Abstract
We aimed to assess whether patients with rheumatoid arthritis (RA) have a higher risk of developing acute and chronic pancreatitis compared to individuals without RA. We identified 54,910 individuals with RA between 2010 and 2017. After exclusion, they were matched in a 1:3 ratio based on age and gender to control population without RA. Cox regression analyses were performed to estimate hazard ratio. During a median follow-up of 5.5 years, 0.18% of the patients with RA and 0.14% of the matched control developed acute pancreatitis. The risk acute pancreatitis was higher in the RA cohort compared to matched control (adjusted hazard ratio [aHR] 1.33; 95% confidence interval [CI] 1.02–1.74). In the case of chronic pancreatitis, 0.11% of patients with RA and 0.09% of the matched control developed chronic pancreatitis. Patients with RA appear to have a marginally elevated risk of chronic pancreatitis compared to matched controls (aHR 1.25, 95% CI 0.90–1.74), though this increase did not achieve statistical significance. The risk of acute pancreatitis is slightly higher in individuals with RA than in matched controls. The risk of chronic pancreatitis did not show statistical significance, but it tended to increase marginally in patients with RA.
Similar content being viewed by others
Introduction
Rheumatoid arthritis (RA) is an autoimmune disorder marked by widespread inflammation throughout the body, affecting both the joints and various extra-articular regions. Numerous studies demonstrated its association with cardiovascular disease1, stroke2, Parkinson disease3, and interstitial lung disease4.
Acute or chronic pancreatitis also involves inflammation as its main pathophysiological mechanism, and it is plausible that RA may increase the risk of these diseases. Apart from several earlier case reports5,6,7, only a few studies examined the association between RA and acute or chronic pancreatitis (Supplementary Table 1). A population-based cohort study conducted in Taiwan indicated that patients with RA have an increased risk of acute pancreatitis (adjusted hazard ratio [aHR] 1.62, 95% CI 1.43–1.83). However, that study specifically focused on the occurrence of acute pancreatitis, and the occurrence of pancreatitis based on serological status of RA was not examined8. Another cross-sectional study conducted in the US suggested that RA may be an independent risk factor for pancreatic manifestations including acute pancreatitis (OR 2.41, 95% CI 2.41–2.60) and chronic pancreatitis (OR 2.97, 95% CI 2.70–3.26). This study benefited from its large sample size, including over 500,000 Western individuals. However, considering the cross-sectional nature of the database, establishing a timeline and the duration of treatment exposure is not possible. Moreover, there may be limitations in diagnostic accuracy since it was evaluated using diagnosis codes from electronic medical records9. Ultimately, there a few long-term studies observing the occurrence of pancreatitis, encompassing both acute and chronic forms, in relation to RA when tracked from the time of RA diagnosis.
In the present study, we investigated the association between RA and subsequent pancreatitis risk including both acute and chronic form using a nation-wide population-based cohort in South Korea, excluding well-known risk factors of pancreatitis, adjusting for underlying diseases or behavioral factors that, to the best of our knowledge, were not considered in previous studies.
Methods
Data source and study setting
In this cohort study, we used data from the National Health Insurance Service (NHIS), which is a single insurer managed by the Korean government and provides a mandatory social insurance program that covers 97% of the Korean population. In addition, administration for the remaining 3% (the Medical Aid population, with the lowest income) are also managed by NHIS. Therefore, the NHIS has information on the whole Korean population and their demographic characteristics, health care use, and diagnostic codes from the International Classification of Diseases, Tenth Revision (ICD-10), medical treatment information.
Additionally, the NHIS provides free biennial national health examination programs for the public, and adults are recommended to undergo a check-up at least once every 2 years10. This health screening program data comprise anthropometric measurements, self-administered questionnaires on health behavior, which were developed for national health screening purposes, and laboratory results. All these resources retained by the NHIS have been utilized to create cohort data for various epidemiologic studies, including investigations of disease risk in patients with RA3,11,12,13. This study was conducted according to the Declaration of Helsinki and was approved by the Institutional Review Board (IRB) of Samsung Medical Center (#SMC 2024-07-196). The IRB has approved a waiver of the requirement to obtain informed consent because the NHIS database is available to researchers and is supplied as anonymized data.
Study population
The current study included patients newly diagnosed with RA between 2010 and 2017 who are 40 years or older. Seropositive rheumatoid arthritis (SPRA) patients were defined by the following criteria: (1) had a registered diagnostic code for RA (ICD-10 M05), (2) received a prescription for any disease-modifying anti-rheumatic drugs (DMARDs) for 270 days or more, and (3) were enrolled in the rare and intractable disease (RID) program. The RID program in Korea offers substantial reductions in copayment for various rare and intractable diseases (5% of copayment vs. typical 30% for other common diseases), including RA. Enrollment in this program as RA requires official certification of diagnosis by a physician, typically a rheumatologist, which is issued when a patient fulfilled 4 or more American College of Rheumatology criteria with positive results from rheumatoid factor or anti-cyclic citrullinated peptide antibody. Seronegative rheumatoid arthritis (SNRA) patients were defined by the following criteria: (1) had a registered diagnostic code for RA (ICD-10 M06 except M06.1 and M06.4), and (2) received a prescription for any DMARDs for 270 days14. The date of RID program registration for SPRA and the first administration of RA ICD-10 code for SNRA were considered as the index date. This operational definition was considered highly valid15 and was repeatedly used in many epidemiological studies conducted in Korea16.
The study enrolled 92,336 individuals diagnosed with RA between 2010 and 2017, including 66,493 with SPRA and 25,840 with SNRA. Of the 92,336 identified cases of RA, we selected 54,910 patients who had undergone a national health screening within 2 years prior to RA diagnosis to obtain covariate information. This allowed us to gather medical and health behavior information from the health screening results. After that, we excluded heavy drinkers (n = 1,577), a common risk factor for both acute and chronic pancreatitis, and those whose records were missing any information (n = 1,925). After these exclusions, a total of 51,318 patients with RA remained (Fig. 1).
Subsequently, we applied different exclusion criteria considering the risk factors associated with each outcome, resulting in the final study populations being defined differently. Specifically, for the analyses of the risk of acute pancreatitis, we excluded 1) individuals who had triglyceride (TG) levels exceeding 400 mg/dl (n = 312), those with a history of chronic pancreatitis (n = 572), those with a history of prior gall stones (n = 3,239), those who had previously been diagnosis with acute pancreatitis before RA diagnosis (n = 1,646), and those who were diagnosed with acute pancreatitis within 1 year after the index date (called 1-year lag period) (n = 382). After these exclusions, patients with RA and individuals without RA were matched (1:3) to RA cases based on age, sex, and index date.
For the analyses of chronic pancreatitis, we excluded individuals with a history of prior acute pancreatitis (n = 2,286), those who had previously been diagnosis with chronic pancreatitis before RA diagnosis (n = 411), and those who were diagnosed with chronic pancreatitis within 1 year after the index date (called 1-year lag period) (n = 418). After these exclusions, patients with RA and individuals without RA were matched (1:3) to RA cases based on age, sex, and index date. The study participants were followed from 1 year after RA diagnosis or corresponding index date to the date of acute or chronic pancreatitis diagnosis, death, or the end of the follow-up period (until December 31, 2019), whichever came first.
Study outcome and follow-up
The end point of the study was newly diagnosed acute pancreatitis or chronic pancreatitis using the ICD-10 code. To mitigate possible reverse causality, the participants were followed from 1 year after the index date (1-year of lag period) to the date of acute or chronic pancreatitis diagnosis, death, or the end of the follow-up period (until December 31, 2019), whichever came first. While there was no formal validation study for the use of ICD 10 codes to identify acute or chronic pancreatitis in our study setting, it is unlikely that these codes were administered solely for administrative or billing purpose without clinical conditions. Moreover, prior population-based cohort studies using claims data related to pancreatitis incidence have utilized ICD codes8,17.
Covariates
We collected data on baseline characteristics including age, sex, body mass index (BMI), smoking status, alcohol use, physical activity, income level, and comorbidities from the dataset. Personal behaviors, such as smoking status, alcohol consumption, and physical activity, were assessed using a self-reported questionnaire. Smoking status was classified into never smoker, ex-smoker, and current smoker. Alcohol intake was divided into none, mild to moderate (< 30 g of alcohol/day), and intake. Regular exercise was defined as moderate exercise for more than 5 days a week or vigorous exercise for more than 3 days a week18. Household income was divided into quartiles according to insurance premium levels, as in Korea, insurance premiums are based on income. Those receiving Medical Aid (poorest 3%) were included in the lowest income quartile. The lowest income quartile was labeled as “low income”19. BMI was calculated by dividing weight (kg) by height (m) squared. Definitions of comorbidities such as diabetes mellitus, hypertension, dyslipidemia, and chronic kidney were based on ICD-10 codes as previously described3,11,18.
Statistical analysis
The baseline characteristics of the study participants were compared based on the presence of RA and the serologic status of RA. Continuous variables were presented as mean (SD) and categorical variables were presented as number and percentage. The incidence rates of acute or chronic pancreatitis were presented per 1,000 person-years. The cumulative incidence of pancreatitis, based on RA status was estimated using the Kaplan-Meier method. Log-rank tests were applied to evaluate differences among the groups. We conducted Cox regression analyses to calculate adjusted hazard ratios (aHR) and 95% confidence intervals (CIs) for the risk of pancreatitis. A multivariable-adjusted proportional hazard model was applied: (1) model 1 was non-adjusted; (2) model 2 was adjusted for age, sex; (3) model 3 was further adjusted for smoking, alcohol drinking, physical activity, low income; (4) model 4 included model 3 plus diabetes, hypertension, hyperlipidemia, chronic kidney disease. Schoenfeld residuals were used to assess the proportional hazards (PH) assumption in Cox regression models. For Model 4, we performed an additional analysis with the Fine-Gray competing risk model as a sensitivity analysis. Statistical analyses were performed in May 2023 using SAS version 9.4 (SAS Institute Inc.), and 2-sided P < 0.05 was considered statistically significant.
Results
Baseline characteristics of study participant
Table 1 presents the baseline characteristics of the study participants for the analysis of acute pancreatitis according to the presence of RA and the serologic status of RA. Among a total of 171,072 study participants at the index date, 38,136 individuals (22.3%) were male, and the mean (SD) age was 58.0 (9.9) years. Alcohol consumption, even in small amounts, was significantly higher in those without RA compared to those with RA (26.4% vs. 21.3%), while current smoking was significantly lower in those without RA compared to those with RA (8.8% vs. 9.6%). The levels of TG were significantly higher in those without RA at 105.7 mg/dl compared to 102.1 mg/dl in patients with RA.
Table 2 describes the baseline characteristics of the study participants for the analysis of chronic pancreatitis according to the presence of RA and the serologic status of RA. The mean (SD) age of study participants (n = 184,660) at the index date was 58.3 (9.9) years; 22.7% were male. The patients with RA were more likely to be current smokers (9.7% vs. 9.0%), consume less alcohol (21.2% vs. 26.1%), and were less likely to be overweight (BMI ≥ 25) or obese (BMI ≥ 30) (29.9% vs. 33.4%).
Risk of acute pancreatitis according to the presence of RA
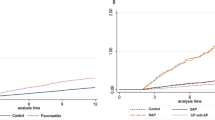
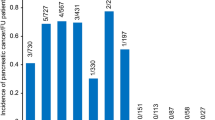
During a median (IQR) follow-up of 5.5 (3.7–7.5) years after a 1-year lag period, 252 participants (77 in the RA group and 175 in the control group) were newly diagnosed with acute pancreatitis. Compared with the control group, patients with RA showed a higher risk for acute pancreatitis (aHR, 1.33 [95%CI, 1.02–1.74], P = 0.032) (Table 2). By serological status, patients with SPRA are at a higher risk (aHR 1.36, 95% CI 1.01–1.84) compared to matched controls, whereas patients with SNRA showed no significant difference in risk (aHR 1.26, 95% CI 0.80–1.98). However, the comparison between SPRA and SNRA showed no significant difference (SPRA vs. SNRA, aHR 1.10, 95% CI 0.66–1.81). (Table 3; Fig. 2A). When the Fine-Gray model was applied, acute pancreatitis did not show a significant association (aHR 1.29, 95% CI 0.99–1.69, P = 0.061); however, the results were generally consistent with the main analyses using the Cox model.
Risk of chronic pancreatitis according to the presence of RA
During a median (Interquartile Range [IQR]) follow-up of 5.47(3.67–7.46) years after a 1-year lag period, 174 participants (50 in the RA group and 124 in the control group) were newly diagnosed with chronic pancreatitis (Fig. 2B). Patients with RA tend to have slightly higher risk of developing chronic pancreatitis (aHR 1.25, 95% CI 0.90–1.74, P = 0.189) than a matched control, although it did not reach predefined statistical significance (Table 2). By serological status, patients with SPRA tended to have marginally higher risk (aHR 1.36, 95% CI 0.95–1.96) than the matched control, while patients with SNRA did not differ in their risk (aHR 0.96, 95% CI 0.52–1.78). Patients with SPRA tended to have higher risk than patients with SNRA (aHR 1.45, 95% CI 0.74–2.84), although this was not statistically significant (Table 3; Fig. 2B). When the Fine-Gray model was applied, chronic pancreatitis did not show a significant association (aHR 1.21, 95% CI 0.87–1.69, P = 0.264), generally consistent with the main analyses using the Cox model.
Discussion
In this nationwide cohort study, we observed that patients with RA had a 1.33-fold higher risk of developing acute pancreatitis compared to those without RA, and we also observed a slight increase in developing chronic pancreatitis among patients with RA, although this was not statistically significant.
Our results showing 1.33-fold risk of developing acute pancreatitis in the RA group compared to the matched control participants aligns with the previous research, although the estimates are lower than findings of previous research from Taiwan (aHR = 1.62) or the US (OR = 2.51).8,9 The specific biological mechanisms connecting RA and acute pancreatitis have not yet been fully understood. However, there is no doubt that an inflammatory immune mechanism likely plays a pivotal role in its development. The pathogenesis of RA involves many immune cells, cytokines growth and differentiation factors, and transcription factors, which induce inflammatory response20. Patients with acute pancreatitis also show increased serum levels of pro-inflammatory or inflammatory cytokines21,22. Examining the pathogenesis of RA and acute pancreatitis reveals similarities in the release of cytokines and chemokines, which fuels systemic hyper-inflammation reactions caused by the activation of NF-κB, transcription factors and the inflammatory reaction resulting from the accumulation of the NLRP3 inflammasome, a critical producer of IL-1 and IL-1821,22,23,24,25,26,27. In both RA and acute pancreatitis patients, the production of IL-1 and IL-18 by the NLRP3 inflammasome is increased, and these cytokines are already known to play a crucial role in the pathogenesis of both diseases20,21,25.
Our study showed no significant difference in acute pancreatitis between SPRA and SNRA patients. Although SNRA did not show significant difference compared to matched control patients, it appears to be due to the small sample size, rather than true lack of association. Consequently, while the results should be interpreted with caution due to the limited sample size, the potential clinical relevance cannot be dismissed.
In the case of chronic pancreatitis, while the limited sample size did not yield statistically significant results, a small increase in chronic pancreatitis was noted among patients with RA. When examined by serological status, it seemed that only SPRA is associated with chronic pancreatitis, although caution is needed for the interpretation because of the small number of events. The mechanism linking RA and chronic pancreatitis remains unclear. However, since chronic pancreatitis is a condition characterized by fibrosis resulting from recurrent episode of acute inflammation, it can be considered similar to RA in terms of chronic inflammation25. Additionally, a pathological study found that in patients with RA, pancreatic tissue evaluation revealed ischemia due to vasculitis, which was accompanied by reactive inflammation and regressive changes in the pancreatic gland28. When examined by serological status, it seemed that only SPRA is associated with chronic pancreatitis, although caution is needed in the interpretation because of the small number of events. Additionally, autoimmune pancreatitis, a form of chronic pancreatitis characterized by lymphocyte infiltration and inflammation29, may have been included in the chronic pancreatitis group. Autoimmune pancreatitis is known to be associated with autoimmune diseases such as Sjögren’s syndrome, rheumatoid arthritis, primary biliary cirrhosis, and primary sclerosing cholangitis and so on30,31.
In patients with RA, gastrointestinal (GI) manifestations can arise from various causes, such as bowel infarction, bowel perforation, pan-colitis or appendicitis32. This study suggests that it may be necessary to consider acute or chronic pancreatitis as a differential diagnosis in cases of GI manifestations. Conversely, if acute or chronic pancreatitis develops in patients with RA, it is important to consider a possible association with RA as a potential cause.
The present study has several methodological advantages over previous ones. First, we applied a more accurate definition of the diagnosis of RA and examined the occurrence of both acute and chronic pancreatitis according to serologic status of RA. While previous studies depended on disease code only9 or prescriptions of DMARDs8 to identify patients with RA, our study used a combination of disease codes, RID program enrollment, and prescription of DMARDs for sufficient amounts of time to define RA, thereby reducing the likelihood of misclassification. Second, we adjusted various socioeconomic, metabolic, and behavioral factors in the multivariate-adjusted model, which were not considered in previous studies8,9, and we found consistent results across all adjustments. This implies RA is an independent risk factor for pancreatitis development.
Several limitations of the present study should also be mentioned. First, due to the nature of claims data, the inability to access RA disease activity led to a restricted assessment of RA severity. Second, although it may be important to consider whether chronic pancreatitis is autoimmune-related when examining its association with RA, the incidence of autoimmune pancreatitis among the cases of chronic pancreatitis was not separately investigated because they are included under the same ICD-10 code. However, the proportion of autoimmune pancreatitis cases within those of chronic pancreatitis is 1–2% and is unlikely to have significantly influenced the results of this study. Nonetheless, considering the potential connection between the mechanisms of RA and autoimmune pancreatitis, further studies that investigate them separately would be valuable33. Third, the number of pancreatitis cases was not large enough to conduct an analysis of the association with DMARDs administration. Fourth, even though our models were adjusted for various potential confounders, information on other potential confounders like family history and genetic information was not available34,35. Moreover, we were unable to adjust for medications and procedures such as post-endoscopic retrograde cholangio-pancreatography that could potentially influence pancreatitis36,37. Fifth, retrospective studies typically encounter surveillance bias, where individuals diagnosed with RA may receive more frequent health care service, potentially leading to a higher likelihood of obtaining a pancreatitis diagnosis. However, diagnosis of acute pancreatitis is very unlikely to have been affected or increased by frequent hospital visits for check-up or other reasons, since acute pancreatitis usually manifests with acute severe pain, requiring a visit to the hospital. Lastly, our study participants were restricted to those undergoing health screenings and may have been healthier and more committed to a healthy lifestyle compared to the general population.
Conclusion
In conclusion, this nationwide cohort study found an association between RA and an increased risk of acute pancreatitis and also possibly chronic pancreatitis. Therefore, as shown in the present study, a diagnosis of acute or chronic pancreatitis should not be overlooked and included as one of the differential diagnosis when patients with RA present with abdominal pain or discomfort.
Data availability
The data can be accessed on the Korean National Health Insurance homepage (http://nhiss.nhis.or.kr), but restrictions apply to the availability of the data, which was used with permission for the current study and therefore not publicly available. The data is available from the corresponding author upon reasonable request and with permission of KNHIS. Applications to use the KNHIS data will be reviewed by the inquiry committee of research support and, once approved, raw data will be provided to the applicant with a fee.
References
Kang, S. et al. Associations between cardiovascular outcomes and rheumatoid arthritis: A nationwide Population-Based cohort study. J. Clin. Med. 11 https://doi.org/10.3390/jcm11226812 (2022).
Nadareishvili, Z., Michaud, K., Hallenbeck, J. M. & Wolfe, F. Cardiovascular, rheumatologic, and Pharmacologic predictors of stroke in patients with rheumatoid arthritis: A nested, case-control study. Arthritis Rheum. 59, 1090–1096. https://doi.org/10.1002/art.23935 (2008).
Kang, J. et al. Rheumatoid arthritis and risk of Parkinson disease in Korea. JAMA Neurol. 80, 634–641. https://doi.org/10.1001/jamaneurol.2023.0932 (2023).
Albrecht, K., Strangfeld, A., Marschall, U. & Callhoff, J. Interstitial lung disease in rheumatoid arthritis: Incidence, prevalence and related drug prescriptions between 2007 and 2020. RMD Open. 9 https://doi.org/10.1136/rmdopen-2022-002777 (2023).
Ben Abdelghani, K. et al. Acute pancreatitis in rheumatoid arthritis: Causes. Joint Bone Spine 81, 377–378. https://doi.org/10.1016/j.jbspin.2014.01.006 (2014).
Kuroda, T. et al. Fatal acute pancreatitis associated with reactive AA amyloidosis in rheumatoid arthritis with end-stage renal disease: A report of three cases. Intern. Med. 50, 739–744. https://doi.org/10.2169/internalmedicine.50.4876 (2011).
Oishi, K. et al. Fatal pancreatitis associated with systemic amyloidosis in a rheumatoid arthritis patient. J. Med. 31, 303–310 (2000).
Chang, C. C. et al. Increased risk of acute pancreatitis in patients with rheumatoid arthritis: A population-based cohort study. PLoS One 10, e0135187. https://doi.org/10.1371/journal.pone.0135187 (2015).
Alkhayyat, M. et al. Pancreatic manifestations in rheumatoid arthritis: A National population-based study. Rheumatology 60, 2366–2374. https://doi.org/10.1093/rheumatology/keaa616 (2020).
Shin, D. W., Cho, J., Park, J. H. & Cho, B. National general health screening program in Korea: History, current status, and future direction. Precis Future Med. 6, 9–31. https://doi.org/10.23838/pfm.2021.00135 (2022).
Jeon, K. H. et al. Rheumatoid Arthritis and Risk of Depression in South Korea. JAMA Netw Open 7, e241139 (2024). https://doi.org/10.1001/jamanetworkopen.2024.1139
Chung, C. et al. Does rheumatoid arthritis increase the risk of COPD? A nationwide retrospective cohort study. Chest 165, 1362–1371. https://doi.org/10.1016/j.chest.2024.02.014 (2024).
Choi, H. et al. Impact of rheumatoid arthritis and seropositivity on the risk of non-cystic fibrosis bronchiectasis. Chest 165, 1330–1340. https://doi.org/10.1016/j.chest.2024.01.001 (2024).
Cho, S. K. et al. Development of an algorithm for identifying rheumatoid arthritis in the Korean National health insurance claims database. Rheumatol. Int. 33, 2985–2992. https://doi.org/10.1007/s00296-013-2833-x (2013).
Cho, M. H. et al. Rheumatoid arthritis and risk of lung cancer: A nationwide cohort study. J. Thorac. Oncol. 19, 216–226. https://doi.org/10.1016/j.jtho.2023.10.006 (2024).
Eun, Y. et al. Menopausal factors and risk of seropositive rheumatoid arthritis in postmenopausal women: A nationwide cohort study of 1.36 million women. Sci. Rep. 10, 20793. https://doi.org/10.1038/s41598-020-77841-1 (2020).
Lai, S. W., Lin, C. L., Liao, K. F. & Ma, C. L. Increased risk of acute pancreatitis following Pneumococcal pneumonia: A nationwide cohort study. Int. J. Clin. Pract. 69, 611–617. https://doi.org/10.1111/ijcp.12590 (2015).
Park, J., Yoon, J. H., Ki, H. K., Han, K. & Kim, H. Lifestyle changes and risk of tuberculosis in patients with type 2 diabetes mellitus: A nationwide cohort study. Front. Endocrinol. (Lausanne) 13, 1009493. https://doi.org/10.3389/fendo.2022.1009493 (2022).
Lee, H. R. et al. Tuberculosis and risk of ischemic stroke: A nationwide cohort study. Stroke 53, 3401–3409. https://doi.org/10.1161/strokeaha.122.039484 (2022).
McInnes, I. B. & Schett, G. The pathogenesis of rheumatoid arthritis. N Engl. J. Med. 365, 2205–2219. https://doi.org/10.1056/NEJMra1004965 (2011).
Malmstrøm, M. L. et al. Cytokines and organ failure in acute pancreatitis: Inflammatory response in acute pancreatitis. Pancreas 41, 271–277. https://doi.org/10.1097/MPA.0b013e3182240552 (2012).
Mayer, J., Rau, B., Gansauge, F. & Beger, H. G. Inflammatory mediators in human acute pancreatitis: Clinical and pathophysiological implications. Gut 47, 546–552. https://doi.org/10.1136/gut.47.4.546 (2000).
Makarov, S. S. NF-kappa B in rheumatoid arthritis: A pivotal regulator of inflammation, hyperplasia, and tissue destruction. Arthritis Res. 3, 200–206. https://doi.org/10.1186/ar300 (2001).
Gukovsky, I., Gukovskaya, A. S., Blinman, T. A., Zaninovic, V. & Pandol, S. J. Early NF-kappaB activation is associated with hormone-induced pancreatitis. Am. J. Physiol. 275, G1402–1414. https://doi.org/10.1152/ajpgi.1998.275.6.G1402 (1998).
Glaubitz, J. et al. Immune response mechanisms in acute and chronic pancreatitis: Strategies for therapeutic intervention. Front. Immunol. 14, 1279539. https://doi.org/10.3389/fimmu.2023.1279539 (2023).
Ferrero-Andrés, A., Panisello-Roselló, A., Roselló-Catafau, J. & Folch-Puy, E. NLRP3 Inflammasome-mediated inflammation in acute pancreatitis. Int. J. Mol. Sci. 21 https://doi.org/10.3390/ijms21155386 (2020).
Hoque, R. et al. TLR9 and the NLRP3 inflammasome link acinar cell death with inflammation in acute pancreatitis. Gastroenterology 141, 358–369. https://doi.org/10.1053/j.gastro.2011.03.041 (2011).
Bély, M. & Apáthy, Á. Pancreatitis in rheumatoid arthritis and the role of systemic vasculitis of autoimmune origin in the pathogenesis of pancreatitis – A postmortem clinicopathologic study of 161 patients. Gastroenterol. Hepatol. Open. Access. 10 https://doi.org/10.15406/ghoa.2019.10.00351 (2019).
Finkelberg, D. L., Sahani, D., Deshpande, V. & Brugge, W. R. Autoimmune pancreatitis. N Engl. J. Med. 355, 2670–2676. https://doi.org/10.1056/NEJMra061200 (2006).
Yildirim, O. & Yildirim, T. Auto-Immune pancreatitis in rheumatoid arthritis. Med. Sci. 4, 1. https://doi.org/10.5455/medscience.2014.03.8212 (2014).
Mustak, M., Boltuch-Sherif, J., Horvath-Mechtler, B., Kowalski-Bodzenta, J. & Erlacher, L. [Autoimmune pancreatitis associated with rheumatoid arthritis: Successful combination therapy with steroids and methotrexate]. Dtsch. Med. Wochenschr. 136, 1842–1844. https://doi.org/10.1055/s-0031-1286354 (2011).
Craig, E. & Cappelli, L. C. Gastrointestinal and hepatic disease in rheumatoid arthritis. Rheum. Dis. Clin. North. Am. 44, 89–111. https://doi.org/10.1016/j.rdc.2017.09.005 (2018).
Beyer, G., Habtezion, A., Werner, J., Lerch, M. M. & Mayerle, J. Chronic pancreatitis. Lancet 396, 499–512. https://doi.org/10.1016/s0140-6736(20)31318-0 (2020).
Kumar, S. et al. Risk factors associated with pediatric acute recurrent and chronic pancreatitis: Lessons from INSPPIRE. JAMA Pediatr. 170, 562–569. https://doi.org/10.1001/jamapediatrics.2015.4955 (2016).
Habtezion, A., Gukovskaya, A. S. & Pandol, S. J. Acute pancreatitis: A multifaceted set of organelle and cellular interactions. Gastroenterology 156, 1941–1950. https://doi.org/10.1053/j.gastro.2018.11.082 (2019).
Thaker, A. M., Mosko, J. D. & Berzin, T. M. Post-endoscopic retrograde cholangiopancreatography pancreatitis. Gastroenterol. Rep. (Oxf). 3, 32–40. https://doi.org/10.1093/gastro/gou083 (2015).
Trivedi, C. D. & Pitchumoni, C. S. Drug-induced pancreatitis: An update. J. Clin. Gastroenterol. 39, 709–716. https://doi.org/10.1097/01.mcg.0000173929.60115.b4 (2005).
Author information
Authors and Affiliations
Contributions
D.W.S., H.K. and J.P. contributed study concept and design, analysis and interpretation, drafting and critical revision of the manuscript. J.H.J. and K.H. designed statistical analysis. Y.E., S.K., S.K. and J.J.H. critically revised the manuscript.All authors reviewed the manuscrip.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Park, J., Eun, Y., Han, K. et al. Rheumatoid arthritis and risk of pancreatitis: a nationwide cohort study. Sci Rep 15, 7607 (2025). https://doi.org/10.1038/s41598-025-91898-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-91898-w