Abstract
Sepsis is a severe complication in patients with malignant tumors, leading to high mortality and increased need for intensive care. This study aimed to investigate the clinical characteristics and prognostic factors influencing sepsis outcomes in patients with malignant tumors. We included 4,858 patients with cancer diagnosed with sepsis between September 2019 and February 2020 whose data were collected from the Korean Sepsis Alliance, a nationwide multicenter cohort study. Cox regression analysis was used to identify predictors of 30-day and in-hospital mortality. In total, 65% of the patients survived, whereas 35% did not. Non-survivors were more likely to require intensive care, including mechanical ventilation and continuous renal replacement therapy. Key predictors of mortality included renal dysfunction, higher Sequential Organ Failure Assessment scores, and reliance on life-sustaining treatments. Non-survivors exhibited lower adherence to the implementation of sepsis care bundles, particularly to later-stage interventions. Gram-negative bacterial infections and multidrug resistance were more prevalent in non-survivors, complicating treatment efficacy. In conclusion, tailored treatment strategies that consider specific patient characteristics and disease dynamics are needed in managing sepsis with malignancy. Early identification and treatment of organ dysfunction, coupled with strict adherence to sepsis treatment protocols, are critical to improving survival in this population.
Similar content being viewed by others
Introduction
Sepsis is a life-threatening condition characterized by organ dysfunction resulting from a dysregulated immune response to infection. When associated with severe circulatory, cellular, and metabolic abnormalities, it escalates to septic shock, which carries a higher risk of mortality than sepsis alone1. Sepsis is associated with high mortality, regardless of the underlying infection or comorbidities, and is a leading cause of death worldwide2. Patients with malignant tumors are more likely to develop sepsis than the general population, and mortality rates for patients with sepsis and malignant tumors are predicted to be higher than those for patients with sepsis without malignant tumors3,4,5. Severely ill patients with sepsis and malignant tumors tend to have longer intensive care unit (ICU) and hospital stays than patients with sepsis without malignant tumors, requiring greater resource utilization6. ICU mortality and length of stay for patients with malignant tumors and sepsis have improved and evolved significantly over the past year but are still high compared to other patient populations7,8,9.
Several factors influence the increased incidence of sepsis and mortality in patients with malignant tumors. Neutropenia, caused by malignant infiltration suppressing the bone marrow or by the effects of chemotherapy on blood cell production, is a major indicator of weakened immunity in patients with cancer. This condition significantly increases their susceptibility to bacterial or fungal infections10,11. Additionally, neutrophils from patients with malignant tumors exhibit various functional defects in chemotaxis, phagocytosis, and bactericidal capacity12,13.
Several studies have examined various clinical and biological factors that influence the prognosis of sepsis in patients with malignant tumors. These factors include age, general physical condition, cancer activity, and biomarkers such as B-type natriuretic peptide, troponin I, neutrophil-to-lymphocyte ratio, and plasma interleukin-33 levels, which are predictive of sepsis outcomes9,14,15,16,17. Effective management of sepsis in these patients may include early recognition of potential sepsis and organ failure, rigorous microbiologic monitoring, regular testing to remove unnecessary invasive devices, and discussion of end-of-life care based on the patient’s needs.
Owing to the multifactorial nature of sepsis and the challenge of predicting different prognostic factors, a comprehensive analysis of various factors is essential. However, such analyses in Korean patients with sepsis are lacking. Therefore, this study aimed to investigate and identify the clinical characteristics of sepsis in patients with malignant tumors. We also aimed to explore prognostic factors that were significantly associated with outcomes in this group. By addressing these gaps, we hope to increase the predictive accuracy of sepsis outcomes and improve the strategic management of this vulnerable patient population.
Methods
Patients and data collection
A comprehensive analysis was performed using data from the Korean Sepsis Alliance, a nationwide prospective multicenter cohort study designed to evaluate the clinical characteristics, treatment modalities, and prognoses of patients with sepsis. Twenty tertiary or university-affiliated medical centers located in 10 of Korea’s 17 metropolitan cities and provinces actively participated in this study. Eligibility criteria included consecutive screening of all individuals who visited the emergency room or were admitted to hospital wards under the care of the rapid response team between September 2019 and December 2021. Individuals aged 19 years and older, diagnosed with cancer and clinically diagnosed with sepsis were included in the registry for further analysis. Malignancy was defined as either hematologic malignancies-including leukemia, lymphoma, and multiple myeloma-or solid tumors in organs or tissues such as breast, colorectal, lung, prostate, and skin. Eligibility was limited to patients diagnosed with cancer within the past five years, excluding those in complete remission (CR).
Sepsis was defined according to The Third International Consensus Definitions for Sepsis and Septic Shock1. Data collected included patient demographics, comorbidities, cancer treatment, initial vital signs, and initial laboratory tests. Additionally, data on treatment specifics such as administration of vasopressors, use of invasive mechanical ventilation, provision of renal replacement therapy, and use of extracorporeal membrane oxygenation were collected. Implementation of the 1-h, 3-h, and 6-h sepsis bundles was also assessed, with the 1-h bundle based on the 2018 update18, the 3-h bundle based on the 2015 revision, and the 6-h bundle based on the 2013 revision19.
We collected information on the suspected site of infection causing sepsis, the identified pathogen, and the appropriateness of initial empiric treatment in the first 24 h. For prognosis, we also included essential outcome measures such as ICU admission, length of stay, discharge status, and mortality. In this study, we assessed patients’ frailty using the Clinical Frailty Score (CFS) based on their pre-admission status20. The CFS is a tool that categorizes patients according to their physical robustness and ability to perform daily activities, with scores ranging from 1 (very fit) to 9 (terminally ill), with each level representing a gradation of dependency and health status.
“Treatment status” was defined as being “on treatment” and included patients actively receiving chemotherapy and/or radiotherapy. Patients were eligible if they had undergone chemotherapy, radiotherapy, or steroid therapy with prednisolone at a dose of 0.3 mg/kg within the past six months. Additionally, those with immunosuppression due to malnutrition, metastatic cancer, or hematologic malignancies were included. However, individuals with human immunodeficiency virus infection were excluded from the study.
The study was approved by the institutional review boards of each hospital, including the Institutional Review Board of Chungnam National University Hospital (2019–11-048). The Institutional Review Board of Chungnam National University Hospital waived the requirement for informed consent as this was a standard care observational study with minimal risk and no intervention. All methods used in this study complied with relevant guidelines and regulations.
Statistical analyses
Patient characteristics and clinical outcomes were compared based on their survival status. Categorical variables are presented as percentages and continuous variables are presented as means and standard deviations or medians and interquartile ranges. Analyses were performed using the survival status of patients with cancer and sepsis to examine differences between survivors and non-survivors using chi-squared t-tests and Welch’s t-test. Univariate and multivariate logistic regression was conducted to investigate the associations between mortality and variable factors on the survival of the patients. All covariates included in the multivariate analysis were selected based on their clinical relevance and statistically significant association with mortality in the univariate analyses. Statistical significance was set at p-values < 0.1 for univariate analysis and < 0.05 for multivariate analysis. All analyses were performed using SPSS version 22.0 (IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
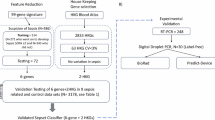
A total of 11,981 patients were diagnosed with sepsis, of which 4,858 (40.5%) were eligible for the study after excluding 7,123 patients who did not have solid tumors or hematologic malignancies. Of these, 3,158 (65%) survived and 1,700 (35.0%) did not (Fig. 1).
Table 1 presents the characteristics of the patients according to their survival status. The mean age of the patients was 68.4 ± 12.5 years, and 3,077 (63.3%) were male. The Clinical Frailty Scale was 5.0 ± 2.2 points, with 4.7 ± 2.2 points for survivors and 5.6 ± 2.1 points for non-survivors (p < 0.001). Eastern Cooperative Oncology Group (ECOG) performance status scores (2.0 vs. 3.0) and Sequential Organ Failure Assessment (SOFA) scores (5.7 ± 2.7 vs. 7.5 ± 3.4) were also lower in the survivor group than in the non-survivor group (p < 0.001). The most common comorbidity was diabetes mellitus, affecting 1,484 (30.5%) patients. Regarding the site of sepsis, lung and abdominal infections were almost equally prevalent; however, abdominal infections were most prevalent in the survivor group at 39.3% (p < 0.001), and lung infections were most prevalent in the non-survivor group at 44.5% (p < 0.001). Infections of unknown primary sites were observed in 719 patients: 13.6% in the survivor group and 17.0% in the non-survivor group (p = 0.002).
Table 2 presents the patients’ vital signs and laboratory findings. Vital signs indicated lower mean blood pressure, faster heart rate, respiratory rate, and lower body temperature in the non-survivor group compared with the survivor group. Initial laboratory results revealed higher levels of white blood cells (WBC), total bilirubin, creatinine, C-reactive protein (CRP), and lactate, and lower levels of hemoglobin, platelets, and albumin in the non-survivor group compared with the survivor group.
Patient pathogen types
Among the pathogens (Table 3), bacteria were the most common (53.3% of the total), with gram-negative bacteria (42.7%) being significantly more common than gram-positive bacteria (17.0%). Gram-positive bacteria were more common in the non-survivor group than in the survivor group (19.4% vs. 15.7%, p = 0.001), and gram-negative bacteria were less common (40.0% vs. 44.2%, p = 0.005). The proportion of multidrug-resistant organisms was 22.4%, and the proportion receiving appropriate antibiotic therapy within 24 h was 89.2% in the survivor group and 84.2% in the non-survivor group (p < 0.001).
Patient prognoses and interventions
Table 4 presents the prognoses and interventions of the patients. Interventions and outcomes differed significantly between these groups. Vasopressors were used in 29.3% of patients, with non-survivors being significantly more likely to receive this treatment than survivors (37.8% vs. 24.7%; p < 0.001). Additionally, a higher percentage of non-survivors required ICU admission than survivors (40.7% vs. 28.7%; p < 0.001). Invasive mechanical ventilation was used in 55.5% of patients admitted to the ICU, with a predominant use in non-survivors compared with survivors (72.5% vs. 42.6%; p < 0.001). Continuous renal replacement therapy (CRRT) was also more frequently required in non-survivors than in survivors (49.3% vs. 15.7%; p < 0.001). Significant differences in outcomes were also observed. The ICU mortality rate was 64.9% among non-survivors. The median length of hospital stay was longer for survivors at 16.0 days compared to 11.0 days for non-survivors (p < 0.001). Additionally, 90.2% of non-survivors had issues related to life-sustaining treatment, significantly higher than the 23.2% of survivors (p < 0.001).
Cox regression analysis: 30-day mortality
Table 5 shows the factors associated with 30-day mortality. In the univariate Cox regression analysis, several factors were significantly associated with 30-day mortality, including age, cancer treatment, Charlson Comorbidity Index, Clinical Frailty Scale, ECOG score, SOFA score, pulmonary infection, abdominal infection, urinary tract infection, systemic infection without clear primary site of infection, WBC count, total bilirubin, albumin, creatinine, CRP, lactate, use of mechanical ventilation, use of CRRT, completion of sepsis 1-h bundle, and completion of sepsis 6-h bundle. In the multivariate analysis, the Charlson Comorbidity Index (odds ratio [OR] 1.034, 95% confidence interval [CI], 1.006–1.064, p = 0.018), clinical frailty scale (OR 1.114, 95% CI 1.071–1.159, p < 0.001), SOFA score (OR 1.048, 95% CI 1.021–1.075, p < 0.001), pulmonary infection (OR 1.833, 95% CI 1.535–2.189, p < 0.001), systemic infection without clear primary site (OR 1.381, 95% CI 1.073–1.776, p = 0.012), low albumin levels (OR 0.791, 95% CI 0.693–0.904, p = 0.001), high lactate levels (OR 1.088, 95% CI 1.067–1.109, p < 0.001), CRRT use(OR 1.900, 95% CI 1.597–2.259, p < 0.001), completion of the 1-h sepsis bundle (OR 0.735, 95% CI 0.594–0.908, p = 0.004) and the 6-h bundle (OR 1.456, 95% CI 1.148–1.847, p = 0.002) were significantly associated with 30-day mortality.
Cox regression analysis: in-hospital mortality
Table 6 shows the factors associated with in-hospital mortality. In the univariate Cox regression analysis, age, cancer treatment, Charlson Comorbidity Index, clinical frailty scale, ECOG, SOFA score, pulmonary infection, abdominal infection, urinary tract infection, WBC count, hemoglobin, total bilirubin, albumin, creatinine, and lactate levels, use of mechanical ventilation, use of CRRT, life-sustaining treatment issues, and completion of the 1-h sepsis bundle were associated with in-hospital mortality. In the multivariate analysis, age (OR 1.010, 95% CI 1.004–1.016, p = 0.001), clinical frailty scale (OR 1.056, 95% CI 1.020–1.094, p = 0.002), SOFA score (OR 1.044, 95% CI 1.020–1.069, p < 0.001), abdominal infection (OR 0.793, 95% CI 0.655–0.960, p = 0. 017), high lactate levels (OR 1.071, 95% CI 1.051–1.092, p < 0.001), use of CRRT (OR 1.332, 95% CI 1.141–1.555, p < 0.001), and life-sustaining treatment issues (OR 8.402, 95% CI 6.762–10.496, p < 0.001) were significantly associated with in-hospital mortality.
Discussion
This study comprehensively evaluated the clinical characteristics and prognostic factors associated with sepsis in patients with malignancy and revealed significant differences between survivors and non-survivors. Non-survivors were more likely to require aggressive interventions such as vasopressors, invasive mechanical ventilation, and CRRT, which was associated with higher ICU admission rates and mortality. The presence of comorbidities, as measured by the Charlson Comorbidity Index, Clinical Frailty Scale, and SOFA scores, strongly predicted 30-day and in-hospital mortality, indicating that a higher initial clinical and physiological burden significantly affects survival outcomes. Additionally, the type of infection, particularly pulmonary and systemic infections without an apparent primary site, was associated with poorer prognosis, highlighting the need for targeted and timely management strategies to improve patient survival.
In the baseline characteristics of the cohort, there were no differences in age or body mass index between survivors and non-survivors, but non-survivors included a higher percentage of men, consistent with studies indicating that men may have a disadvantage due to cell-mediated immune responses and reduced cardiovascular function, which can increase sepsis susceptibility.21,22. Indicators of a patient’s systemic condition and mobility, such as the Charlson Comorbidity Index, Clinical Frailty Scale, and ECOG status, were also higher in non-survivors, which have been reported to be highly relevant indicators in cancer and sepsis23,24. Conditions such as chronic kidney disease (CKD), liver disease, and diabetes mellitus are more prevalent in patients with sepsis and are associated with worse outcomes; however, in our study group, only CKD exhibited a significant difference25. Respiratory, abdominal, and urinary tract infections are the most common causes of sepsis26. Several studies have discussed their prognosis, but the results vary27,28. In this study, pulmonary infections had a worse prognosis, and infections of unknown cause were more common in non-survivors owing to the challenge of targeting the appropriate antibiotics.
Patients’ vital signs and lab results showed significant differences between survivors and non-survivors of sepsis. Specifically, non-survivors had significantly lower mean blood pressures, higher heart and respiratory rates, and elevated body temperatures compared with survivors. These findings are consistent with previous studies29,30 that have reported differences in early vital signs as predictors of mortality in patients with sepsis. Laboratory values such as WBC counts, hemoglobin, platelets, creatinine, inflammatory markers, such as CRP, and lactate levels also exhibited significant differences between the two groups, with non-survivors having worse profiles. This pattern is consistent with findings from other studies indicating that high levels of lactate31 and CRP32 are associated with an increased risk of death in patients with sepsis, underscoring the importance of these biomarkers in assessing sepsis severity. Additionally, our findings regarding thrombocytopenia are consistent with previous research33, indicating that while sepsis can trigger thrombocytopenia, thrombocytopenia can also exacerbate sepsis and contribute to worse outcomes. These findings emphasize the importance of early identification of these markers to guide timely, intensive management and improve survival.
Previous epidemiologic data on sepsis have revealed that Gram-negative bacteria are the predominant causative agents; however, the prevalence of Gram-positive bacteria has increased over time. Currently, the proportions of these two groups of bacteria are approximately equal or marginally different34,35. This study of patients with malignancy revealed a significantly higher proportion of Gram-negative bacteria, highlighting a distinct microbial pattern in this subgroup. Additionally, multidrug resistance was very high at 22.4%, with specific resistance observed in strains such as Pseudomonas aeruginosa36 and various enterobacteria37. These resistance patterns can lead to suboptimal outcomes when standard antibiotic therapy is administered after the initial diagnosis of sepsis. Furthermore, patients with malignancy have different strain dynamics and higher rates of drug resistance compared with the general sepsis population38. These findings suggest that antimicrobial coverage in patients with malignancy and sepsis should be carefully considered to identify specific resistance patterns prevalent in this population and to ensure timely and targeted antimicrobial use. These findings emphasize the need for precise antimicrobial coverage to address resistance and optimize outcomes in this population, highlighting the importance of monitoring and adjusting treatments based on prevalent microbial trends.
Intervention strategies in the study population reflected disease severity and organ dysfunction, with significant differences in treatment approaches between groups. Non-survivors required more aggressive treatments such as mechanical ventilation, use of vasopressors, and CRRT, indicative of disease severity and organ failure. This finding aligns with other studies revealing that these intensive treatment measures are more often required in patients with poor prognostic indicators39,40. Additionally, issues with life-sustaining treatments were significantly more common among non-survivors, suggesting a complex interaction between patient prognosis and end-of-life decisions, a phenomenon observed in other studies. Although there is growing interest in LST and do-not-resuscitate orders in Korea, many patients continue to receive invasive treatments toward the end of life. In Korean society, the timing of LST decisions is often late, often at the terminal stage, as many families feel that pursuing all possible treatments honors cultural values such as familial devotion and respect for older population41,42. This societal tendency may delay end-of-life discussions, sometimes leading to potentially unnecessary invasive interventions. In addition, adherence to the sepsis bundle was variable, with no significant difference observed between survivors and non-survivors for the 1-h and 3-h bundles. However, adherence to the 6-h sepsis bundle differed, suggesting a potential gap in the ongoing management of sepsis over time. These differences highlight the need for aggressive application of sepsis treatment protocols to improve outcomes, as supported by other papers43,44,45, indicating that strict adherence to the recommended sepsis bundle improves survival in various healthcare settings.
This study used a Cox regression analysis to identify predictors associated with 30-day mortality and in-hospital mortality in patients with cancer and sepsis. Consistent with previous studies of sepsis in patients with cancer, particularly those with hematologic malignancies or poor performance status as measured by ECOG scores, this study highlights the importance of underlying disease status in patient prognosis16,46. We could not distinguish between hematologic and solid tumors owing to data limitations. Furthermore, the retrospective nature of the study and constraints in data collection prevented us from accounting for key variables such as the type of cancer treatment, time since the last treatment, specific type of solid tumor, and stage of disease. Despite these limitations, the mortality analysis identified key factors such as the Charlson Comorbidity Index and clinical frailty scale as valuable predictors of outcomes47,48. Organ dysfunction, particularly renal dysfunction, was also a significant factor in mortality, aligning with recent studies emphasizing renal dysfunction as a prognostic factor for sepsis-related mortality49,50,51. However, liver dysfunction and poor cardiac function, as indicated by total bilirubin levels and clinical symptoms, respectively, did not significantly affect mortality in our cohort, consistent with findings from other studies16,52. These findings contrast with some reports in which such dysfunctions were associated with higher mortality in patients with sepsis. This variability may be due to differences in patient characteristics or definitions of organ dysfunction between studies. Thus, context is crucial in interpreting the prognostic value of organ dysfunction in sepsis. Additionally, the SOFA score was significantly associated with patient outcomes, supporting previous studies that have identified it as a reliable predictor of sepsis severity and mortality in various patient populations, including those with cancer53,54. Age is often considered an important determinant of sepsis prognosis53,55; however, in this study, it had a significant effect on in-hospital mortality but not on 30-day mortality. This discrepancy may be because certain systemic diseases associated with cancer alter the effect of age on sepsis outcomes. Furthermore, the strong correlation between the issues of life-sustaining treatment and in-hospital mortality, which is common in similar studies56,57, underscores the need for timely medical intervention, rapid response, and appropriate management strategies to improve survival in vulnerable populations.
However, this study has several limitations. First, the retrospective design inherently introduces potential biases and missing data that could affect the results and their interpretation. Although data collection was performed by experienced investigators and supplemented by the coordinating center, some data may not have been fully verified because it relied on institutional management practices. Second, the study population consisted exclusively of patients with solid tumors or hematologic malignancies. While this focused approach provides valuable insights into a specific subgroup, it limits the generalizability of the findings to a broader sepsis population. Third, the reliance on existing medical records introduced variability in data quality and completeness across participating centers. This heterogeneity may have affected the robustness and consistency of the analysis. Despite efforts by experienced investigators to standardize data collection and ensure accuracy, these limitations remain inherent in the study design. Fourth, although the Cox regression analysis identified important predictors of mortality, the study could not account for all relevant variables or potential confounders. Critical cancer-related variables-such as cancer type, disease stage, specific treatment regimens, and time since last treatment-were not collected at enrollment. These factors58 are important confounders that could have influenced the study results and limited the scope of the analysis. Finally, the observational design of the study prevents causality from being established between the identified factors and mortality, and caution should be exercised in drawing definitive conclusions regarding cause-and-effect relationships. Further prospective studies with detailed cancer-related variables and a broader sepsis population are warranted to validate these associations and explore underlying causal mechanisms.
Conclusions
Our study identified key survival determinants in patients with malignancy and sepsis, including the higher prevalence of pulmonary infections, the dominance of Gram-negative bacteria, and the critical role of frailty and SOFA scores. These findings highlight the need for early detection and tailored treatment strategies to improve outcomes in this vulnerable population. The presence of multidrug-resistant infections further necessitates the development of personalized sepsis management protocols tailored to the specific physiological and therapeutic needs of patients with cancer. Implementing evidence-based interventions that focus on early detection and individualized care can significantly improve survival and quality of life for these patients.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Singer, M. et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315(8), 801–810 (2016).
Keegan, J. & Wira, C. R. 3rd. Early identification and management of patients with severe sepsis and septic shock in the emergency department. Emerg. Med. Clin. N. Am. 32(4), 759–776 (2014).
Williams, J. C., Ford, M. L. & Coopersmith, C. M. Cancer and sepsis. Clin. Sci. (Lond) 137(11), 881–893 (2023).
Azoulay, E. et al. Outcomes of critically ill patients with hematologic malignancies: Prospective multicenter data from France and Belgium–a groupe de recherche respiratoire en réanimation onco-hématologique study. J. Clin. Oncol. 31(22), 2810–2818 (2013).
Liu, Z., Mahale, P. & Engels, E. A. Sepsis and risk of cancer among elderly adults in the United States. Clin. Infect. Dis. 68(5), 717–724 (2019).
Williams, M. D. et al. Hospitalized cancer patients with severe sepsis: analysis of incidence, mortality, and associated costs of care. Crit. Care (London, England) 8(5), R291-298 (2004).
Larché, J. et al. Improved survival of critically ill cancer patients with septic shock. Intensive Care Med. 29(10), 1688–1695 (2003).
Camou, F. et al. Long-term prognosis of septic shock in cancer patients. Support Care Cancer 28(3), 1325–1333 (2020).
Pène, F. et al. Temporal changes in management and outcome of septic shock in patients with malignancies in the intensive care unit. Crit. Care Med. 36(3), 690–696 (2008).
Safdar, A. & Armstrong, D. Infections in patients with hematologic neoplasms and hematopoietic stem cell transplantation: neutropenia, humoral, and splenic defects. Clin. Infect. Dis. 53(8), 798–806 (2011).
Seo, S. K., Liu, C. & Dadwal, S. S. Infectious disease complications in patients with cancer. Crit. Care Clin. 37(1), 69–84 (2021).
Staudinger, T. & Pène, F. Current insights into severe sepsis in cancer patients. Revista Brasileira de terapia intensiva 26(4), 335–338 (2014).
Lortholary, O. et al. Epidemiological trends in invasive aspergillosis in France: the SAIF network (2005–2007). Clin. Microbiol. Infect. 17(12), 1882–1889 (2011).
Yang, Y., Leng, J., Tian, X., Wang, H. & Hao, C. Brain natriuretic peptide and cardiac troponin I for prediction of the prognosis in cancer patients with sepsis. BMC Anesthesiol. 21(1), 159 (2021).
Soares, M. et al. Characteristics and outcomes of patients with cancer requiring admission to intensive care units: A prospective multicenter study. Crit. Care Med. 38(1), 9–15 (2010).
Rosolem, M. M. et al. Critically ill patients with cancer and sepsis: clinical course and prognostic factors. J. Crit. Care 27(3), 301–307 (2012).
Yang, Y. et al. Development and validation of a nomogram for predicting the prognosis in cancer patients with sepsis. Cancer Med. 11(12), 2345–2355 (2022).
Levy, M. M., Evans, L. E. & Rhodes, A. The surviving sepsis campaign bundle: 2018 update. Crit. Care Med. 46(6), 997–1000 (2018).
Barochia, A. V., Cui, X. & Eichacker, P. Q. The surviving sepsis campaign’s revised sepsis bundles. Curr. Infect. Dis. Rep. 15(5), 385–393 (2013).
Lee, H. Y. et al. Preexisting clinical frailty is associated with worse clinical outcomes in patients with sepsis. Crit. Care Med. 50(5), 780–790 (2022).
Kempker, J. A. & Martin, G. S. The changing epidemiology and definitions of sepsis. Clin. Chest Med. 37(2), 165–179 (2016).
Angele, M. K., Pratschke, S., Hubbard, W. J. & Chaudry, I. H. Gender differences in sepsis: cardiovascular and immunological aspects. Virulence 5(1), 12–19 (2014).
Welford, J. et al. The Clinical Frailty Scale can indicate prognosis and care requirements on discharge in oncology and haemato-oncology inpatients: A cohort study. Eur. J. Cancer Care (Engl) 31(6), e13752 (2022).
Jouffroy, R. et al. Relationship between prehospital modified Charlson Comorbidity Index and septic shock 30-day mortality. Am. J. Emerg. Med. 60, 128–133 (2022).
Guidet, B., Aegerter, P., Gauzit, R., Meshaka, P. & Dreyfuss, D. Incidence and impact of organ dysfunctions associated with sepsis. Chest 127(3), 942–951 (2005).
Lagunes, L., Encina, B. & Ramirez-Estrada, S. Current understanding in source control management in septic shock patients: A review. Ann. Transl. Med. 4(17), 330 (2016).
Wu, K. S. et al. Factors associated with outcomes of septic shock patients receiving high dose noradrenaline according to three primary infection sites. J. Int. Med. Res. 48(2), 300060519874545 (2020).
Volakli, E. et al. Infections of respiratory or abdominal origin in ICU patients: what are the differences?. Crit. Care (London, England) 14(2), R32 (2010).
Candel, B. G. et al. The association between vital signs and clinical outcomes in emergency department patients of different age categories. Emerg. Med. J. 39(12), 903–911 (2022).
Asiimwe, S. B., Abdallah, A. & Ssekitoleko, R. A simple prognostic index based on admission vital signs data among patients with sepsis in a resource-limited setting. Crit. Care (London, England) 19(1), 86 (2015).
Liu, Z. et al. Prognostic accuracy of the serum lactate level, the SOFA score and the qSOFA score for mortality among adults with Sepsis. Scand. J. Trauma Resuscitation Emerg. Med. 27(1), 51 (2019).
Qu, R. et al. C-reactive protein concentration as a risk predictor of mortality in intensive care unit: A multicenter, prospective, observational study. BMC Anesthesiol. 20(1), 292 (2020).
Vardon-Bounes, F. et al. Platelets are critical key players in sepsis. Int. J. Mol. Sci. 20(14), 1 (2019).
Martin, G. S., Mannino, D. M., Eaton, S. & Moss, M. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 348(16), 1546–1554 (2003).
Mayr, F. B., Yende, S. & Angus, D. C. Epidemiology of severe sepsis. Virulence 5(1), 4–11 (2014).
Cattaneo, C. et al. P. aeruginosa bloodstream infections among hematological patients: An old or new question?. Ann. Hematol. 91(8), 1299–1304 (2012).
Satlin, M. J. et al. Bacteremia due to carbapenem-resistant Enterobacteriaceae in neutropenic patients with hematologic malignancies. J. Infect. 73(4), 336–345 (2016).
Martinez-Nadal, G. et al. Inappropriate empirical antibiotic treatment in high-risk neutropenic patients with bacteremia in the era of multidrug resistance. Clin. Infect. Dis. 70(6), 1068–1074 (2020).
Wang, D. H. et al. Attributable mortality of ARDS among critically ill patients with sepsis: A multicenter, retrospective cohort study. BMC Pulm. Med. 24(1), 110 (2024).
Uusalo, P., Hellman, T. & Järvisalo, M. J. Mortality and associated risk factors in perioperative acute kidney injury treated with continuous renal replacement therapy. Perioper. Med. (Lond) 10(1), 57 (2021).
Kim, S. & Tak, S. H. Family members’ knowledge and attitude toward life-sustaining treatment decisions for patients in the intensive care unit. J. Hosp. Palliat. Nurs. 23(3), 256–263 (2021).
Park, S. Y., Lee, B., Seon, J. Y. & Oh, I. H. A national study of life-sustaining treatments in South Korea: What factors affect decision-making?. Cancer Res. Treat 53(2), 593–600 (2021).
Memon, J. I. et al. Impact of 6-hour sepsis resuscitation bundle compliance on hospital mortality in a Saudi hospital. Crit. Care Res. Pract. 2012, 273268 (2012).
Gao, F., Melody, T., Daniels, D. F., Giles, S. & Fox, S. The impact of compliance with 6-hour and 24-hour sepsis bundles on hospital mortality in patients with severe sepsis: a prospective observational study. Crit. Care (London, England) 9(6), R764-770 (2005).
Levy, M. M. et al. Mortality changes associated with mandated public reporting for sepsis: The results of the New York state initiative. Am. J. Respir. Crit. Care Med. 198(11), 1406–1412 (2018).
Torres, V. B. et al. Sepsis-associated outcomes in critically ill patients with malignancies. Ann. Am. Thorac. Soc. 12(8), 1185–1192 (2015).
Munshi, L. et al. Long-term survival and functional outcomes of critically ill patients with hematologic malignancies: A Canadian multicenter prospective study. Intensive Care Med. 50(4), 561–572 (2024).
Asdahl, P. H., Christensen, S., Kjærsgaard, A., Christiansen, C. F. & Kamper, P. One-year mortality among non-surgical patients with hematological malignancies admitted to the intensive care unit: A Danish nationwide population-based cohort study. Intensive Care Med. 46(4), 756–765 (2020).
Kim, D. W. et al. Hospital mortality and prognostic factors in critically ill patients with acute kidney injury and cancer undergoing continuous renal replacement therapy. Kidney Res. Clin. Pract. 41(6), 717–729 (2022).
Can, W., Rong, L. & Lixia, L. Incidence and risk factors of acute kidney injury in patients with malignant tumors: A systematic review and meta-analysis. BMC Cancer 23(1), 1123 (2023).
Bou Chebl, R. et al. Sepsis in patients with haematological versus solid cancer: A retrospective cohort study. BMJ Open 11(2), e038349 (2021).
Nesseler, N. et al. Clinical review: The liver in sepsis. Crit. Care (London, England) 16(5), 235 (2012).
MacPhail, A. et al. Sepsis mortality among patients with haematological malignancy admitted to intensive care 2000–2022: A binational cohort study. Crit. Care (London, England) 28(1), 148 (2024).
Gudiol, C., Albasanz-Puig, A., Cuervo, G. & Carratalà, J. Understanding and managing sepsis in patients with cancer in the era of antimicrobial resistance. Front. Med. (Lausanne) 8, 636547 (2021).
Martin-Loeches, I. et al. Risk factors for mortality in elderly and very elderly critically ill patients with sepsis: A prospective, observational, multicenter cohort study. Ann. Intensive Care 9(1), 26 (2019).
Chang, Y. C. et al. Effect of do-not-resuscitate orders on patients with sepsis in the medical intensive care unit: A retrospective, observational and propensity score-matched study in a tertiary referral hospital in Taiwan. BMJ Open 9(6), e029041 (2019).
Huang, C. T., Chuang, Y. C., Tsai, Y. J., Ko, W. J. & Yu, C. J. High mortality in severe sepsis and septic shock patients with do-not-resuscitate orders in East Asia. PloS One 11(7), e0159501 (2016).
Nates, J. L. et al. Septic shock in the immunocompromised cancer patient: A narrative review. Crit. Care (London, England) 28(1), 285 (2024).
Acknowledgements
We thank the Korean Sepsis Alliance (KSA) for their contribution to data collection and study design. The full list of members can be found at the end of the manuscript under ‘Consortium’.
Funding
This work was supported by the Research Program funded by the Korea Disease Control and Prevention Agency (fund codes 2019E280500, 2020E280700, and 2021-10-026) and the Korean Sepsis Alliance (KSA) affiliated with the Korean Society of Critical Care Medicine (KSCCM).
Author information
Authors and Affiliations
Consortia
Contributions
GRH, HKJ and SIL contributed to conception and design; All authors contributed to data analysis and interpretation; GRH, HKJ and SIL contributed to drafting the manuscript for intellectual content; GRH, HKJ and SIL contributed to revision of the manuscript. All the authors have read and approved the final manuscript. The Korean Sepsis Alliance (KSA) contributed to data collection, study design. The consortium was represented by Chae-Man Lim (Chair).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hong, G., Ju, H., Oh, D.K. et al. Clinical characteristics and prognostic factors of sepsis in patients with malignancy. Sci Rep 15, 7078 (2025). https://doi.org/10.1038/s41598-025-87457-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-87457-y