Abstract
In this study, a severely damaged official document produced in the Qing Dynasty (1911 A.D.), was characterized using a range of techniques and subsequently restored using the traditional Chinese conservation methods. The pH and crystalline index of the paper were measured using a pH meter, attenuated total reflectance Fourier-transform infrared spectroscopy (ATR-FTIR), and X-ray Diffraction (XRD) spectroscopy. The results indicate that the paper archive is acidic and degraded, which is also supported by the broken fibres as observed using scanning electron microscopy (SEM). Then, the properties and durability of the conservation materials, including the conservation paper made of Wikstroemia fibre and wheat starch paste, were explored by determination of colour difference, whiteness, pH, tensile strength, and observation of cross-sectional features before and after dry- and moist-heat degradation. The results demonstrate the conservation materials presented good durability and were then used to perform subsequent conservation treatments, including the assembly of paper pieces and the application of backing paper, to provide additional mechanical support for the preservation of the historical paper archive.
Similar content being viewed by others
Introduction
Paper literature archives are an essential part of tangible cultural heritage, recording historical, cultural, and societal records on paper as a medium1,2. A variety of papermaking techniques and storage environments often lead to several deterioration issues, including acidification, tearing, staining, etc.3,4,5, which significantly affects readability and usability6. Facing with these problems, it is of great importance to carry out conservation treatments for such paper artefacts7.
An official document from the Qing Dynasty (Fig. 1), produced in the third year of the Xuantong reign (1911 A.D.) in Nanyang College, was relocated to Xi’an Jiaotong University (XJTU) during the “Westward Relocation”. It is a movement where part of Jiaotong University was transferred from Shanghai in eastern China to Xi’an in the west to support the national industrial development strategy in the western region during the 1950s. This paper archive not only preserves a historical record of Xi’an Jiaotong University but also embodies the enduring spirit of the Westward Relocation, passing down this legacy down through generations.
Given exposure to undesirable storage conditions, this paper archive has suffered from severe acidification, cracking, discolouration, mold growth, and water stains, and some parts of the paper have been completely lost. These issues highlight the urgent need for conservation measures, as recommended by the XJTU Archives. In this study, we conducted deacidification and reinforcement treatments as well as characterization for this paper archive, in order to mitigate further potential damages and extend its lifetime for future preservation.
Deacidification treatments are widely recognized for their effectiveness in neutralizing acids and providing an alkaline reserve within paper6,8,9,10,11, which has been widely used in practices12,13. A study by Irene Alexopoulou and Spiros Zervos (2016) indicated that the aqueous deacidification methods using calcium hydroxide and magnesium bicarbonate account for 66.1% and 27.4% worldwide respectively based on a survey of 402 samples from institutions practicing paper conservation, including national libraries, archives, museums, etc7. Calcium hydroxide is known as an efficient traditional deacidification agent, but it reduces the tensile strength of the paper and may cause yellowing of lignin-containing papers due to its high pH14. The use of the magnesium bicarbonate solution demonstrated a better effect on maintaining the folding strength of the paper15, making it suitable for deacidifying historical newspapers6, publication of books, and archival files13.
Reinforcement treatments are necessary for the paper archives that are severely damaged and have lost mechanical strength4,7. Various types of papers have been reported for backing and reinforcement, including Japanese washi paper, Chinese Xuan paper, papers made of mulberry bark, and etc6. During the traditional Chinese conservation process, handmade papers are commonly used as backing papers and reinforcing strips16,17, and the wheat starch paste18,19, serves as an adhesive due to its good adhesion, reversibility, low cost and environmental friendliness4,20. In some cases, the methyl cellulose (MC) can also be used as a substitute of starch paste7.
Based on the above background and suggestions given by the conservators in Engineering Research Center of Historical Cultural Heritage Conservation, Shaanxi Normal University, conservation treatments were conducted by using the handmade paper made of Wikstroemia fibres the mounting paper, wheat starch paste as the adhesive, and magnesium bicarbonate solution as an deacidification agent respectively. In addition, characterization was carried out for the paper archive before and after conservation treatment, in order to investigate the material properties21,22. In the study, paper pH, fibre morphology, and crystalline index were explored using the non-invasive pH measurement, scanning electron microscopy (SEM), attenuated total reflectance Fourier-transform infrared spectroscopy (ATR-FTIR), and X-ray diffraction (XRD) for the paper archive before conservation treatment. Also, the properties and durability of the conservation materials were investigated by examining the whiteness, colour difference, pH, tensile strength, and fibre cross-section analyses. Conservation treatments were then carried out for this historical paper archive, including the assembly of the pieces of the paper archive and pasting backing paper, to provide extra mechanical support.
Experimental section
Samples
Three types of paper samples were used in this research. The first sample type is a small piece of residue dropped from the historical paper archive, i.e., an official document from 1911 A.D. It was used to explore the crystalline index of the paper sample using ATR-FTIR and XRD spectroscopy.
The second type of sample was made from the conservation materials by the curators with years of experience. The wheat starch paste was used to adhere two contemporary mounting papers (made from Wikstroemia fibres) together, in order to evaluate the stability and durability of these conservation materials as applied in practical conservation treatments. Then this sample was exposed to both dry-heat degradation under 105 °C for 72 h in the oven and moist-heat degradation under 80 °C and 65% relative humidity for 72 h in the environmental chamber (HCP150, Memmert) following the standard ISO 5630-1:200823 and ISO 5630-3:200824 respectively.
The third type of model acid paper sample was prepared by acidifying A4 paper with phosphoric acid to match the pH level of the historical paper archive, in order to explore the deacidification effect using the saturated magnesium bicarbonate solution.
pH measurement
Non-destructive pH measurements were conducted for the historical paper sample using a portable pH meter (3310 SET 2, WTW) coupled with a flat-tip electrode (SenTix41, Munich). A drop of ultrapure water was dropped on the paper surface, subsequently, and the flat-tip electrode head was placed on the water drop until the pH value stabilised. Calibration of the pH meter was performed before each measurement.
pH measurement using the cold extraction method was performed for the second and third type of samples. 0.5 g paper sample was weighed and cut into 5 mm2 pieces according to ISO 6588-1:202125 and 25 mL of ultrapure water was added by shaking the beaker to wet the paper. The beaker was left at room temperature for 1 h, during which time the beaker was shaken once. Finally, the resulting solution was transferred into a small clean beaker and the pH of the resulting solution was tested using a pH meter (S210, Mettler Toledo). The pH meter was calibrated with a standard buffer solution before testing.
SEM and observation under polarising light microscope
The historical paper sample and the deacidified model paper samples were fixed on the sample stage with conductive adhesive and then sprayed with gold for 90 s (Q150R+, Quorum Technologies). A tungsten scanning electron microscope (SU3500, Hitach) was used for analysis with an accelerating voltage of 10 kV. Also, SEM-EDS elemental mapping was carried out to observe micromorphology and homogeneity of magnesium bicarbonate in the third type of sample.
In order to better observe cross-sections of the contemporary mounting papers, i.e., the second type of paper sample used in this study, this paper sample before and after degradation was embedded in a resin mold (Transparent Cold Mount, Shandong Laizhou). After solidification at room temperature, the sample was extracted using a low-density cutter operating g at 440 rpm (DTQ-5, Veiyee). The cross-section of the sample was then observed using a polarising light microscope (X53M, Olympus) with an objective lens at 20 × magnification, and then this sample was observed under the SEM with a magnification at 250 × magnification, and a resolution of 200 μm.
ATR-FTIR spectroscopy and XRD
Non-destructive FTIR analysis of the paper samples was performed using an attenuated total reflectance Fourier-transform infrared (ATR-FTIR) spectrometer (PE-Frontier, Perkin Elmer) equipped with a diamond cell from 4000 to 400 cm− 1 at a resolution of 4 cm− 1 with a total of 32 scans. The crystalline index of the historical paper residue was quantified by the empirical Eq. (1)26,27. I1732 cm−1 and I2900 cm−1 are the intensity of the absorption peak at 1732 cm− 1 and 2900 cm− 1, respectively.
A high-resolution X-ray diffractometer (Smart Lab, Rigaku Corporation) was also used to explore the crystalline index for this sample, utilizing Cu Kα radiation (λ = 1.5406 Å) over a 2θ range of 5–45°, with an acceleration voltage of 45 kV, tube current of 200 mA, and a scanning speed of 5°/min. The crystalline index of paper fibres is a calculated according to Eq. (2)26,28.
where I002 is the maximum diffraction intensity of the 002 crystal plane at 2θ = 22.8°, and Iam is the diffraction intensity at approximately 2θ = 18°, i.e. the diffraction intensity in the amorphous region.
Colour difference test and whiteness test
The CIE L*a*b* system29 was used to evaluate the colour difference before and after degradation for the conservation material samples, i.e., the second type of model sample, using a non-contact spectrophotometer (VS450, X-Rite). The total colour difference (ΔE*) was calculated using the following equation:
where ΔE* is the colour difference, ΔL* is the brightness difference, Δa* is the red and green deviation, and Δb* is the yellow and blue deviation. According to the standard ISO 11476:201030, a whiteness tester (WSB-2, Pingxuan) was used to test the whiteness of the reinforcement material samples.
Tensile strength
The tensile strength tests were conducted based on the standard ISO 1924-3:200531. The conservation material samples were cut into strips of 150 mm × 15 mm. The tensile strength was measured using a universal material testing machine (QT-1136 PC, Quatest) with a tensile speed of 20 mm/min. The paper samples were cut in machine direction (MD) and cross direction (CD) to assess the strength properties.
Statistical analysis
Each measurement was performed at least three times. Statistical analysis was carried out using the SPSS 26.0 statistical package (SPSS Inc., Chicago, IL), and Duncan’s multiple range test was applied to determine significant differences between data. The significance levels were defined as follows, ns: P > 0.05; *: P ≤ 0.05; **: P ≤ 0.01.
Results and discussion
Material properties of the historical paper archive
FTIR spectra were collected to explore the extent of ageing by calculating the crystalline index, as shown in Fig. 2a. The peaks at 3336 and 2897 cm− 1, are attributed to the O-H stretching and C-H stretching vibrations, respectively. The absorption peak at 1640 cm− 1 is attributed to the water content, indicating that the samples are hygroscopic32. The absorption bands at 1423, 1370, and 1317 cm− 1 are assigned to C-H asymmetric bending, and 1029 cm− 1 is assigned to C-O bond stretching, separately. The vibrational peak of the glycosidic bond at 898 cm− 1. These peaks are characteristic of cellulose11,33,34. According to the Eq. (1), the crystalline index of the filter paper made from cotton fibres is 46.95% and that of the archival paper sample is 44.54%. The lower crystalline index for the historical paper sample indicates its degradation, which is also confirmed by the use of the XRD technique.
As shown in Fig. 2b, the diffraction peak intensities of the filter paper and the historical archival paper are approximately 420 and 80 at 22.8°, and the diffraction peak intensities at 18.8° trough are 80 and 30, respectively. The crystalline index of the filter paper and the archival paper were calculated to be 81% and 63% using the Eq. (2), respectively, demonstrating the lower content of crystalline of the historical paper.
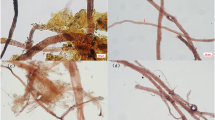
The pH of the top, centre and bottom parts of the historical archival paper was determined to be 3.31, 3.39, and 3.30 respectively, with minimal measurement uncertainties using the non-invasive method, demonstrating that the archive has been severely acidified and damaged, probably due to the inappropriate storage conditions over the last century. This is also supported by the observation of the fibre morphology as shown in Fig. 2c and d, where the crisscrossed fibres form a network structure35. Most of the fibres have broken, leading to numerous short single fibres. These broken fibres indicate that the bonding strength between the fibres has decreased and thus the paper archive has degraded to a large extent, suggesting the urgency and significance of subsequent reinforcement and conservation treatment.
Evaluation of reinforcement materials
Paper degradation commonly results in changes in colours, which were evaluated by measuring L*, a*, and b* parameters18. The restoration materials, i.e., the second type of sample, were found to be darker and more yellowish after both dry- and moist-heat degradation, especially under the condition of moist-heat degradation, as shown in Fig. 3a. The colour changes in Table 1 also support this finding. Compared to the samples before degradation, the L*, a*, and b* parameters after dry-heat degradation showed statistically significant differences (p ≤ 0.05), while after moist-heat degradation, they exhibited highly significant differences (p ≤ 0.01). ΔE* of this model paper is above 5 before and after degradation under both conditions, belonging to the significant changes, according to National Bureau of Standards (NBS) units of colour difference36. The colour change under moist-heat degradation was greater than that of dry-heat degradation. Given that both cellulose and hemicellulose in the paper can degrade and yield a large number of typical chromogenic groups, such as carboxyl and carbonyl groups37. It is also possible that the polysaccharide, i.e., the presence of starch in this model sample, could also degrade and produce the chromophore group18.
Figure 3b shows the values of visible light reflectance from the surface of the second type of paper sample, i.e., made from the restoration materials. The reflectance values decreased to a large extent in the 400–700 nm on the surface of the samples under both degradation conditions. This decline in reflectance further confirms that the chromogenic groups lead to the change in colour of the paper samples and affect their reflectance performance.
The whiteness of paper samples is shown in Table 1. Paper whiteness is the ability of paper to fully reflect light when exposed to light, i.e. the brightness of the paper expressed as a percentage38. The whiteness of the paper sample before degradation is 60.18%, which is reduced by 4.43% after dry-heat degradation, and by 8.01% after moist-heat degradation, both of which showed a highly significant difference (p ≤ 0.01), indicating that both temperature and moisture could affect the paper whiteness4. Therefore, in order to extend the longevity of such objects, it is necessary to maintain stable and moderate environmental conditions.
The pH of the second paper sample before and after degradation is listed in Table 1. Before degradation, the pH of the sample was 6.52—slightly acidic, which can be attributed to the inherent mild acidity of starch paste used as an adhesive39, given that the paper pH of the conservation paper itself was determined to be 7.85. There was no statistically significant difference in the pH values before and after the dry-heat degradation (p > 0.05), while a highly significant difference was observed for pH values before and after the moist-heat degradation (p ≤ 0.01). After degradation, slight pH decreases indicate that high temperature and humidity contribute to further acidification of the sample11,40.
The tensile strength is another important indicator to evaluate paper stability41. As presented in Fig. 4, comparing with the samples before degradation, the CD tensile strength of the dry-heat degraded paper samples decreased by 10.63% with a statistically significant difference (p ≤ 0.05), while the MD tensile strength decreased by 13.85% with no statistically significant difference (p > 0.05). The CD tensile strength of the moist-heat degraded paper samples was maintained at 94.21% with no statistically significant difference (p > 0.05), and the MD tensile strength remained at 81.50%, showing a statistically significant difference (p ≤ 0.05). This suggests that the mechanical properties of this paper sample type made from the conservation materials can be maintained after both dry- and moist-heat degradation.
To assess whether the starch adhesive retains its pasting properties after degradation under two conditions, i.e., whether the two layers of restoration papers would separate after degradation, the cross sections of the second type of paper sample were observed using a microscope, as presented in Fig. 5a1-c1. No laminated structure can be observed, with a thickness of approximately 94 μm for this sandwich-like structure, with a 250 × magnification (Fig. 5a2-c2), demonstrating the good pasting properties and stability of the wheat starch paste.
Effectiveness of deacidification
After deacidification using a saturated magnesium bicarbonate solution, the pH of the acidified model paper (i.e., the third type of paper sample) increased from 2.71 to 8.87, indicating a good deacidifying effect. No obvious changes were observed in the paper sample before and after deacidification when viewed with the naked eye (Fig. 6a1 and a2). Upon examining the sample with SEM (Fig. 6b1 and b2), the morphology of the fibres in the deacidified paper also did not change significantly. Additionally, magnesium bicarbonate particles were observed on the fibre surface and within the fibre gaps. The presence of evenly distributed Mg was verified by the EDS results (Fig. 6c1 and c2) and the elemental mapping image (Fig. 6d).
Pictures of the acidified paper sample (a1) before and (a2) after deacidification using saturated magnesium bicarbonate solution; SEM images of the acidified paper sample (b1) before and (b2) after deacidification using saturated magnesium bicarbonate solution; EDS results of the acidified paper sample (c1) before and (c2) after deacidification; (d) Mg elemental mapping image of the acidified paper sample after deacidification.
Restoration process step by step
Rough assembly: A sheet of dry Xuan paper was placed on the flat conservation table as assisting paper (labeled as assisting paper No. 1). The folded historical paper archive was then carefully unfolded from left to right completely onto assisting paper No. 1, keeping the front side (the side with characters) facing up (Fig. 7a). The pieces of the paper archive were arranged to match their original format.
Wetting the historical paper archive: Another sheet of Xuan paper was prepared and used as assisting paper No. 2. This paper was sprayed with water, and excessive water was absorbed using a wet towel to ensure the moisture was evenly distributed so it could be attached to the conservation table (Fig. 7b).
Fine assembly: Each piece of the historical paper archive on assisting paper No. 1 was moved to assisting paper No. 2, maintaining the front sides were kept up. Water was sprayed onto the historical paper to keep it wet, allowing it to adhere to assisting paper No. 2. Then, these pieces of the historical paper archive were assembled under the wet condition in their original format (Fig. 7c).
Reverse the paper archive upside down: A third sheet of wet Xuan paper was used as assisting paper No. 3, and placed on the assembled historical paper archive. In order to make this sandwich structure (i.e., assisting paper No. 3, historical paper archive, assisting paper No. 2 from top to bottom) adhere completely, excessive air was removed using the hog-bristle brush. This sandwich structure was reversed upside down on the conservation table (Fig. 7d), with the back side of the historical paper archive facing up, where the assisting paper No. 2 was on the very top, and the assisting paper No. 3 was on the bottom.
Micro-assembly: The assisting paper No. 2 was then removed, and the pieces of the historical paper archive were assembled again using the brush pen (Fig. 7e). The dry Xuan paper was used to absorb excessive water.
Application of the backing paper: The conservation paper, i.e., bast paper (made from Wikstroemia fibre), was dyed to match the colour of the archival paper. Then, the diluted wheat starch paste was applied homogeneously to this conservation paper. The conservation paper with the starch paste was pasted to the backside of the historical paper archive, continuously removing the air using the brush (Fig. 7f). This step aims to mend holes and tears in the paper archive and to provide additional support for display or handling.
Gently tapping: The reinforcing strips (3 mm wide) were then pasted on the backing paper using the starch paste (Fig. 7g). A soft hammer was used to gently tap the paper archive with the backing paper to attach the paper archive and the backing paper tightly.
Adjusting the backing paper: Finally, the restored paper archive attached to the backing paper and the assisting paper No. 3 were reversed back to the initial status of the paper archive in Step 1, and the assisting paper No. 3 was removed from the paper archive (Fig. 7h). Then, the restored paper archive attached to the backing paper was air-dried for 2 days, after which the edge of the backing paper was trimmed to make its size the same as the size of the historical paper archive.
Images of the historical paper archive before and after conservation and restoration are shown in Figs. 8 and 9, where the paper pieces were well assembled, with holes and cracks mended and repaired, and the backing paper gives extra physical support for better reading or handling. The average thickness of the historical paper archive increased from 56 μm to 120 μm after restoration, due to the conservation paper being adhered to the back of the archive. The pH of the restored paper archive increased to 7.58, which gives appropriate alkaline reserve to the paper archive for better long-term preservation and management42.
Conclusion
In this study, a series of techniques were applied to characterize, conserve, and deacidify a severely damaged paper archive stored in the XJTU Archives, produced in 1911 A.D. during the Qing Dynasty with an initial pH at ~ 3.33. This historical paper was characterized using SEM, ATR-FTIR, and XRD spectroscopy, with the results showing a decrease in the crystalline index and significant breakage of the fibres, highlighting the urgent need for deacidification and conservation treatments.
Also, the durability of the conservation materials selected by the conservators, made from bast (from Wikstroemia) paper pasted using the wheat starch paste, was investigated. The colour difference and whiteness results indicate that the paper sample becomes slightly yellowish after both dry- and moist-heat degradation. Additionally, the mechanical properties of the paper sample and the adhesive properties of the wheat starch paste exhibited good stability even after degradation under the two conditions, as explored using the tensile strength measurements and observation of the cross-sectional images of this sample. Deacidification using a saturated magnesium bicarbonate solution shows effective deacidifying effects for the acidic model paper samples, with homogeneous magnesium bicarbonate particles distributed in the paper fibres.
Based on the damaging condition of the historical paper archive, good durability of the conservation materials, and deacidification effects using the saturated magnesium bicarbonate solution, the paper archive was ultimately conserved and deacidified using the traditional Chinese conservation mounting method, in order to improve fitness-for-use and prolong its lifetime.
Data availability
All datasets used and/or analysed in this study are available on request from the corresponding author.
References
Liang, X. et al. Electrochemical removal of stains from paper cultural relics based on the electrode system of conductive composite hydrogel and PbO2. Sci. Rep. 7, 8865–8877. https://doi.org/10.1038/s41598-017-08907-w (2017).
Wang, Q., Guo, X., Xu, Z. & Fan, H. The ageing and color development of writing in paper archives. Chem. Pap. 78, 6553–6562. https://doi.org/10.1007/s11696-024-03554-8 (2024).
Isca, C., Fuster-López, L., Yusá-Marco, D. J. & Casoli, A. An evaluation of changes induced by wet cleaning treatments in the mechanical properties of paper artworks. Cellulose 22, 3047–3062. https://doi.org/10.1007/s10570-015-0712-1 (2015).
Liu, J. et al. A new reinforcement method for the conservation of fragile, double-sided, printed paper cultural relics. Herit. Sci. 9, 123–133. https://doi.org/10.1186/s40494-021-00597-y (2021).
Malesic, J., Marinsek, M. & Cigic, I. K. Evaluation of bookkeeper mass deacidification based on historical book papers. Cellulose. 29, 6889–905. https://doi.org/10.1007/s10570-022-04681-9 (2022).
Liu, J. et al. An essential role of polymeric adhesives in the reinforcement of acidified paper relics. Polymers 14, 207–220. https://doi.org/10.3390/polym14010207 (2022).
Alexopoulou, I. & Zervos, S. Paper conservation methods: an international survey. J. Cult. Herit. 21, 922–930. https://doi.org/10.1016/j.culher.2016.04.001 (2016).
Cheng, L. Protection and restoration of the Jiuwang daily published in Guilin in 1940. Sci. Conserv. Archaeol. 30, 85–90. https://doi.org/10.16334/j.cnki.cn31-1652/k.2018.04.011 (2018).
Lin, Y. Introduction to restoration techniques for foreign paper archives. Beijing Arch. 12, 32–33 (1999).
Baty, J. W., Maitland, C. L., Minter, W., Hubbe, M. A. & Jordan-Mowery, S. K. Deacidification for the conservation and preservation of paper-based works: A review. Bioresources 5, 1955–2023 (2010).
Zhang, C. et al. Exploration of effects by the ‘alum-glue solution’ on Xuan paper degradation using a 23 factorial design experiment. J. Cult. Herit. 64, 42–48. https://doi.org/10.1016/j.culher.2023.08.012 (2023).
Yu, C. A comparative study of restoration of archives and antiquities-paper archives and antiquities as an example. East. China Pap. 50, 1–7 (2020).
Sui, L. Paper-based revolutionary cultural relics protection and restoration of the first exploration-to the eight route army Jiaodong military region organs of the former site of the memorial hall cultural relics as an example. Orient. Collect. 2, 110–112 (2023).
Ni, Q. Research on the deacidification agents and methods for paper archive. China Pulp Pap. Ind. 42, 32–36 (2021).
Xi, S. The summary of deacidification and consolidation of paper cultural relics. Sci. Conserv. Archaeol. 20, 85–94. https://doi.org/10.16334/j.cnki.cn31-1652/k.2008.s1.012 (2008).
Liu, P. et al. Comprehensive evaluation of paper matching for restoration of paper cultural relics-taking the golden light Sutra as an example. J. Fudan Univ. (Nat. Sci.). 60, 665–670. https://doi.org/10.15943/j.cnki.fdxb-jns.2021.05.010 (2021).
Mullock, H. Xuan paper. Pap. Conserv. 19, 23–30. https://doi.org/10.1080/03094227.1995.9638410 (2010).
Zhao, H., Zhang, H., Xu, Q., Zhang, H. & Yang, Y. Thermal, rheological, structural and adhesive properties of wheat starch gels with different potassium alum contents. Molecules 28, 6670–6686. https://doi.org/10.3390/molecules28186670 (2023).
Microscopy for paper conservation: Comparing various adhesives and examining wheat starch paste preparation methods (2011).
Ma, X. The comparison of the functions between CMC paste and wheat starch paste in the file mountingtechnology. J. Guangxi Univ. Nationalities. 2, 81–86 (1996).
Ma, W. Some problems in the restoration of painting and calligraphy relics—Qing dynasty -Huang Xiangjian squabbling mountains axle restoration documentary. Herit. Restor. Res. 00, 381–388 (2014).
Liu, P. et al. Analysis of Qing dynasty calligraphy by Cao Hongxun. Anal. Lett. 57, 2572–2581. https://doi.org/10.1080/00032719.2023.2299438 (2024).
ISO 5630-1:2008, Paper and board—Accelerated ageing—Part 1: Dry heat treatment at 105°C (2008).
ISO 5630-3:2008, Paper and board—Accelerated ageing—Part 3: Moist heat treatment at 80°C and 65% relative humidity (2008).
ISO 6588-1:2021, Paper, board and pulps—Determination of pH of aqueous extracts—Part 1: Cold extractio (2021).
Lv, Z. et al. Identification of dominant taxa of sooty moulds and their impact on the leaf Microbiome. Environ. Microbiol. 25, 853–866. https://doi.org/10.1111/1462-2920.16321 (2023).
Carvalho, B. F. et al. Occurrence of mycotoxins and yeasts and moulds identification in corn silages in tropical climate. J. Appl. Microbiol. 120, 1181-92. https://doi.org/10.1111/jam.13057 (2016).
Alía, A. et al. Identification and control of moulds responsible for black spot spoilage in dry-cured Ham. Meat Sci. 122, 16–24. https://doi.org/10.1016/j.meatsci.2016.07.007 (2016).
McLaren, K. XIII—The development of the CIE 1976 (L* a* b*) uniform colour space and colour-difference formula. J. Soc. Dyers Colour. 92, 338–341. https://doi.org/10.1111/j.1478-4408.1976.tb03301.x (2008).
ISO 11476. Paper and board—Determination of CIE whiteness, C/2° (indoor illumination conditions) (2010) (2010).
ISO 1924-3:2005, Paper and board—Determination of tensile properties — Part 3: Constant rate of elongation method (100 mm/min) (2005).
Li, Y. et al. Deacidification and consolidation of brittle book paper using bacterial cellulose composite with zinc oxide nanoparticles. J. Cult. Herit. 64, 83–91. https://doi.org/10.1016/j.culher.2023.09.003 (2023).
Luo, Y., Liu, Y., Wei, Q. & Strlic, M. NIR spectroscopy in conjunction with multivariate analysis for non-destructive characterization of Xuan paper. Herit. Sci. 12, 175–184. https://doi.org/10.1186/s40494-024-01287-1 (2024).
Wu, C., Jin, C., Zhu, Z., Liu, P. & Zhang, H. Research on measuring methods of crystal structure of paper cellulose. J. Fudan Univ. (Nat. Sci.) 61, 589–97. https://doi.org/10.15943/j.cnki.fdxb-jns.20221014.001 (2022).
Manandhar, S., Shrestha, B., Sciortino, F., Ariga, K. & Shrestha, L. K. Recycling waste paper for further implementation: XRD, FTIR, SEM, and EDS studies. J. Oleo Sci. 71, 619–626. https://doi.org/10.5650/jos.ess21396 (2022).
Mao, T. et al. Study on the performance of acrylic polyurethane for the protection of handwriting on paper relics. Coatings 13, 822–838. https://doi.org/10.3390/coatings13050822 (2023).
Hubbe, M. A. et al. Archival performance of paper as affected by chemical components: A review. Bioresources 18, 6430–6498. https://doi.org/10.15376/biores.18.3.Hubbe (2023).
Liu, C. & Li, Y. Study and analysis of optical properties of paper surfaces. Print Today. 5, 63–64. https://doi.org/10.16004/j.cnki.pt.2017.05.016 (2017).
Zhang, W., Han, X., Guo, H., Wang, M. & Ji, L. Study on improvement of traditional Chinese medicine paste. Adhesion 50, 1–5 (2023).
Yang, S. et al. Research on ageing and acidification performance of commercial archival paper. Ferroelectrics 593, 158–165. https://doi.org/10.1080/00150193.2022.2076452 (2022).
Havlínová, B., Katuščák, S., Petrovičová, M., Maková, A. & Brezová, V. A study of mechanical properties of papers exposed to various methods of accelerated ageing. Part I. The effect of heat and humidity on original wood-pulp papers. J. Cult. Herit. 10, 222–231. https://doi.org/10.1016/j.culher.2008.07.009 (2009).
Di Napoli, B. et al. Gellan gum microgels as effective agents for a rapid cleaning of paper. ACS Appl. Polym. Mater. 2, 2791–801. https://doi.org/10.1021/acsapm.0c00342 (2020).
Acknowledgements
The authors are grateful to the Archives of Xi’an Jiaotong University for the support of the historical archive for this research, also appreciate Lei Zhang and Yangchu Yan for their contributions to the restoration of the paper relic, and appreciate Xi Yu for the acidified paper samples.
Funding
This research was supported by the Key Research and Development Program of Shaanxi Province, China (2021ZDLSF06-03), the Young Scientist Initiative Project of the School of Materials Science and Engineering at the Shaanxi Normal University (2023YSIP-MSE-SNNU003), Fundamental Research Funds for the Central Universities (GK202304013), Shaanxi Key Research and Development Program (2024GX-YBXM-560), Youth Open Project from Nanjing Museum (NBKFKT0101) and Emperor Qinshihuang’ s Mausoleum Site Museum (Qkfkt202407). Special thanks go to the Fundamental Innovation Project in the School of Materials Science and Engineering (SNNU).
Author information
Authors and Affiliations
Contributions
PL (PanpanLiu): Writing original draft, Investigation. YL (Yanli Li): Writing review, Funding acquisition. HL (Huimin Li): Making samples. WZ (Wenyue Zhang): Restoring literature. YQ (Yunpeng Qi): Data curation. XZ (Xiaoya Zhang): Conceptualization. YL (Yujia Luo): Writing review, Funding acquisition. YL (Yuhu Li): Supervision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, P., Li, Y., Li, H. et al. Characterisation and restoration of a severely damaged paper archive from the Qing Dynasty. Sci Rep 15, 7380 (2025). https://doi.org/10.1038/s41598-025-92134-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-92134-1