Abstract
This study examines the correlation between nailfold videocapillaroscopy (NVC) abnormalities and pulmonary hypertension (PH) in connective tissue disease (CTD) patients, evaluating its diagnostic and predictive value for microcirculation alterations. A cross-sectional study included 351 CTD patients and 30 non-CTD healthy people, with NVC assessments conducted qualitatively, semi-quantitatively, and quantitatively by two independent physicians. Clinical and laboratory data were analyzed, comparing CTD patients with pulmonary arterial hypertension (CTD-PAH) and those without (CTD-non-PAH). Among the patients, 16.5% (n = 58) had pulmonary hypertension. CTD-PAH patients showed higher nailfold videocapillaroscopy scores (5.73 ± 3.54 vs. 4.30 ± 2.98, P = 0.001) and larger capillary diameters (17.06 ± 8.22 vs. 14.41 ± 9.25, P = 0.044) compared to CTD-non-PAH patients. Factors significantly influencing the nailfold videocapillaroscopy score included Raynaud’s phenomenon, pulmonary hypertension, and the presence of anti-Scl-70 antibody. The ROC analysis yielded an AUC of 0.621 nailfold videocapillaroscopy score for predicting PAH. Additionally, pulmonary artery systolic pressure in CTD-PAH patients was positively correlated with both nailfold videocapillaroscopy score (R = 0.618, B = 3.26, P < 0.001) and capillary diameter (R = 0.541, B = 1.23, P < 0.001). Nailfold videocapillaroscopy abnormalities, such as higher scores and increased capillary diameters, are associated with pulmonary hypertension in patients with connective tissue diseases (CTD). This method demonstrates potential diagnostic and predictive value for detecting microcirculation alterations in these patients.
Similar content being viewed by others
Introduction
Capillaroscopy serves as an essential tool for diagnosing and prognosing various pathologies, with anatomic pathological lesions playing a crucial role in their progression1. Nailfold videocapillaroscopy presents as a safe, inexpensive, swift, and accurate non-invasive approach for examining microcirculatory abnormalities in connective tissue diseases, such as systemic sclerosis, dermatomyositis, and systemic lupus erythematosus2. Key uses of this technique incorporate the identification of primary and secondary Raynaud’s phenomenon, and the evaluation of systemic sclerosis3. Notably, abnormalities of nailfold microcirculation in systemic sclerosis were included in the 2013 ACR/EULAR classification criteria4. Culoto M. et al. expounded on the typical “scleroderma pattern” of abnormalities in the nailfold microcirculation in patients with systemic sclerosis which included features factors such as reduced capillary density, increased dimension, abnormal morphology, and the incidence of haemorrhage, Capillary diameter has been identified as an independent predictor of Raynaud’s phenomenon secondary to systemic sclerosis. Additionally, a correlation exists between microvascular dysfunction in systemic sclerosis patients and internal organ damage, particularly in the lungs.5,6. They further detailed the variations of the “scleroderma pattern” across early, active, and late stages7. Besides the typical “scleroderma pattern”, numerous other connective tissue diseases like systemic lupus erythematosus, Sjögren’s syndrome, and dermatomyositis often exhibit "non-specific abnormalities". These are characterized by decreased capillary density, abnormal capillary dimensions, altered blood flow velocity and patterns, accompanied by occasional haemorrhages and exudation8.
Pulmonary hypertension, a common complication of connective tissue disease affecting the cardiovascular system, has a prevalence reaching up to 13% (95% Confidence Interval CI 9.1%−18.1%) among patients with such conditions9. It is mainly characterized by changes driving increased pulmonary vascular resistance and pulmonary artery pressure. If not adequately treated, these changes may lead to right heart failure and ultimately, death9,10. Systemic sclerosis, the most common autoimmune connective tissue disease associated with pulmonary hypertension, accounts for approximately 75% of CTD-PAH cases. Other autoimmune disorders such as systemic lupus erythematosus, rheumatoid arthritis, dermatomyositis, and Sjögren’s syndrome also carry a significant risk for pulmonary hypertension11. Pulmonary hypertension is clinically defined by a mean pulmonary arterial pressure (mPAP) > 20 mmHG or concomitant presence of mPAP > 20 mmHg, PAWP ⩽15 mmHg and pulmonary vascular resistance ⩾3 WU for pre-capillary PH using right heart catheterizationthe12.. Due to the invasive and costly nature of right heart catheterization13, an alternative clinical estimation for pulmonary artery pressure is often performed using echocardiography. This method involves using Doppler ultrasound to convert tricuspid regurgitation velocity to tricuspid transvalvular pressure and provides an indirect estimate of pulmonary artery pressure, offering additional insights when considered in conjunction with right atrial size14.
Despite the fact that lesions in PAH primarily manifest in large vessels, such as the pulmonary arteries, significant associations have been observed between cardiovascular risk events, atherosclerosis, and microvascular abnormalities in patients with connective tissue disease. This is particularly noted in patients with systemic sclerosis, suggesting a more widespread systemic vascular pathology, inclusive of nailfold microcirculation, in those affected by CTD-PAH patients15. This study builds upon prior research demonstrating microcirculatory abnormalities in patients with connective tissue disease (CTD) and pulmonary arterial hypertension (PAH) 16, providing a novel perspective by specifically examining the extent of peripheral microcirculation impairment in CTD-PAH patients. By focusing on the distinctive abnormalities in nailfold microcirculation observed in this population, the study highlights the unique pathophysiological features of CTD-PAH. Additionally, it pioneers the exploration of nailfold videocapillaroscopy as a non-invasive diagnostic tool tailored to this patient group, emphasizing its innovative contribution to the field.
Methods
Participants
We specified a 5% tolerance, a 0.95 confidence level (1-α), an estimated proportion of CTD patients with significant perinatal abnormalities of approximately 50%, and a required sample size of 350, as calculated using PASS (v11.0) software. All participants were recruited from the Department of Rheumatology and Immunology at Nanjing Drum Tower Hospital, Jiangsu, China, with ages ranging from 35 to 65 during the period from September 2022 to September 2023, Individuals clinically diagnosed with rheumatoid arthritis (meet 2010 ACR/EULAR classification criteria for rheumatoid arthritis17), systemic lupus erythematosus (meet 2019 ACR/EULAR classification criteria for systemic lupus erythematosus18), dermatomyositis (meet 2017 ACR/EULAR classification criteria for adult and juvenile idiopathic inflammatory myopathies19), Sjögren’s syndrome (meet 2016 ACR/EULAR classification criteria for Primary Sjögren’s Syndrome20) and systemic sclerosis (meet 2013 ACR/EULAR classification criteria for systemic sclerosis21) within the study period were all included. Participants had a disease duration of at least six months and were in a relatively stable condition, with no acute exacerbations or crises. Additionally, the study included 30 non-CTD healthy people. All participants demonstrated an understanding of the study procedures and provided informed consent following stringent ethical standards in compliance with the Declaration of Helsinki.
Patients with conditions affecting microcirculation, such as diabetes mellitus, severe cardiovascular diseases (e.g., coronary heart disease, heart failure), peripheral vascular diseases (excluding Raynaud’s phenomenon), or hematological disorders (e.g., anemia, leukemia), were excluded from this study. Exclusion criteria also included patients with periungual skin abnormalities (e.g., infections like paronychia, trauma, ulcers) or those who had undergone periungual surgery or invasive procedures within the past month. Additionally, individuals who had used medications known to affect microcirculation within the previous three months (e.g., chemotherapeutic agents, certain antibiotics), those with a history of heavy smoking (over 20 cigarettes per day), alcohol or drug abuse, pregnant or lactating women, patients with known allergies to study reagents or equipment, and individuals with severe psychiatric illnesses or cognitive impairments that could prevent compliance with study requirements were also excluded. The study design and methodology were reviewed and approved by the Ethics Committee of Nanjing Drum Tower Hospital.
Clinical assessment
Complete medical histories, physical examinations, and laboratory tests were collected. All the data were collected in a standardized computerized electronically filled form including demographics, past medical history with date of diagnosis, past surgeries, comorbidities, smoking habits, and current therapies. All patients underwent nailfold videocapillaroscopy, electrocardiograms, and lung CT scans within a week. Cardiopulmonary arterial systolic pressure (SARP) was estimated using cardiac Doppler ultrasound, which gauged the tricuspid regurgitation velocities. Patients with a prior history of pulmonary hypertension or a SARP greater than 35 mmHg were identified as being in the pulmonary hypertension group.22.
Laboratory tests
Routine hematologic and biochemical tests were performed, and the results were analyzed in the hospital’s laboratory. Plasma was tested for antinuclear antibodies (ANA) and anti-topoisomerase-I antibody (anti-Scl-70) using indirect immunofluorescence with HEp-2 cells as the antigen substrate (Immuno Concepts, Sacramento, USA). The presence of anti-SSA, anti-SSB and anti-Ro-52 antibodies was confirmed by enzyme-linked immunosorbent assay (ELISA) (Quanta Lite ENA 6; Inova Diagnostics). anti-SM/RNP antibody was determined using an enzyme-linked immunoassay (Euroimmun, Medizinische Labor diagnostic, AG). and anti-dsDNA antibodies were measured by ELISA (Alpha Diagnostic, TX, USA),
Nailfold videocapillaroscopy and image analysis
Patients were advised to abstain from caffeine and smoking at least six hours before the examination. Additionally, they were recommended to relax in an environment maintained at a temperature between 20 to 25 °C. Trained experts performed standardized examinations and analyses using a TR8000D microcirculation microscope set at 200 × magnification (Jiangsu Tongren Medical). To improve image clarity during the examination, a drop of cedar oil was applied around the nail23. Examinations were performed on the third and fourth fingers of both hands, positioned at heart level, and four consecutive observation areas in the middle of each nailfold were assessed. Each patient’s examination was constrained to a duration between 15 to 30 min. Acquired images were analyzed and archived using specialty software (Videocap Scalar Ltd.).
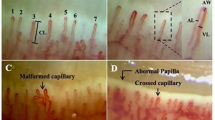
The parameters encompassed the distribution and morphology of capillaries, capillary diameters (including diameter of the afferent loops, diameter of the efferent loops and total diameter of the loop), the count of irregular capillaries (irregularly dilated capillaries, giant capillaries, vascular array disorganization, capillary ramifications), capillary flow rate (calculated by measuring the distance and time of erythrocyte movement using video software to analyze their trajectory during capillaroscopy.), erythrocyte aggregation (characterized by Rouleaux formation or visible clumps of erythrocytes, which led to the slowing or stagnation of blood flow), exudation, and hemorrhaging8,24. Capillary images were independently scored semi-quantitatively by two specially trained physicians for the above capillary abnormalities and a total score was calculated (Supplementary Fig. 1), employing scoring criteria extrapolated from prior studies (0 = no change, 1 = less than 33% capillary abnormalities, 2 = 33–66% capillary abnormalities, 3 = more than 66% capillary abnormalities)25. For each patient, the final nailfold optic papillary score and the total malformation score were calculated as the average of two individual scores..
Statistical methods
Continuous variable data were represented as either the mean ± standard deviation or the median (interquartile range). The Kolmogorov–Smirnov test was used to examine data normality, while the Student’s t-test or the Mann–Whitney U test was utilized for analyzing differences in quantitative data between two independent samples. Independent sample data group differences were inspected using ANOVA or the Kruskal–Wallis test, with multiple sample post-hoc analysis conducted using the LSD test.
Ordinal data were expressed as percentages of the total, and differences between two ordinal data groups were evaluated using the Mann–Whitney U test. Kappa values were calculated to assess consistency, Kruskal–Wallis tests were utilized for analyzing differences between multiple ordinal data groups, and categorical data were statistically examined using the chi-square test.
Correlation between pulmonary artery pressure, laboratory diagnostic indicators and nailfold videocapillaroscopy abnormalities were determined using Pearson’s correlation analysis or Spearman’s rank correlation, with the correlation strength indicated by the correlation coefficient. Logistic and multiple linear regression were employed to evaluate correlations between different variables. The receiver operating characteristic curve (ROC) and its area under the curve (AUC) was used to analyze the efficiency of diagnosing pulmonary hypertension by nailfold videocapillaroscopy, demonstrating the optimal diagnostic cutoff.
All statistical analyses were performed utilizing IBM SPSS Statistics (version 26.0, IBM Corp., Armonk, N.Y. USA) and GraphPad Prism (version 9.0.0, San Diego, California USA). P values < 0.05 were identified as statistically significant. For missing data, we applied Multiple Imputation, a method that generates several imputed datasets, which are then analyzed and merged.
Ethical Statement
All studies have been approved by the ethics committee of Nanjing Drum Tower Hospital and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All persons gave their informed consent prior to their inclusion in the study.
Results
Participant baseline characteristics
This study enrolled 351 patients (64 with rheumatoid arthritis, 106 with systemic lupus erythematosus, 59 with dermatomyositis, 61 with Sjögren’s syndrome and 61 with systemic sclerosis) consisted of 58 patients with CTD-PAH patients (89.7% female, mean age 51.1 ± 15.2 years), and 293 patients with connective tissue disease but without PAH (CTD-non-PAH) (82.3% female, mean age 46.4 ± 15.7 years). A further 30 non-CTD healthy people(76.7% female, mean age 47.1 ± 6.9 years) were included in the study. Disease characteristics and laboratory assessments for both groups are summarized in Table 1. The presence of Raynaud’s phenomenon was significantly higher in patients with CTD-PAH compared to those with connective tissue disease-non-pulmonary arterial hypertension (CTD-non-PAH) (53.4% vs. 32.4%, P = 0.002). Patients with CTD-PAH showed significantly lower platelets (167 ± 77.0 vs. 215.2 ± 88.9, P = 0.001) and glutamic-pyruvic transaminase (ALT) levels (24.30 ± 24.29 vs. 36.72 ± 72.55, P = 0.021) compared to CTD-non-PAH, while thyrotropin levels were statistically elevated (3.484 ± 4.230 vs. 2.598 ± 2.468, P = 0.038). There were no significant differences observed in the other laboratory parameters or autoantibodies.
Alterations in nailfold videocapillaroscopy in patients with pulmonary hypertension
Among all participants, patients with systemic sclerosis presented significantly higher total scores in nailfold videocapillaroscopy (7.06 ± 3.46, P < 0.001) compared to those with other diseases (RA 2.81 ± 1.63, SLE 3.56 ± 2.52, DM 5.78 ± 2.91, SS 4.29 ± 3.15), with the lowest scores in non-CTD healthy people group(0.93 ± 0.88), corroborating previous findings23, All patients with connective tissue diseases, except rheumatoid arthritis, exhibited higher nailfold videocapillaroscopy scores compared to normal controls. Patients with dermatomyositis had slightly lower scores than those with systemic sclerosis but significantly higher scores than patients with other connective tissue diseases.(Fig. 1). It’s worth noting that patients with CTD-PAH had significantly higher scores in areas such as irregularly enlarged capillaries (1.02 ± 1.11 vs. 0.61 ± 0.92, P = 0.004), giant capillaries (0.25 ± 0.66 vs. 0.17 ± 0.52, P = 0.029), haemorrhages (0.70 ± 0.78 vs. 0.49 ± 0.70, P = 0.043), loss of capillaries (1.47 ± 1.12 vs. 0.95 ± 1.04, P = 0.001), and total score (5.73 ± 3.54 vs. 4.30 ± 2.98, P = 0.001) in comparison with CTD-non-PAH patients(Table 2). These differences were more pronounced in patients with rheumatoid arthritis and Sjögren’s syndrome(Supplementary Table 1&2). However, there were no significant differences between the two groups concerning the disorganization of the vascular array and capillary ramifications (Table 2). Besides, the capillary diameter was significantly larger in CTD-PAH patients compared to CTD-non-PAH patients (17.06 ± 8.22 vs. 14.41 ± 9.25, P = 0.044), especially noticeable in patients diagnosed with Sjögren’s syndrome (Supplementary Table 3). Additionally, compared to patients with CTD-non-PAH, the blood flow rate was significantly slower in CTD-PAH (χ2 = 6.835, P = 0.009). No statistically significant difference was observed, however, in the hemorrhage, nail papilla, nail papilla venous plexus and erythrocyte aggregation within the capillaries(Fig. 2).
Differences in nailfold videocapillaroscopy score for different connective tissue diseases. Differences of nailfold videocapillaroscopy in different connective tissue diseases and non-CTD healthy people obtained by one-way ANOVA, * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001, RA rheumatoid arthritis, SLE systemic lupus erythematosus, DM dermatomyositis, SS Sjögren’s syndrome, SSc systemic sclerosis.
Difference in nailfold microcirculation between CTD-PAH and CTD-non-PAH. Evaluation of differences in Nail Papillae, Venous plexus of the nail papilla, Hemorrhage, Erythrocyte Aggregation, and Capillary Blood Flow in nailfold videocapillaroscopy between patients with CTD-PAH and CTD-non-PAH using a Chi-Square test.
Analysis of factors influencing nailfold videocapillaroscopy score
There was substantial agreement between the scores of the two physicians [Kappa = 0.589, 95% CI (0.561–0.616), P < 0.001]. A multiple linear regression analysis of clinical and experimental indices against nailfold videocapillaroscopy scores across all patients revealed negative correlations with erythrocyte sedimentation rate, creatinine, urinary occult blood, and urinary protein. In contrast, age, antinuclear antibody, anti-Ro52 antibody, anti-Scl-70 antibody, pulmonary arterial hypertension, and Raynaud’s phenomenon showed positive correlations with nailfold videocapillaroscopy scores (Table 3). A subsequent multivariable linear regression analysis, including all nailfold videocapillaroscopy score-related influencing factors, demonstrated Raynaud’s phenomenon [B = 2.755, P = 0.000, 95% CI (2.103, 3.406)], pulmonary hypertension [B = 0.914, P = 0.029, 95% CI (0.094, 1.733)], and anti-Scl-70 antibody [B = 1.038, P = 0.043, 95% CI (0.035, 2.041)] as significant factors affecting nailfold videocapillaroscopy score.
Relation between nailfold videocapillaroscopy parameters and pulmonary artery hypertension
Univariate linear regression revealed a positive correlation between pulmonary artery pressure (as estimated by cardiac ultrasound) and the nailfold videocapillaroscopy score in CTD-PAH (R = 0.618, B = 3.26, P < 0.001) (Fig. 3a). Subsequently, variables that had P < 0.05 in the univariate logistic regression analysis were introduced into the multivariate logistic regression model. This analysis identified age, nailfold videocapillaroscopy score, and thyroid-stimulating hormone levels as independent risk factors for pulmonary hypertension, while the platelet count was found to be an independent protective factor. (Supplementary Table 4). Linear regression analysis also showed a positive correlation between the capillary diameter and the systolic blood pressure in the pulmonary artery of CTD-PAH patients (R = 0.541, B = 1.23, P < 0.001) (Fig. 3b).
Linear correlation of SARP with nailfold videocapillaroscopy score and capillary diameter. Scatterplot of linear positive correlation of pulmonary artery systolic pressure with nailfold videocapillaroscopy score (a) and capillary diameter (b) in CTD-PAH patients, Univariate linear regression was used, R-values and regression equations were obtained. P < 0.05 was considered statistically significant. PASP pulmonary artery systolic pressure, CTD-PAH pulmonary arterial hypertension associated with connective tissue disease.
Efficacy of nailfold videocapillaroscopy in the diagnosis of pulmonary artery hypertension
The diagnostic relevance of the nailfold videocapillaroscopy score for PAH was assessed via Receiver Operating Characteristic (ROC) analysis, (Fig. 4). The Area Under Curve (AUC) for PAH diagnosis via nailfold videocapillaroscopy score was determined to be 0.621(P = 0.004, 95% CI (0.539, 0.703)) with an optimal cut-off value of 5.25. This cut-off value presented a sensitivity of 0.517, a specificity of 0.706, and a Youden index of 0.223. A higher diagnostic significance for the nailfold videocapillaroscopy score was found in patients diagnosed with rheumatoid arthritis (AUC = 0.793, P = 0.012) and Sjögren’s syndrome accompanied by pulmonary arterial hypertension (AUC = 0.710, P = 0.018) (Supplementary Fig. 2&3).
ROC curve of nailfold videocapillaroscopy score for PAH. ROC curve of nailfold videocapillaroscopy score for PAH was obtained from ROC analysis, AUC and cut-off values were acquired, P < 0.05 was considered statistically significant, PAH pulmonary arterial hypertension, ROC receiver operating characteristic, AUC Area under the curve.
Discussion
Nailfold videocapillaroscopy is a commonly used examination modality to evaluate microcirculatory abnormalities. However, most previous studies have focused on peri-nail microcirculatory abnormalities in patients with systemic sclerosis26, while other abnormalities related to connective tissue diseases remain understudied. Pulmonary hypertension is a common comorbidity of connective tissue diseases. Previous studies have primarily examined pathological changes in large vessels9,27, with less focus on microvascular lesions. In this article, we focused on the pathological changes of the microcirculation in patients with connective tissue diseases comorbid with pulmonary hypertension and evaluated microcirculatory injury through nailfold videocapillaroscopy. This study found more severe nailfold abnormalities, including dilated capillaries, hemorrhage, capillary loss, and reduced blood flow, in CTD-PAH patients compared to CTD-non-PAH patients, consistent with previous findings28. Nailfold scores and capillary diameter were positively correlated with pulmonary artery systolic pressure, and ROC analysis supported its potential for assessing PAH. These findings highlight nailfold videocapillaroscopy as a useful tool in evaluating PAH severity.
Pulmonary hypertension, a common cause of mortality in connective tissue disease, emphasizes the importance of early detection and evaluation of risk factors for prognosis and efficient treatment in cases of connective tissue disease co-occurring with Pulmonary Arterial Hypertension (PAH)29. Traditionally, the primary pathological changes in PAH are understood to encompass haemodynamic abnormalities within the pulmonary vasculature, alongside damage to the heart, lungs, and other organs. However, numerous clinical experiments have established that inflammation and immune system abnormalities constitute important pathogenic mechanisms in idiopathic pulmonary hypertension patients, impaired microcirculation in the small pulmonary arteries and capillary remodeling represent significant pathologic changes in PAH30,31. Corrado, A. et al. observed that nailfold videocapillaroscopy in patients with idiopathic pulmonary arterial hypertension revealed decreased capillary density and an increased diameter in the capillary26, The findings of this study further confirm the value of nailfold videocapillaroscopy in diagnosing PAH. Compared to CTD-non-PAH patients, those with CTD-PAH were generally older, aligning with findings from previous epidemiological studies of PAH32. Additionally, a higher prevalence of Raynaud’s phenomenon was observed in CTD-PAH patients, possibly due to the shared pathogenesis of both disorders involving vascular function abnormalities. In terms of laboratory parameters, CTD-PAH patients exhibited higher levels of TSH, relevant observational studies have demonstrated that elevated TSH levels are associated with increased mortality in pulmonary hypertension and that thyroid function significantly impacts the prognosis of PAH33. This association may be attributed to hypothyroidism-related factors such as slowed metabolism, vasoconstriction, endothelial dysfunction, or impaired cardiac function. Additionally, elevated TSH levels may directly affect vascular endothelial cell function, contributing to vasoconstriction or structural remodeling of the pulmonary arteries34. Furthermore, platelet counts were higher in CTD-non-PAH patients, a phenomenon that might be associated with vascular remodeling and platelet depletion during thrombosis in PAH patients. Moreover, a more pronounced slowing of microcirculatory blood flow velocity was observed in CTD-PAH patients, which could be associated with increased blood viscosity and reduced endothelial cell function specific to PAH patients. These findings suggest a possible link to autoimmune abnormalities and vascular inflammation in patients with connective tissue diseases. The concurrent presence of PAH and connective tissue disease, which exacerbates peri-nail microcirculation abnormalities in CTD-PAH patients, offers valuable insights for identifying comorbidities in patients with both connective tissue disease and pulmonary hypertension.
Previous studies primarily targeted the abnormalities of nailfold videocapillaroscopy in patients suffering from systemic sclerosis and systemic lupus erythematosus, concomitant with PAH, elucidating the pathogenesis of pulmonary hypertension and microcirculatory injury35,36. However, fewer studies focus on other connective tissue diseases. This association may stem from the fact that pulmonary hypertension is a comparatively infrequent complication of rheumatoid arthritis and Sjögren’s syndrome. The heightened severity of abnormalities observed in nailfold videocapillaroscopy examinations was markedly accentuated in patients diagnosed with co-existing connective tissue disorders and PAH. Interestingly, despite higher nailfold videocapillaroscopy scores in systemic sclerosis patients, abnormalities in nailfold microcirculation due to PAH were more pronounced in patients with rheumatoid arthritis and Sjögren’s syndrome, contradicting previous assumptions37. This could be attributed to the inherent microcirculation impairment in systemic sclerosis and systemic lupus erythematosus38. Therefore, the difference in microcirculatory impairment is not as significant in these conditions when combined with PAH, compared to rheumatoid arthritis and Sjögren’s syndrome, where such impairment is less common3,39. Consequently, patients with the latter diseases combined with PAH may show more pronounced capillary microcirculatory abnormalities than those without PAH. These findings underscore the importance of nailfold videocapillaroscopy in detecting PAH in these conditions.
As one of the diagnostic criteria for systemic sclerosis, Abnormalities of the nailfold videocapillaroscopy in patients with systemic sclerosis are easily identifiable and demonstrate a typical scleroderma pattern, characterized by decreased capillary density, increased capillary diameter, morphological abnormalities, and capillary hemorrhage8. This scleroderma pattern is also observed in other connective tissue disorders, such as systemic lupus erythematosus and dermatomyositis40. However, it is predominantly manifested through various non-specific abnormalities in capillaroscopic examinations. Connective tissue diseases frequently exhibit systemic inflammatory responses and immune abnormalities involving multiple organs, wherein microcirculatory damage may reflect systemic organ involvement to a certain degree. Ornowska, S. et al. discovered that nailfold videocapillaroscopy patterns in patients with mixed connective tissue diseases could be indicative in predicting organ-specific involvement, such as lung disease and myositis 41. Although not as extensively researched, damage to the microcirculation in patients with Sjögren’s syndrome and rheumatoid arthritis has been observed using capillaroscopy42. In addition to nailfold videocapillaroscopy, Dynamic Optical Coherence Tomography (D-OCT) Capillaroscopy and Fluorescence Optical Imaging (FOI) are emerging techniques for detecting abnormalities in nailfold microcirculation43.
The observed abnormalities in nailfold videocapillaroscopy may be attributed to specific inflammatory factors and autoantibodies44. In this study, we identified that the anti-topoisomerase antibody and Raynaud’s phenomenon were most specifically independent influential factors precipitating nailfold videocapillaroscopy abnormalities. As a specific marker for systemic sclerosis, the anti-Scl-70 antibody is thought to induce abnormalities in nailfold videocapillaroscopy by facilitating endothelial cell damage, vascular inflammation, remodeling, and fibrosis, resulting in a compromised blood supply to the microcirculation in affected patients45. Raynaud’s phenomenon, often associated with connective tissue disease, is characterized by an exaggerated response of the small blood vessels in the microcirculation to cold or emotional stress, leading to spasms, and these abnormalities or necrosis. This spasm could be a primary factor in nailfold videocapillaroscopy abnormalities. Schlager, O. et al. determined that anti-Scl-70 and anti-filament antibodies are correlated with capillary abnormalities in patients with Raynaud’s phenomenon46.In addition, Mueller, M. et al. observed a notably higher mortality rate in patients exhibiting Raynaud’s phenomenon combined with anti-Scl-70 positivity, ANA antibody positivity, and nailfold videocapillaroscopy abnormalities47. This finding reinforces the importance of microcirculation examination in the early stages of autoimmune diseases for assessing patient conditions. Although ANA and anti-Ro-52 antibodies are likewise linked to nailfold videocapillaroscopy abnormalities, the precise mechanisms remain unclear and could be connected to autoimmune abnormalities. Regarding the association between creatinine, urinary protein, urinary occult blood, and nailfold videocapillaroscopy abnormalities, this may be attributed to microcirculatory damage resulting from renal injury. This necessitates further investigation into the possible roles of other autoantibodies and inflammatory factors in the amalgamation of pulmonary hypertension and microcirculatory injury in patients diagnosed with connective tissue diseases48.In addition, the diameters of periungual capillaries were found to have a significant positive correlation with the diameters of pulmonary arteries in patients with pulmonary hypertension, which may be related to peripheral endothelial dysfunction and increased pulmonary vascular resistance49.
However, there are some limitations of the study. First, this study includes the inability to accurately assess pulmonary artery systolic pressure due to the invasive nature of right heart catheterization and considerations for patient compliance; therefore, relying solely on cardiac ultrasound results could introduce bias in the determination of PAH50. Another limitation is compounded by the absence of detection of hemodynamic markers such as endothelin and prostacyclin that play prominent roles in the pathogenesis of CTD-PAH32. This could potentially act as a confounding factor for the observed abnormalities in nailfold videocapillaroscopy. Third, The sample size of CTD-PAH patients in this study was limited, necessitating an expansion of the sample size to enhance the credibility of the results. Finally, the findings of this study suggest that microvascular injury may be associated with the development of PAH. However, the absence of control for idiopathic PAH may temper the efficacy of nailfold videocapillaroscopy in assessing PAH51.
In conclusion, our results demonstrated significant abnormalities in nailfold videocapillaroscopy of CTD-PAH patients, which correlated with pulmonary artery systolic pressure. The proposed semi-quantitative method for assessing abnormalities detected through nailfold videocapillaroscopy offered a more specific counting method and resulted in an improved inter-observer agreement. The use of this supporting test for early PAH diagnosis holds the potential to further optimize patient management and prognosis of individuals with CTD-PAH.
Data availability
The data that support the fndings of this study are available from the corresponding author on reasonable request.
References
Taormina, V. et al. Automated stabilization, enhancement and capillaries segmentation in videocapillaroscopy. Sensors (Basel). 23(18), 7674 (2023).
Etehad Tavakol, M. et al. Nailfold capillaroscopy in rheumatic diseases: which parameters should be evaluated?. Biomed. Res. Int. 2015, 1–17 (2015).
Chojnowski, M. M., Felis-Giemza, A. & Olesińska, M. Capillaroscopy – a role in modern rheumatology. Rheumatology 54(2), 67–72 (2016).
van den Hoogen, F. et al. Classification criteria for systemic sclerosis: An American college of rheumatology/european league against rheumatism collaborative initiative. Arthritis Rheumatism 65(11), 2737–2747 (2013).
Trombetta, A. C. et al. Quantitative alterations of capillary diameter have a predictive value for development of the capillaroscopic systemic sclerosis pattern. J. Rheumatol. 43(3), 599–606 (2016).
D’Oria, M. et al. Correlation between microvascular damage and internal organ involvement in scleroderma: focus on lung damage and endothelial dysfunction. Diagnostics (Basel). 13(1), 55 (2022).
Smith, V. et al. Fast track algorithm: How to differentiate a “scleroderma pattern” from a “non-scleroderma pattern”. Autoimmun. Rev. 18(11), 102394 (2019).
Smith, V. et al. Standardisation of nailfold capillaroscopy for the assessment of patients with Raynaud’s phenomenon and systemic sclerosis. Autoimmun. Rev. 19(3), 102458 (2020).
Thakkar, V. & Lau, E. M. T. Connective tissue disease-related pulmonary arterial hypertension. Best Pract. Res. Clin. Rheumatol. 30(1), 22–38 (2016).
Ruopp, N. F. & Cockrill, B. A. Diagnosis and treatment of pulmonary arterial hypertension: A Review. JAMA : J. Am. Med. Assoc. 327(14), 1379–1391 (2022).
Cansu, D. Ü. & Korkmaz, C. Pulmonary hypertension in connective tissue diseases: epidemiology, pa thogenesis, and treatment. Clin. Rheumatol. 42(10), 2601–2610 (2023).
Simonneau, G. et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 53(1), 1801913 (2019).
Celermajer, D. S. & Marwick, T. Echocardiographic and right heart catheterization techniques in patien ts with pulmonary arterial hypertension. Int. J. Cardiol. 125(3), 294–303 (2008).
Willens, H. J. et al. Noninvasive differentiation of pulmonary arterial and venous hypertens ion using conventional and Doppler tissue imaging echocardiography. J. Am. Soc. Echocardiogr. : Official Publ. Am. Soc. Echocardiogr. 21(6), 715–719 (2008).
Giuggioli, D. et al. Peripheral Microangiopathy Changes in Pulmonary Arterial Hypertension Related to Systemic Sclerosis: Data From a Multicenter Observational Study. Front. Cardiovasc. Med. 9(11), 924899 (2022).
Ruaro, B. et al. The Relationship between Pulmonary Damage and Peripheral Vascular Mani festations in Systemic Sclerosis Patients. Pharmaceuticals (Basel, Switzerland) 14(5), 403 (2021).
Aletaha, D. et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 62(9), 2569–2581 (2010).
Aringer, M. EULAR/ACR classification criteria for SLE. Sem. Arthritis Rheum. 49(3S), S14–S17 (2019).
Lundberg, I. E. et al. 2017 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Adult and Juvenile Idiopathic Inflammatory Myopathies and Their Major Subgroups. Arthritis Rheumatol. 69(12), 2271–2282 (2017).
Shiboski, C. H. et al. 2016 American college of Rheumatology/European League against rheumatism classification criteria for primary Sjogren’s Syndrome: A consensus and data-driven methodology involving three international patient cohorts. Arthritis Rheumatol. 69(1), 35–45 (2017).
van den Hoogen, F. et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 65(11), 2737–2747 (2013).
Fisher, M. R. et al. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am. J. Respir. Crit. Care Med. 179(7), 615–621 (2004).
El Miedany, Y. et al. Nailfold capillaroscopy: tips and challenges. Clini. Rheumatol. 41(12), 3629–3640 (2022).
Ferrari, G. et al. Antiphospholipid antibodies and anticoagulant therapy: capillaroscopic findings. Arthritis Res. Ther. 23(1), 175 (2004).
Sulli, A. et al. Scoring the nailfold microvascular changes during the capillaroscopic analysis in systemic sclerosis patients. Ann. Rheum. Dis. 67(6), 885–887 (2008).
Corrado, A. et al. Nailfold capillaroscopic changes in patients with idiopathic pulmonary arterial hypertension and systemic sclerosis-related pulmonary arterial hypertension. Microvasc. Res. 114, 46–51 (2017).
Hassoun, P. M. Pulmonary arterial hypertension complicating connective tissue disease s. Sem. Respir. Crit. Care Med. 30(4), 429–439 (2009).
Arvanitaki, A. et al. Nailfold videocapillaroscopic changes in patients with pulmonary arterial hypertension associated with connective tissue diseases. Rheumatol. Int. 41(7), 1289–1298 (2021).
Levine, D. J. Pulmonary arterial hypertension: updates in epidemiology and evaluatio n of patients. Am. J. Manag. Care 27(3 Suppl), S35–S41 (2021).
Rabinovitch, M. et al. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ. Res. 115(1), 165–175 (2014).
Dimopoulos, S. et al. Peripheral muscle microcirculatory alterations in patients with pulmonary arterial hypertension: a pilot study. Respir. Care 58(12), 2134–2141 (2013).
Lau, E. et al. Epidemiology and treatment of pulmonary arterial hypertension. Nat. Rev. Cardiol. 14(10), 603–614 (2017).
Richter, M. J. et al. The prognostic impact of thyroid function in pulmonary hypertension. J. Heart Lung Transplant 35(12), 1427–1434 (2016).
Pi, H. et al. Thyroid-stimulating hormone and mortality in pulmonary arterial hypertension. BMJ Open Respir. Res. 9(1), e001348 (2022).
Haque, A. et al. Pulmonary hypertension phenotypes in patients with systemic sclerosis. Eur. Respiratory Rev.: An Off. J. Eur. Resp. Iratory Soc. 30(161), 210053 (2021).
Jais, X. et al. Immunosuppressive therapy in lupus- and mixed connective tissue diseas e-associated pulmonary arterial hypertension: a retrospective analysis of twenty-three cases. Arthritis Rheumatism 58(2), 521–531 (2008).
Arvanitaki, A. et al. Nailfold videocapillaroscopic changes in patients with pulmonary arterial hypertension associated with connective tissue diseases. Rheumatol Int 41(7), 1289–1298 (2021).
Kello, N. et al. Secondary thrombotic microangiopathy in systemic lupus erythematosus a nd antiphospholipid syndrome, the role of complement and use of eculiz umab: Case series and review of literature. Sem. Arthritis Rheumatism 49(1), 74–83 (2019).
Anyfanti, P. et al. Nailfold videocapillaroscopy for the evaluation of peripheral microang iopathy in rheumatoid arthritis. Life (Basel, Switzerland) 12(8), 1167 (2022).
Mugii, N. et al. Association between nail-fold capillary findings and disease activity in dermatomyositis. Rheumatology (Oxford) 50(6), 1091–1098 (2011).
Ornowska, S. et al. Microvascular damage - a marker of specific organ involvement in mixed connective tissue disease?. Reumatologia 59(2), 115–120 (2021).
Lercara, A. et al. Microvascular status in juvenile Sjogren’s disease: the first nailfold videocapillaroscopy investigation. Clin. Rheumatol. 43(2), 733–741 (2024).
Rothe, F. et al. Fluorescence optical imaging feature selection with machine learning for differential diagnosis of selected rheumatic diseases. Front. Med. (Lausanne) 10, 1228833 (2023).
Hysa, E. et al. Specific autoantibodies and microvascular damage progression assessed by nailfold videocapillaroscopy in systemic sclerosis: Are there pecul iar associations? An Update. Antibodies (Basel, Switzerland) 12(1), 3 (2023).
Dol, C., et al., [High anti-topoisomerase-1 autoantibodies levels are associated with the extension of skin fibrosis and vascular progression in patients with systemic sclerosis]. Rev Med Interne, 2024.
Schlager, O. et al. Associations of nailfold capillary abnormalities and immunological markers in early Raynaud’s phenomenon. Scand. J. Rheumatol. 43(3), 226–233 (2014).
Mueller, M. et al. Relation of nailfold capillaries and autoantibodies to mortality in patients with raynaud phenomenon. Circulation 133(5), 509–517 (2016).
Donnarumma, J. F. S. et al. Nailfold capillaroscopy as a risk factor for pulmonary arterial hypertension in systemic lupus erythematosus patients. Adv. Rheumatol. 59(1), 1 (2019).
Farrero, M. et al. Pulmonary hypertension is related to peripheral endothelial dysfunction in heart failure with preserved ejection fraction. Circ. Heart Fail. 7(5), 791–798 (2014).
Humbert, M. et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary h ypertension. Eur. Heart J. 43(38), 3618–3731 (2022).
Haque, A. et al. Pulmonary hypertension phenotypes in patients with systemic sclerosis. Eur. Respir. Rev.: An Official J. Eur. Resp. Iratory Soc. 30(161), 210053 (2017).
Funding
National Natural Science Foundation of China,81802126
Author information
Authors and Affiliations
Contributions
Zhicheng Tang and Haolin Wu performed nailfold videocapillaroscopy examination, analysed the data and wrote the manuscripts. Fan Yang and Ying Zhao analysed the data, Jingyi Shen, Huiming Hong and Fanzhang Yin conceptualized the idea, Xiaolei Ma and Linyu Geng revised the manuscript, Xue Xu and Yu Wei conceptualized the idea, provided technical support and revised the manuscript, Huayong Zhang conceptualized the idea, revised the manuscript, granted project funding and supervised the project. All authors approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical statement
The Ethics Committee of the Nanjing Drum Tower Hospital approved the study(No. 2024–160-01), and informed consent was obtained from all participants.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tang, Z., Yang, F., Wu, H. et al. Alterations in nailfold videocapillaroscopy among patients with connective tissue diseases combined with pulmonary arterial hypertension: A cross-sectional study. Sci Rep 15, 8647 (2025). https://doi.org/10.1038/s41598-025-92093-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-92093-7