/L. The laboratory technicians who performed the HE4 analysis were blinded to the clinical details of the patients.
Statistical analysis
We employed a receiver operating characteristic (ROC) curve to evaluate the accuracy of HE4 in diagnosing MPE. The area under the curve (AUC) was used to measure the global diagnostic accuracy of HE4. Two methods were used to test the effects of age and sex on the accuracy of HE4. First, participants were categorized into subgroups according to their sex (male and female) and age (< 55 and ≥ 55 years old), and the AUCs of HE4 in each group were calculated. A threshold of 55 years of age was selected because a previous study revealed that HE4 increased with age, particularly among women ≥ 55 years20. We used Delong’s method to compare the AUCs21. Second, we resampled the participants with different upper age limits and calculated the AUC of HE4 in each resampled dataset. The principles of this statistical method have been described in our previous studies22,23,24. All the statistics and graphs were produced with R (version 4.3.2). A p-value of less than 0.05 was considered to indicate statistical significance.
Results
Characteristics of the participants
The Hohhot cohort comprised 66 patients with MPE and 86 patients with BPE, whereas the Changshu cohort comprised 26 patients with MPE and 32 patients with BPE. The participant selection process and essential characteristics of the participants in both cohorts were described in a previous study13.
Factors affecting the accuracy of HE4 for diagnosing MPE
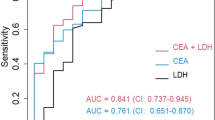
Figure 1A shows the results of the subgroup analyses. We failed to observe a significant difference in the AUC of HE4 according to sex (p > 0.05 for all). However, the AUCs of HE4 in younger patients (< 55 years old) were greater than those in older patients (≥ 55 years old) in the Hohhot cohort (p = 0.02) and the combined cohort (p < 0.001) (Fig. 1B).
In the next step, the participants were resampled using different upper age limits, and the AUCs of HE4 were calculated for each resampled dataset. Figure 2 shows the relationship between the AUCs and the upper age limits in each resampled dataset. The AUCs of HE4 decreased as the upper age limit increased in both cohorts.
Discussion
Our previous study revealed that HE4 has a moderate diagnostic accuracy for MPE13; however, the factors that can affect the accuracy of HE4 remain unclear. With two independent cohorts, this study revealed that sex did not affect the accuracy of HE4. However, we found that the accuracy of HE4 decreased as age increased. Therefore, we conclude that age should be considered when interpreting the accuracy of pleural HE4 for detecting MPE. To our knowledge, no study has analysed the effects of age and sex on the accuracy of pleural HE4 for diagnosing MPE.
This study used two methods to explore how age and sex affect the accuracy of HE4 diagnosis: subgroup analyses and resampling of participants with different upper age limits. Subgroup analyses are commonly used to investigate how certain factors impact the accuracy of a diagnostic test. However, these methods have limitations when analysing the accuracy of continuous data. The thresholds used to define subgroups can theoretically affect the results. One strength of this study is that we resampled the participants using different upper age limits and calculated the AUCs of each dataset for comparison. This method considers nearly all the possible thresholds and thus provides more theoretically reliable results than subgroup analyses. Both analytical methods revealed that older patients had a decreased AUC, suggesting that age negatively affects the accuracy of HE4. Another strength of this study was that we used two cohorts to assess the effects of age on the accuracy of HE4, making our findings reliable.
Other studies have reported that age can influence serum HE4 levels15,25,26. Our study revealed that HE4 has an AUC of approximately 0.75, whereas previous studies reported that the AUC of HE4 was > 0.80 for diagnosing MPE27,28,29. This inconsistency may be attributed to the median age of the participants in our study being > 70 years. Conversely, the average ages in previous studies ranged from 60 to 65 years. These findings also support our conclusion that HE4 has a low diagnostic accuracy for MPE in older patients. The adverse effects of age on the accuracy of HE4 are biologically plausible. Serum HE4 is affected by various factors, such as heart failure30, diabetes31, hypertension31, and renal impairment25. The prevalence of these disorders increases in older patients, thus impairing the diagnostic accuracy of HE4. Although sex does have an impact on serum HE4 concentrations in healthy individuals, the effects are minor15,32. Various disorders, such as cancer and inflammation, can significantly increase HE4 levels in body fluids33. Consequently, the complex pathogenesis of pleural effusion may outweigh the influence of sex on pleural HE4 levels.
Despite being the first study to investigate the confounding factors affecting the diagnostic value of HE4 for MPE, there were certain limitations. The small sample size is the first limitation, which may impair the precision of our results. Although there were two cohorts in our study and the findings were reproduced in both cohorts, the sample size of the Changshu cohort was small. Therefore, external cohorts are needed to validate our findings. The second limitation is that > 90% of the participants in our study had a normal renal function, hindering the study of renal-function impact. The third limitation is that the stored pleural fluid specimens were used to measure HE4 levels; however, the long-term stability of pleural HE4 remains unknown.
In summary, the diagnostic accuracy of pleural HE4 for MPE decreased in older patients, whereas sex had no significant impact. Therefore, age should be considered when using pleural fluid HE4 levels to diagnose MPE. Owing to the limitations of this study, further research with larger sample sizes is necessary to validate our findings.
Data availability
Data generated from this study are available from the corresponding author upon reasonable request.
References
Porcel, J. M., Esquerda, A., Vives, M. & Bielsa, S. Etiology of pleural effusions: analysis of more than 3,000 consecutive thoracenteses. Arch. Bronconeumol. 50, 161–165 (2014).
Zheng, W. Q. & Hu, Z. D. Pleural fluid biochemical analysis: the past, present and future. Clin. Chem. Lab. Med. 61, 921–934 (2023).
Bibby, A. C. et al. ERS/EACTS statement on the management of malignant pleural effusions. Eur. Respir J. 52, (2018).
Porcel, J. M. et al. Clinical features and survival of lung cancer patients with pleural effusions. Respirology 20, 654–659 (2015).
Quek, J. C., Tan, Q. L., Allen, J. C. & Anantham, D. Malignant pleural effusion survival prognostication in an Asian population. Respirology 25, 1283–1291 (2020).
Bibby, A. C. et al. ERS/EACTS statement on the management of malignant pleural effusions. Eur. J. Cardiothorac. Surg. 55, 116–132 (2019).
Kulandaisamy, P. C., Kulandaisamy, S., Kramer, D. & McGrath, C. Malignant pleural Effusions-A Review of Current guidelines and practices. J. Clin. Med. 10, 5535 (2021).
Porcel, J. M. et al. The diagnosis of pleural effusions. Expert Rev. Respir Med. 9, 801–815 (2015).
Roberts, M. E., Neville, E., Berrisford, R. G., Antunes, G. & Ali, N. J. Management of a malignant pleural effusion: British thoracic Society Pleural Disease Guideline 2010. Thorax 65 (Suppl 2), ii32–40 (2010).
Arnold, D. T. et al. Investigating unilateral pleural effusions: the role of cytology. Eur. Respir J. 52, 1801254 (2018).
Kassirian, S. et al. Diagnostic sensitivity of pleural fluid cytology in malignant pleural effusions: systematic review and meta-analysis. Thorax 52, 7959 (2022).
Wang, X. J. et al. Efficacy and safety of diagnostic thoracoscopy in undiagnosed pleural effusions. Respiration 90, 251–255 (2015).
Yang, Q. et al. Value of human epididymis secretory protein 4 in differentiating malignant from benign pleural effusion: an analysis of two cohorts. Ther. Adv. Respir Dis. 17, 17534666231216566 (2023).
Nah, E. H. et al. Establishment and validation of reference intervals for tumor markers (AFP, CEA, CA19-9, CA15-3, CA125, PSA, HE4, Cyfra 21 – 1, and ProGRP) in primary care centers in Korea: a cross-sectional retrospective study. Health Sci. Rep. 6, e1107 (2023).
Li, Y. et al. Age-stratified and gender-specific reference intervals of six tumor markers panel of lung cancer: a geographic-based multicenter study in China. J. Clin. Lab. Anal. 35, e23816 (2021).
Bossuyt, P. M. et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. Radiology 277, 826–832 (2015).
Han, Y. Q. et al. A study investigating markers in PLeural Effusion (SIMPLE): a prospective and double-blind diagnostic study. BMJ Open. 9, e027287 (2019).
Cha, S. N. et al. Pleural carbohydrate antigen 50 and malignant pleural effusion: a prospective, double-blind diagnostic accuracy test. Transl Lung Cancer Res. 13, 1061–1068 (2024).
Cao, X. S., Zheng, W. Q. & Hu, Z. D. Diagnostic value of soluble biomarkers for parapneumonic pleural effusion. Crit. Rev. Clin. Lab. Sci. 60, 233–247 (2023).
Urban, N. et al. Interpretation of single and serial measures of HE4 and CA125 in asymptomatic women at high risk for ovarian cancer. Cancer Epidemiol. Biomarkers Prev. 21, 2087–2094 (2012).
DeLong, E. R., DeLong, D. M. & Clarke-Pearson, D. L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44, 837–845 (1988).
Zhang, M. et al. Age affects the diagnostic accuracy of serum N-terminal pro-B-type natriuretic peptide for heart failure in patients with pleural effusion. Clin. Biochem. 114, 52–58 (2023).
Huang, J. H. et al. Age affects the diagnostic accuracy of the cancer ratio for malignant pleural effusion. BMC Pulm Med. 23, 198 (2023).
Zhao, W., Jiang, T. W., Zheng, W. Q. & Hu, Z. D. Diagnostic accuracy of adenosine deaminase for tuberculous pleural effusion: age does matter. Clin. Chem. Lab. Med. 62, e116–e118 (2024).
Lycke, M., Ulfenborg, B., Lauesgaard, M., Kristjansdottir, J., Sundfeldt, K. & B. & Consideration should be given to smoking, endometriosis, renal function (eGFR) and age when interpreting CA125 and HE4 in ovarian tumor diagnostics. Clin. Chem. Lab. Med. 59, 1954–1962 (2021).
Cheng, H. Y. et al. Age and menopausal status are important factors influencing the serum human epididymis secretory protein 4 level: a prospective cross-sectional study in healthy Chinese people. Chin. Med. J. (Engl). 133, 1285–1291 (2020).
Elsammak, M. Y. et al. Evaluation of pleural fluid human epididymis 4 (HE4) as a marker of malignant pleural effusion. Tumour Biol. 33, 1701–1707 (2012).
Lv, M., Wang, F., Wang, X. & Zhang, C. Diagnostic value of human epididymis protein 4 in malignant pleural effusion in lung cancer. Cancer Biomark. 26, 523–528 (2019).
Demirbas, S., Yerlikaya, F. H., Yosunkaya, S., Can, U. & Celalettin, K. The investigation of levels of endothelial cell-specific molecule, progranuline, clusterin, and human epididymis protein 4 in the differential diagnosis of malignant pleural effusions. Med. (Baltim). 101, e32471 (2022).
Piek, A. et al. HE4 serum levels are Associated with Heart failure severity in patients with Chronic Heart failure. J. Card Fail. 23, 12–19 (2017).
Ahmed, A. A. & Abdou, A. M. Diagnostic accuracy of CA125 and HE4 in ovarian carcinoma patients and the effect of confounders on their serum levels. Curr. Probl. Cancer. 43, 450–460 (2019).
Ferraro, S., Schiumarini, D. & Panteghini, M. Human epididymis protein 4: factors of variation. Clin. Chim. Acta. 438, 171–177 (2015).
Hertlein, L. et al. Human epididymis protein 4 (HE4) in benign and malignant diseases. Clin. Chem. Lab. Med. 50, 2181–2188 (2012).
Funding
This study was supported by the Zhiyuan Talent Project of Inner Mongolia Medical University, Shanxue Talent Project (ZY20242140). The funder played no role in the study design, data analysis, paper preparation, or submission.
Author information
Authors and Affiliations
Contributions
Qian Yang: Formal analysis; Investigation; Methodology; Software; Visualization; Writing-original draft.Yan Niu: Methodology; Validation; Writing-original draft.Jian-Xun Wen: Methodology; Validation; Writing-original draft.Qianghua Zhou: Methodology; Resources; Writing-review & editing.Dan-Ni Yang: Methodology; Validation; Writing-original draft.Hong-Zhe Zhu: Methodology; Software; Writing-original draft.Cheng Yan: Methodology; Validation; Writing-original draft.Su-Na Cha: Formal analysis; Methodology; Writing-original draft.Ting-Wang Jiang: Conceptualization; Investigation; Resources; Writing-review & editing.Li Yan: Formal analysis; Investigation; Resources; Writing-review & editing.Wen-Qi Zheng: Conceptualization; Methodology; Writing-review & editing.Jian-Xun Wen: Methodology; Formal analysis; Validation; Investigation; Writing-review & editing.Zhi-De Hu: Conceptualization; Data curation; Formal analysis; Funding acquisition; Methodology; Project administration; Resources; Supervision; Writing-review & editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yang, Q., Niu, Y., Zhou, Q. et al. Influences of age and sex on the diagnostic accuracy of human epididymis secretory protein 4 for malignant pleural effusion. Sci Rep 15, 3217 (2025). https://doi.org/10.1038/s41598-025-86929-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-86929-5