Abstract
COVIDTrach is a UK-wide, prospective cohort study evaluating tracheostomised COVID-19 patient outcomes and operator disease transmission. Early in the pandemic controversy surrounded optimal timing of tracheostomy insertion, however meta-analyses have since addressed this uncertainty. We report on our cohort’s data and outcomes to help inform the management of this disease and compare our findings to the literature. Our inclusion criteria were COVID-19 patients aged ≥ 18 undergoing tracheostomy following invasive ventilation. We recorded relevant characteristics, clinical parameters, intra-operative details and outcome data. Predictors for mortality and time to ventilatory wean were determined. Among 1982 patients, there was a 21% post-tracheostomy mortality and median intubation to tracheostomy time of 15 days (IQR 11–21). The median time to successful ventilatory wean post-tracheostomy was 12 days (IQR 7–20). Advancing age, greater FiO2 and PEEP requirements and inotrope or anticoagulant use were associated with increased mortality (p < 0.05) and time to wean success (p < 0.01). Higher CRP predicted increased mortality (p < 0.05), while NIV use and extended pre-tracheostomy ventilation predicted prolonged wean time (p < 0.01). The death risk for tracheostomy performed ≤ 7 or ≥ 14 days of ventilation was equivocal (OR 1.01, 95% CI [0.37–2.72]) but lower between 8 and 14 days (OR = 0.64, 95% CI [0.47–0.86]) (p = 0.01). Eight operators tested positive within two weeks of performing a tracheostomy. Our mortality rates were similar to cohort studies but lower than early versus late tracheostomy designs. In contrast to the literature, we found reduced mortality when tracheostomy was performed 8–14 days post-intubation, with more favourable wean time and wean and decannulation rates.
Similar content being viewed by others
Introduction
COVIDTrach is a UK-wide, multicentre, prospective cohort study which has evaluated the outcomes of tracheostomised severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) positive patients and the incidence of disease transmission to tracheostomy operators since the emergence of the virus in 2019. Two previous publications by our collaborative1,2 reported on the earliest 563 cases in the pandemic from 78 hospitals, followed by a larger first wave review of 1605 patients from 126 contributing hospitals between April and August, 2020. The second wave of COVID-19 between September 2020 and May 20213 provided further outcome data which have been combined with the former to produce a total cohort of 1982 patients in the present analysis.
SARS-CoV-2 infection not uncommonly leads to invasive mechanical ventilation (IMV), as evidenced by a systemic review of over 12,000 COVID-19 hospital presentations across seven countries wherein the critical care admission rate was 21%, 69% of whom ultimately required IMV4. While the optimal timing of tracheostomy in any critical illness is still a source of debate5, it is traditionally indicated for periods of IMV exceeding approximately 10 days to facilitate ventilatory weaning and pulmonary toilet, reduce sedation requirements, mitigate complications such as pneumonia and laryngotracheal stenosis and improve patients’ comfort, speech and oral intake6,7,8. Despite these apparent benefits, around 50% of those tracheostomised for prolonged ventilation are dead by one year (secondary to their index illness and co-morbidities), with considerable long-term effects on quality of life in survivors9.
At the outset of the COVID-19 pandemic, delayed tracheostomy until as late as 14–21 days post-intubation in combination with negative PCR testing10,11, or complete avoidance of surgery altogether if the patient was still felt to be infectious12,13, was advocated—both to avoid viral transmission to the surgical team and operating in cases of futility14. As our understanding of the disease has progressed, it has become clearer that the presence of viral RNA as detected by PCR testing does not equate to infectiousness, particularly if antibodies are also measurable9,15. Subsequently, the relative safety of tracheostomy in COVID-19 patients has been supported by several studies16,17,18 which, although citing fairly poor-quality evidence, estimate an overall risk of COVID-19 transmission to healthcare workers of approximately 1%. In parallel, seven systematic reviews and meta-analyses have provided more data on optimal timing of tracheostomy and outcomes following the procedure16,17,18,19,20,21,22, informing clinical management and decision-making around severe COVID-19 and empowering practitioners to tracheostomise earlier in the disease course. Our interim COVIDTrach data was included in three of these major meta-analyses17,18,20, however to our knowledge the present study, with data for 1982 tracheostomised patients accrued across two pandemic waves, represents the single largest cohort to date in the field. Furthermore, contemporaneous comparisons with the cohort characteristic data from the Intensive Care National Audit & Research Centre (ICNARC) attest to the generalisability of our results to the UK as a whole2.
Herein we report on the characteristics of our patient population, timing of tracheostomy and associated weaning and mortality outcomes in order to inform the management of this newly endemic and global disease. We also compare our findings to the wealth of data and meta-analysis that has been generated in the timeframe since our last publication.
Methods
Inclusion and study design
COVIDTrach was supported by the National Institute for Health and Care Research Clinical Research Network (NIHR CRN) as one of its portfolio studies. In order to maximise engagement with every UK department undertaking tracheostomies in COVID-19 patients, COVIDTrach participation was advertised via the Intensive Care Society fora, UK Federation of Surgical Speciality Associations and affiliated groups, medical society digital platforms and social media. In total, 126 hospitals consented to study inclusion across England, Ireland, Scotland and Wales. All research was performed in accordance with relevant guidelines.
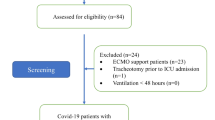
Our inclusion criteria were COVID-19 patients aged 18 or above undergoing elective tracheostomy following a period of IMV at any of the contributing sites, provided positivity for COVID-19 had been confirmed with quantitative reverse transcription (RT)-PCR testing. In the absence of viral RNA testing or a positive result, COVID-19 infection was assumed on the basis of a clear history and concordant serological and radiological evidence. Considering the scarcity of testing in the early phase of the pandemic and widespread prevalence of the disease at this time, other diagnoses presenting in this context were extremely unlikely and inclusion of patients on the above grounds justifiable.
Data collection and governance
For every patient the following data was collected: treating institution, characteristics, comorbidities, anthropometry, pre-admission symptoms, COVID-19 swab results, presence of inotropic support and anticoagulation use, pyrexial episodes, C reactive protein (CRP), pre-tracheostomy ventilatory requirements and intra-operative details including use of personal protective equipment (PPE), operator specialty and tracheostomy technique employed.
Outcome data collected were tracheostomy decannulation rates, mortality and the following intervals: admission to intubation; intubation to tracheostomy; tracheostomy to successful ventilatory wean; intubation to critical care discharge and tracheostomy to hospital discharge.
Successful ventilatory wean was defined as liberation from ventilatory pressure support for greater than 24 h, and COVID-19 positivity among tracheostomy operators in the two weeks following the procedure was also recoded.
REDCap is a secure online database management application used throughout the COVIDTrach collaboration23, enabling practitioners to upload clinical data contemporaneously and in an anonymised fashion. Patient care was not impacted and consent was not required for study inclusion as per the UK Control Of Patient Information (COPI) notice (Department of Health and Social Care, 2020).
Statistical analysis
Multivariable models for mortality and time to wean success
Data were analysed using the Stata 18 statistical software package. We used logistic regression to investigate which variables were associated with mortality. Individual data points for a given parameter were omitted if there was no recorded tracheostomy method or male or female sex, or if a hybrid technique was used. Multiple imputation was used to impute other missing predictor values and all numerical variables except age and body mass index (BMI) were log-transformed. We then fitted a multivariable logistic regression model including all predictor variables and applied backward elimination at the 10% level.
A similar approach was taken to investigate which predictors were associated with time to wean success, although we used multiple regression after log-transforming the outcome because of skewness.
Results
Characteristics and medical history
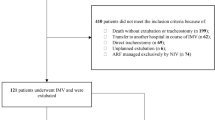
Data were collected between 6th April 2020 and 1st September 2021. Following removal of duplicates (n = 39), 1982 patients from a total of 126 hospitals were included, with the number of patients for whom data was submitted from each institution ranging from 1 to 124. The mean (standard deviation, SD) patient age was 58 ± 11 years, with a male preponderance of 68%. Table 1 details patient characteristics and medical history and Table 2 summarises COVID-19 testing results among our cohort.
Tracheostomy-related data
The median time (interquartile range, IQR) from intubation to tracheostomy was 15 days (11, 21) (Table 1, Fig. 1). On the day of surgery, the mean (SD) CRP was 107 (88) mg/L and down trending in 67% of cases (n = 1287); 37% of patients (n = 711) required inotropic support and 23% (n = 431) harboured a temperature greater than 37.5 °C. ‘Anticipated prolonged wean’ was the primary indication in 92% (n = 1833) of tracheostomies inserted, followed by ‘failed extubation’ in 12% (n = 246). Open and percutaneous tracheostomies were performed in equal numbers (995 versus 917), predominantly by ENT surgeons and ITU physicians, respectively (Fig. 2). Median (IQR) ventilatory parameters at the time of tracheostomy were an inspired oxygen concentration (FiO2) of 40% (30, 45) and a positive end-expiratory pressure (PEEP) of 8cmH20 (6, 10), with a corresponding PaO2 of 9.8 kPa (9, 11).
Mortality and time to successful ventilatory wean
There was an all-cause mortality of 21% (n = 403) following tracheostomy for COVID-19 in our study. One patient died intra-operatively, 383 died between tracheostomy and wean from ventilation and 19 died between successful wean and hospital discharge (Table 3). The median time (IQR) from tracheostomy to death was 15 days (6, 15). Eighty-eight percent of mortalities (n = 349) were attributed to COVID-19 infection and 3% (n = 13) to tracheostomy-related complications.
Tables 4 and 5 detail the variables independently associated with mortality and time to ventilatory wean success using multivariable logistic regression, expressed as odds ratios and regression coefficients, respectively. Advancing age, higher CRP, increasing interval between last pre-operative pyrexia and tracheostomy, inotropic support, anticoagulant use and higher FiO2 and PEEP requirements were all associated with higher mortality (p < 0.05). Of the 383 patients who died before ventilatory wean, data on timing of death were available for 378: twenty percent (n = 75) had received 7 days or less of pre-tracheostomy ventilation, 37% (n = 142) between 8 and 14 days and 43% (n = 161) more than 14 days. The death risk when performing a tracheostomy after 7 days or less or more than 14 days of ventilation was equivocal (OR 1.01, 95% confidence interval [CI] 0.37–2.72), whereas the odds ratio was lower (OR = 0.64, 95% CI 0.47–0.86) when the tracheostomy was performed between days 8 and 14 (p = 0.01).
Advancing age, use of NIV, longer duration of ventilation pre-tracheostomy, percutaneous tracheostomy, higher FiO2 and PEEP requirements and use of inotropes or anticoagulants were all associated with an increased time to wean success (p < 0.01). A higher partial pressure of oxygen (PO2) was not associated with increased mortality or time to wean in our analysis. The tracheostomy method coefficient antilog was 1.11 (p = 0.006), suggesting an average 11% increase in time to successful wean of percutaneous versus open tracheostomy.
Among 1579 survivors, 92% (n = 1447) had been successfully weaned from IMV and 86% (n = 1359) had been decannulated at the time of analysis. The median (IQR) time to ventilatory wean after tracheostomy in survivors was 12 days (7, 20), with a median (IQR) critical care stay duration of 34 days (25, 45). The median (IQR) time from tracheostomy to hospital discharge in survivors was 31 days (22, 44). Supplemental Table 1 provides a breakdown of patient characteristics by mortality.
COVID-19 infection in operators
Ninety-seven percent of operators used either a filtering face piece class 3 (FFP3, n = 1780) or powered air-purifying respirator (PAPR, n = 385) (supplementary Fig. 1). The question ‘did the operator test positive for COVID-19 within two weeks of the procedure?’ was answered in 96% of cases (n = 1912), of which 0.4% (n = 8) tested positive from four hospitals. Of the eight associated procedures in which the operator subsequently tested positive for COVID-19, six were percutaneous and performed on ITU and two were open (one was undertaken on ITU and one in theatre). Among the COVID-19 positive operators, six used an FFP3 respirator during the procedure, one used a PAPR and one used a fluid resistant hood only. Of note, five of the six ITU percutaneous tracheostomies mentioned above were undertaken at the same hospital.
Discussion
Comparison with the COVID ORL ESP Collaborative
Our all-cause mortality was 21% following tracheostomy for COVID-19, with a median time from intubation to tracheostomy of 15 days (IQR 11–21). The COVID ORL ESP Collaborative Group, with their similarly-sized cohort of 1890 tracheostomised COVID-19 patients, had a slightly higher mortality of 23.7% at one month post-procedure and a median time from intubation to tracheostomy of 12 days (IQR 4, 42)24. While their data collection was confined to the first wave only (March to May of 2020), the degree of bias this introduces is unclear as the literature is contradictory in its purported lethality of the various COVID-19 variants, with claims of no statistically significant difference25, a marked reduction26,27 or even increase28 in delta wave hospital death rates when compared to the alpha variant.
There is also a marked divergence of results concerning the median time to successful wean post-tracheostomy. In our cohort this was 12 days (IQR 7–20) and 93% of survivors had weaned at the time of censoring, whereas the COVID ORL ESP collaborative had only weaned 68% of survivors at one-month post-procedure. Decannulation success rates for COVIDTrach and COVID ORL ESP of 97% and 81%, respectively, provide another surrogate marker of the perhaps greater difficulty encountered by the latter group in recovering their patients from the sequelae of severe COVID-19, however the reasons for this cannot be clearly elucidated. It should be noted that the authors cite their study design—which included the use of an instant messaging platform for data collection and a relatively limited post-tracheostomy follow up period of one month—as limitations contributing to their incomplete results, with 24% of the cohort still ventilated at censoring.
Comparison with tracheostomy in COVID-19 systematic reviews
It is acknowledged among the systematic reviews16,17,18,19,20,21,22 examining tracheostomy outcomes in COVID-19 infection that considerable heterogeneity exists between studies, thus rendering meta-analysis difficult. As a consequence, we could not always reliably extract data for a given parameter from every review for comparison, instead drawing on a few in each case where there was concordance in content and reporting style. Most of the studies included have fewer than 200 patients each, however the aggregated cohorts (typically 2000–5000 per systematic review) do permit some salient analogies with COVIDTrach data. The values for n given below (where available) are the total numbers of patients for whom pertinent data were available for each variable within a systematic review.
Timing of tracheostomy
Two meta-analyses provided overall mean intervals from intubation to tracheostomy: Benito et al. reported a mean 13.6 days (17 studies) and Battaglini et al. 16.5 days (n = 5268)19,20. Our median time from intubation to tracheostomy was 15 days, straddling the common cut-off definition of ‘early’ and ‘late’ tracheostomy employed (at least in part) in many other studies16,17,20,21,22.
Mortality data
The mortality of tracheostomised COVID-19 patients in the systematic reviews published between 2021 and 2022 varies considerably. Benito and colleagues reported the most favourable overall death rate at 13% (n = 2980), however their censor date of September 2020 was the earliest among the systemic reviews and it is suggested their figures were not abreast with the true and rapidly evolving disease burden at that time17. Similar to our own cohort mortality of 21%, figures of 18%18, 19%17 and 22%20 were given by three other meta-analyses examining 41 studies and 3876 and 5218 patients, respectively. It is interesting that considerably higher death rates were noted by Ji and colleagues and Chong and colleagues (between 29 and 33%)21,22, both of which only included studies with comparative outcomes data for early versus late tracheostomy in COVID-19. It is argued that meta-analyses not looking solely at early versus late tracheostomy data may have suffered from more clinical and statistical heterogeneity, or that selection criteria for tracheostomy were more stringent and biased towards those likely to survive22. However, ‘early’ and ‘late’ tracheostomy cut-off points are also somewhat arbitrary and are themselves heterogeneously defined within the literature, impairing comprehensive meta-analysis. For example, Staibano and colleagues cited a 28% mortality in a sub-group of tracheostomised patients when compared to non-tracheostomised COVID-19 sufferers (n = 342), however this fell to 13% in one of their separate analyses of early versus late tracheostomy studies (n = 526)16. The marked variance in the same outcome within a meta-analysis calls into question both the size and homogeneity of included studies.
Wean success and decannulation rates
Ferro and colleagues’ analysis quoted a successful wean rate of 61% (n = 3510) and a decannulation rate of 44% (n = 3326) within a weighted mean follow-up time of 42 days post-tracheostomy17; Benito and colleagues reported 55% weaned and 35% decannulated (n = 3234) within a weighted mean follow-up duration of 29 days19; for Sharma and colleagues, just 48% were weaned and 42% decannulated (6 studies and 31 studies respectively)18, however no follow-up timeframe was specified. In all three publications the number successfully weaned and decannulated were expressed as percentages of the total patient cohort, hence for COVIDTrach the equivalent figures would be calculated at 73% and 69%, respectively. Among COVIDTrach survivors only (n = 1579), our wean and decannulation success rates were 92% and 86%, respectively, with a median time to wean of 12 days (IQR 7, 20). It should be noted that our data are incomplete, and so the true values for these parameters may be higher.
Time to wean
Ferro and colleagues reported an overall weighted mean time to ventilatory wean post-tracheostomy of 24 days (8 studies), with a similar finding by Battaglini and colleagues of 23 days (18 studies)17,20. For the early versus late tracheostomy comparison by Chong and colleagues the mean times to wean were 14 and 16 days, respectively (7 studies)22. Taken together, it would appear that for tracheostomised COVID-19 patients within British critical care units, the time to successful wean is considerably shorter and potentially more likely to be successful than the prevailing literature would suggest, however the exact reasons for this remain unclear and are beyond the scope of this paper. Even among the meta analyses with mortality rates similar to our own (Ferro and colleagues, Sharma and colleagues and Battaglini and colleagues), their post-tracheostomy recovery data as described above is generally indicative of a longer recovery and poorer prognosis. Whether in time more of their patients would have been weaned and decannulated could only be answered by longer-term follow-up studies of tracheostomised COVID-19 patients, data for which is scarce at present29.
Factors affecting mortality
There is universal agreement between meta-analyses (n = 526, n = 422, 19 studies, n = 5218) and COVIDTrach that tracheostomy technique does not impact on mortality in COVID-19 disease16,17,18,20. When examining timing of tracheostomy following intubation, three meta-analyses failed to show an impact of early or late tracheostomy on mortality (n = 203, n = 2343, 12 studies)17,21,22. Similarly, Battaglini and colleagues demonstrated no impact of tracheostomy timing on mortality within their pooled cohort (n = 5218), however early and late tracheostomy sub-group analysis revealed a statistically significant higher mortality in those tracheostomised beyond 16.5 days20. When compared to tracheostomy less than 8 days following intubation, we found a reduced death risk if it was performed between day 8 and 14, but not if it was undertaken beyond 14 days.
Factors affecting time to wean
In general, concordance was seen between studies: Staibano and colleagues reported no difference in wean or decannulation rates between early and late tracheostomy (n = 259) as part of their comparison of open and percutaneous sub-groups, while Battaglini and colleagues found no difference in IMV duration between those tracheostomised early or late and a similar time to decannulation in the context of percutaneous or surgical techniques16,20. Ji and colleagues also concluded that early or late tracheostomy had no bearing on time to wean (n = 1715)21, and while Chong and colleagues showed that patients undergoing early tracheostomy had a reduced overall IMV duration (6 studies), the duration from tracheostomy to IMV weaning was similar between early and late tracheostomies (7 studies). Within COVIDTrach we found that a longer duration of ventilation pre-tracheostomy and a percutaneous tracheostomy technique were associated with increased time to wean success.
Study limitations
Heterogeneity of design and data collection across studies examining tracheostomy outcomes in COVID-19 patients makes some comparisons difficult, however patient characteristics and time from intubation to tracheostomy within COVIDTrach and other cohorts are broadly similar, thus increasing the validity of our findings.
The exceptional circumstances of the pandemic and burden on healthcare staff undoubtedly impacted our data collection, yet despite this adversity our results do exhibit above 90% completeness in most parameters (see supplemental Table 2). Not every UK institution caring for tracheostomised COVID-19 patients enrolled in this study, and our data are more skewed towards the beginning rather than the end of the period spanning April 2020 to September 2021. As a result, while our findings can be considered reflective, they cannot be seen to be a comprehensive overview of outcomes during the most virulent era of the COVID-19 age to date.
Reported mortality rates between our study and others do vary considerably, and even when they can be approximated the wean rates and time to wean appear more favourable in our cohort. Longer-term follow-up would be required in all studies to determine if the end-point of patients still intubated at the time of censoring is death or successful extubation.
Smoking-related data were available for 1301 patients and suggested that only 8% of our cohort were current smokers at the time of data capture, however the incidence of smoking in adults aged 18 years or over in the UK is estimated at 11.9%30. There is evidence to show that smoking is linked to increased disease severity and mortality in hospitalised COVID-19 patients, however this risk is difficult to quantify and population-based studies are required to investigate whether this association is causal or otherwise31.
Conclusion
According to our results, there is an all-cause mortality of 21% following tracheostomy for COVID-19, with a median time from intubation to tracheostomy of 15 days (IQR 11–21). The median time to successful wean post tracheostomy was 12 days (IQR 7–20), and 93% of survivors had weaned at the time of censoring.
Our mortality rates were similar to those of single cohort studies but lower than early versus late tracheostomy study designs. Of note, and in contrast with the prevailing literature, there was a reduced mortality when tracheostomy was performed between 8- and 14 days post-intubation (perhaps reflective of a window of opportunity between the acute phase of infection and the longer-term sequelae of intubation), and a small but statistically significant increase in time to wean when a percutaneous tracheostomy was performed instead of using a surgical technique. Our time to wean post-tracheostomy and overall wean and decannulation rates were considerably more favourable than other reports.
Data availability
The datasets generated and/or analysed during the current study are not publicly available due to privacy and ethical considerations, but are available from the corresponding author on reasonable request.
Abbreviations
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- IMV:
-
Invasive mechanical ventilation
- NIV:
-
Noninvasive ventilation
- CRP:
-
C-reactive protein
- BMI:
-
Body mass index
- IQR:
-
Interquartile range
- SD:
-
Standard deviation
- PEEP:
-
Positive end-expiratory pressure
- CI:
-
Confidence interval
- FiO2:
-
Inspired oxygen concentration
- PO2:
-
Partial pressure of oxygen
References
COVIDTrach Collaborative. COVIDTrach: the outcomes of mechanically ventilated COVID-19 patients undergoing tracheostomy in the UK: Interim Report. Br. J. Surg. 107(12), e583–e584 (2020).
COVIDTrach Collaborative. COVIDTrach: A prospective cohort study of mechanically ventilated patients with COVID-19 undergoing tracheostomy in the UK. BMJ Surg. Interv. Heal Technol. 3(1), e000077 (2021).
Sutherland, E., Headicar, J., & Delong, P. Coronavirus (COVID-19) Infection Survey technical article: waves and lags of COVID-19 in England, June 2021. Office for National Statistics Census 2021 (2021).
Chang, R., Elhusseiny, K., Yeh, Y. & Sun, W. COVID-19 ICU and mechanical ventilation patient characteristics and outcomes-A systematic review and meta-analysis. PLoS ONE 16(2), e0246318 (2021).
Hosokawa, K., Nishimura, M., Egi, M. & Vincent, J.-L. Timing of tracheotomy in ICU patients: A systematic review of randomized controlled trials. Crit. Care. 19, 424 (2015).
Cheung, N. & Napolitano, L. Tracheostomy: Epidemiology, indications, timing, technique, and outcomes. Respir. Care. 59(6), 895–919 (2014).
Groves, D. & Durbin, C. Tracheostomy in the critically ill: Indications, timing and techniques. Curr. Opin. Crit. Care. 13, 90–97 (2007).
Nouraei, S., Battson, R., Koury, E., Sandhu, G. & Patel, A. Adult post-intubation laryngotracheal stenosis: An underestimated complication of intensive care?. J. Intensive Care Soc. 10(3), 229 (2009).
McGrath, B. et al. Tracheostomy in the COVID-19 era: Global and multidisciplinary guidance. Lancet Respir. Med. 8(7), 717–725 (2020).
Miles, B. et al. Tracheostomy during SARS-CoV- 2 pandemic: Recommendations from the New York Head and Neck Society. Head Neck. 42(6), 1282–1290 (2020).
Laryngolgogical AB. COVID-19 DOCUMENT COVID-19 Tracheostomy Guideline [Internet]. 2020 [cited 2023 Mar 15]. Available from: https://www.britishlaryngological.org/sites/default/files/BLATracheostomyguideline-BLAApril2020FINAL.pdf.
Sommer, D. et al. Recommendations from the CSO-HNS taskforce on performance of tracheotomy during the COVID-19 pandemic. J. Otolaryngol. Head Neck Surg. 49(1), 23 (2020).
Michetti, C., Burlew, C. & Bulger, E. Davis K Performing tracheostomy during the Covid-19 pandemic: Guidance and recommendations from the Critical Care and Acute Care Surgery Committees of the American Association for the Surgery of Trauma. Trauma Surg. Acute Care Open. 15(5), e000482 (2020).
Brenner, M., Feller-Kopman, D. & De Cardenas, J. Tracheostomy in patients with COVID-19: Should we do it before 14 days?. Yes. Chest. 159(5), 1723–1727 (2021).
Hakki, S. et al. Onset and window of SARS-CoV-2 infectiousness and temporal correlation with symptom onset: A prospective, longitudinal, community cohort study. Lancet Respir. Med. 10(11), 1061–1073 (2022).
Staibano, P., Levin, M., McHugh, T., Gupta, M. & Sommer, D. Association of tracheostomy with outcomes in patients with COVID-19 and SARS-CoV-2 transmission among health care professionals: A systematic review and meta-analysis. JAMA Otolaryngol. Head Neck Surg. 147(7), 646–655 (2021).
Ferro, A., Kotecha, S., Auzinger, G., Yeung, E. & Fan, K. Systematic review and meta-analysis of tracheostomy outcomes in COVID-19 patients. Br. J. Oral Maxillofac. Surg. 59(9), 1013–1023 (2021).
Sharma, A. et al. Tracheostomy outcomes in coronavirus disease 2019: A systematic review and meta-analysis. Anaesthesiol. Intensive Ther. 53(5), 418–428 (2021).
Benito, D., Bestourous, D., Tong, J., Pasick, L. & Sataloff, R. Tracheotomy in COVID-19 patients: A systematic review and meta-analysis of weaning, decannulation, and survival. Otolaryngol. Head Neck Surg. 165(3), 398–405 (2021).
Battaglini, D. et al. Tracheostomy outcomes in critically ill patients with COVID-19: A systematic review, meta-analysis, and meta-regression. Br. J. Anaesth. 129(5), 679–692 (2022).
Ji, Y., Fang, Y., Cheng, B., Li, L. & Fang, X. Tracheostomy timing and clinical outcomes in ventilated COVID-19 patients: A systematic review and meta-analysis. Crit. Care. 26(1), 40 (2022).
Chong, W. & Tan, C. Clinical outcomes of early versus late tracheostomy in coronavirus disease 2019 patients: A systematic review and meta-analysis. J. Intensive Care Med. 37(9), 1121–1132 (2022).
Harris, P. et al. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 42(2), 377–381 (2009).
Martin-Villares, C., Perez Molina-Ramirez, C., Bartolome-Benito, M. & Bernal-Sprekelsen, M. Outcome of 1890 tracheostomies for critical COVID-19 patients: A national cohort study in Spain. Eur. Arch. Otorhinolaryngol. 278(5), 1605–1612 (2021).
Carbonell, R. et al. Mortality comparison between the first and second/third waves among 3,795 critical COVID-19 patients with pneumonia admitted to the ICU: A multicentre retrospective cohort study. Lancet Reg Heal Eur. 11, 100243 (2021).
Meschiari, M. et al. First and second waves among hospitalised patients with COVID-19 with severe pneumonia: a comparison of 28-day mortality over the 1-year pandemic in a tertiary university hospital in Italy. BMJ Open. 12(1), 1 (2022).
Oladunjoye, O. et al. Mortality due to COVID-19 infection: A comparison of first and second waves. J. Community Hosp. Intern. Med. Perspect. 11(6), 747–752 (2021).
Zirpe, K. et al. The second- vs first-wave COVID-19: More of the same or a lot worse? A comparison of mortality between the two waves in patients admitted to intensive care units in nine hospitals in Western Maharashtra. Indian J. Crit. Care Med. 25(12), 1343–1348 (2021).
Corona, A. et al. Tracheostomy in critically ill patients with SARS 2 COVID-19 infection: a prospective observational multi-center study of short- and long-term outcomes. Can. J. Respir. Ther. 58, 155–161 (2022).
Office for National Statistics. Adult smoking habits in the UK: 2023 [Internet]. 2023 [cited 2025 Feb 10]. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/healthandlifeexpectancies/bulletins/adultsmokinghabitsingreatbritain/2023.
WHO. Smoking and COVID-19 [Internet]. World Health Organisation. 2020 [cited 2025 Feb 10]. Available from: https://www.who.int/news-room/commentaries/detail/smoking-and-covid-19.
Acknowledgements
The authors would like to thank BMJ Surgery, Interventions, & Health Technologies for granting us permission to reuse data from this collaboration’s second publication2.
Funding
The study was supported by the Wellcome Trust UCL COVID-19 Rapid Response award and the NIHR Clinical Research Network (IRAS 258019).
Author information
Authors and Affiliations
Consortia
Contributions
Data interpretation and drafting, revision and approval of the final manuscript: the writing group (MH, GA, CW, AA, TJ, AGM, NIH). Project conception and overall guarantor: NIH. Statistical analysis: GA. Project steering, national coordination and data collection: NIH. Patient-level data collection and dataset validation: collaborators.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study received ethical approval (20/HRA/3766) from the London Westminster Research & Ethics Committee.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Haywood, M., Ambler, G., Walker, C. et al. The COVIDTrach prospective cohort study on outcomes in 1982 tracheostomised COVID-19 patients during the first and second UK pandemic waves. Sci Rep 15, 23013 (2025). https://doi.org/10.1038/s41598-025-93391-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-93391-w