Abstract
Cervical cancer significantly affects women’s health, while current preoperative imaging methods for predicting lymph node status are suboptimal. This study aims to evaluate the predictive value of CT attenuation (Hounsfield units) for lymph node metastasis (LNM) in cervical cancer patients and to develop a nomogram integrates clinical and imaging features to improve the accuracy of preoperative assessment. A retrospective analysis was conducted on 132 cervical cancer patients treated at the Second Affiliated Hospital of Dalian Medical University from January 2021 to August 2024. The cohort was divided into a training set (70%) and a validation set (30%) for constructing and evaluating the nomogram. CT attenuation was identified as robust predictors of LNM. Multivariate logistic regression showed that preoperative SCC levels, tumor histology, uterine corpus involvement, and lymphovascular space invasion were independent risk factors for LNM. The nomogram incorporating CT attenuation demonstrated excellent discrimination and calibration. This study presents a simple but robust nomogram that integrates clinical and imaging data to predict lymph node status in cervical cancer patients. The model exhibits high accuracy and reliability, highlighting the utility of CT imaging as a non-invasive preoperative tool for assessing LNM.
Similar content being viewed by others
Introduction
Cervical cancer is the fourth most prevalent cancer affecting women globally and represents the leading malignancy of the female reproductive system1. According to the 2018 International Federation of Gynecology and Obstetrics (FIGO) staging system, lymph node metastasis (LNM) is identified as an independent prognostic factor for cervical cancer. Patients with LNM are classified as Stage IIIC, irrespective of other clinical or pathological factors, highlighting the critical importance of lymph node status in treatment planning and prognosis2,3,4.
Accurate preoperative evaluation of LNM status spares patients from unnecessary interventions. Surgical staging is the most widely used method for assessing lymph node status and is considered the gold standard for detecting LNM5. However, early-stage cervical cancer patients have a lower rate of LNM6 and are at higher risk of complications from systematic lymph node dissection, such as lymphoceles and lower extremity lymphedema7. Consequently, there is no consensus on the necessity of lymph node dissection in early-stage, low-risk cervical cancer patients8. Moreover, the status of the para-aortic lymph nodes is critical for determining the radiation therapy target volume. The Uterus-11 prospective study reported a false-negative rate of 8–22% when using PET-CT to assess para-aortic lymph nodes in locally advanced cervical cancer patients. Such inaccuracies can delay chemoradiation therapy and negatively impact patient outcomes, as surgical intervention does not provide survival benefits for these individuals9.
In clinical practice, a variety of imaging techniques are utilized to assess lymph node status. Among these, multi-slice spiral computed tomography (CT) remains a commonly employed modality. However, reactive lymph node enlargement and smaller LNM can complicate accurate diagnosis based on size alone10. Moreover, studies have concluded that diffusion-weighted magnetic resonance imaging (DW-MRI) had the highest sensitivity (87%) and [18F] FDG-PET/CT has the highest specificity (97%) for detecting LNM11. However, high cost and technical requirements limit their widespread clinical application. Consequently, exploration of additional imaging parameters is crucial to improving diagnostic precision.
CT attenuation, measured in Hounsfield units (HU), quantifies X-ray attenuation by tissues and serves as a critical imaging parameter. Extensive research has demonstrated its significant value in diagnosing LNM across various malignancies. However, studies specifically addressing CT attenuation for LNM in cervical cancer remain limited12,13,14,15,16.
In recent years, radiomics has been increasingly applied in medicine. It leverages artificial intelligence (AI) to extract spatial and inter-pixel relationships of signal intensities, quantifying texture information and revealing disease features invisible to the naked eye17. However, current research suffers from limited reproducibility and clinical utility. Over the past few years, the development of preoperative nomograms has garnered increasing attention. Nomograms integrate multiple clinical and imaging variables into a single model to provide individualized probability estimates of clinical events, making them widely utilized in oncology18.
This study employs a radiomics-inspired approach to extract readily available CT attenuation values as quantitative data from CT images, comprehensively evaluating the predictive value of CT attenuation combined with clinical and pathological factors for LNM in cervical cancer. Based on these findings, we developed a preoperative nomogram to provide reliable and personalized references for optimizing the treatment strategies of cervical cancer patients.
Materials and methods
Patient general information
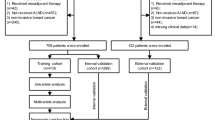
This retrospective study analyzed clinical data from 150 patients with pathologically confirmed cervical cancer treated at the Second Affiliated Hospital of Dalian Medical University from January 2021 to August 2024. All participants completed a written informed consent form for data collection and storage. The specific inclusion and exclusion criteria were as follows:
Inclusion criteria:
-
Availability of complete clinical data.
-
Preoperative serum squamous cell carcinoma antigen(SCC) levels and pelvic-abdominal contrast-enhanced CT examinations performed within two weeks prior to surgery.
-
Initial treatment comprising pelvic lymphadenectomy ± radical hysterectomy ± aortic lymphadenectomy.
Exclusion criteria:
-
Patients who underwent preoperative radiotherapy, chemotherapy, immunotherapy, or other antitumor treatments, or had a history of prior cervical conization.
-
Patients diagnosed with other malignancies.
This well-defined cohort ensured the reliability of the dataset for constructing and validating the preoperative nomogram.
Clinical variables
Preoperative clinical and laboratory data, including serum squamous cell carcinoma antigen (SCC) levels and human papillomavirus (HPV) test results, were systematically collected for all patients. Preoperative clinical staging was assessed by two senior gynecologists upon admission. Postoperative pathological data, including tumor type, differentiation grade, lymphovascular space invasion (LVSI), depth of stromal invasion, and involvement of key anatomical regions (vaginal apex, uterine corpus, and parametrial tissue), were meticulously documented.
Imaging assessment
The imaging evaluation of LNM in cervical cancer patients was conducted by two experienced gynecologists to assess the diagnostic accuracy of enhanced CT scans. A standardized protocol was followed, utilizing an enhanced CT scan with a slice thickness of 5 mm to systematically identify and delineate suspicious lymph nodes in the para-aortic and bilateral pelvic regions as regions of interest (ROIs). The system’s measurement tools were employed to determine the maximum short-axis diameter and CT attenuation of these lymph nodes during the non-contrast, arterial, venous, and delayed phases, as illustrated in Fig. 1. Image interpretation was performed using a synchronized double-blind method to ensure unbiased results. After initial delineation, a second reviewer independently reviewed and confirmed or modified the ROIs. In cases of disagreement, consensus was reached through discussion, and disputed samples were excluded from the analysis.
Suspicious lymph nodes were defined as those with a short-axis diameter greater than 1 cm, marked or heterogeneous enhancement, and irregular margins. For each patient, only one suspicious lymph node was selected for preoperative assessment; when both pelvic and para-aortic lymph nodes were suspicious, priority was given to the para-aortic region. The selected lymph node was sent for individual pathological examination during surgery to ensure accurate correspondence between the suspected lymph node and the pathological gold standard.
Among the 150 enrolled cervical cancer patients, all CT images were acquired using uniform scanning parameters (slice thickness ≤ 5 mm). A total of 18 patients were excluded from the study, as their CT scans were performed at external institutions with a slice interval of 1.2 cm, to ensure the accuracy and consistency of the imaging data, thereby providing a solid foundation for subsequent analysis and model development.
Nomogram training and validation
The cohort was randomly divided into a training set (n = 92) and a validation set (n = 40) at a 7:3 ratio. The training set was used for nomogram development, while the validation set was utilized for internal validation and efficacy assessment. Lymph nodes were classified as metastatic or non-metastatic based on postoperative pathological findings, which served as the gold standard for statistical analysis.
Data analysis was conducted using SPSS software (version 27.0). Initially, univariate analysis was performed to identify potential risk factors for LNM. Variables with a P-value < 0.05 in the univariate analysis were subsequently included in multivariate analysis using binary logistic regression to determine independent predictors of LNM. Odds ratios (OR) with 95% confidence intervals (CI) were calculated to quantify the strength of associations between the predictors and LNM.
Based on the significant variables identified in the multivariate analysis, a predictive nomogram for LNM in cervical cancer patients was constructed using R software (version 4.4.1). The R packages utilized included “car”, “rms”, “pROC”, and “rmda”. The model’s performance was evaluated in terms of discrimination, calibration, and clinical utility.
-
Discrimination The model’s predictive accuracy was evaluated using the receiver operating characteristic (ROC) curve, with the area under the curve (AUC) serving as an indicator of discrimination performance.
-
Calibration Calibration was assessed using calibration curves and the Hosmer–Lemeshow goodness-of-fit test to evaluate the agreement between predicted probabilities and observed outcomes.
-
Clinical utility Decision curve analysis (DCA) was conducted to quantify the clinical benefit of the model across different threshold probabilities.
To validate the nomogram’s robustness, data from the validation set were input into the model. The validation set was analyzed to assess discrimination (using AUC), calibration (using calibration curve and the Hosmer–Lemeshow goodness-of-fit test), and clinical utility (using DCA). This comprehensive evaluation provided a thorough assessment of the model’s performance in predicting LNM.
Analysis of the diagnostic value of CT attenuation measurements
Potential indicators with P < 0.05 in CT attenuation and CT attenuation differences, identified through univariate analysis, were used to plot ROC curves. Sensitivity, specificity, and AUC were calculated at the optimal cut-off values to assess the diagnostic performance of CT values in identifying lymph node metastasis.
Statistical analysis
The Shapiro–Wilk test was used to assess the normality of continuous variables. Normally distributed data were reported as mean ± standard deviation, and intergroup comparisons were conducted using the independent samples t-test. Non-normally distributed data were reported as median (interquartile range), with intergroup differences assessed using the Mann–Whitney U test. Categorical variables were reported as frequencies and percentages (%), and group comparisons were performed using the χ2 test or Fisher’s exact test, as appropriate.
Results
A total of 132 patients were included in this study, with 92 patients assigned to the training set and 40 patients to the validation set. Among these 132 patients, 7 were identified as having advanced disease based on preoperative evaluation and therefore underwent only pelvic lymphadenectomy. The LNM rates were 46.7% in the training set and 45.0% in the validation set. As shown in Table 1, no statistically significant differences were observed in CT attenuation or clinicopathological characteristics between the training and validation sets (P > 0.05), confirming the comparability of the two datasets.
In the training set, patients were further categorized into a metastatic group (n = 43, 46.74%) and a non-metastatic group (n = 49, 53.26%) based on postoperative lymph node pathology results. Univariate analysis revealed significant differences between the metastatic and non-metastatic groups for the following variables:
-
Preoperative SCC levels
-
Maximum short axis of lymph node
-
CT attenuation in the plain, arterial, venous, and delayed phases
-
Histopathological type
-
Uterine corpus involvement
-
Lymphovascular space invasion (LVSI)
These variables were statistically significant (P < 0.05) and are detailed in Table 2, forming the basis for subsequent multivariate analysis and nomogram construction.
Analysis of the diagnostic value of CT attenuation measurements
ROC curves were plotted to evaluate the predictive performance of CT attenuation in the plain, venous, and delayed phases for LNM. The results, summarized in Table 3 and illustrated in Fig. 2, demonstrated the following optimal cutoff values:
-
Non-contrast phase: CT attenuation cutoff value of 21.36, with a sensitivity of 65.1% and specificity of 87.8%, yielding an area under the curve (AUC) of 0.803.
-
Arterial phase: CT attenuation cutoff value of 37.09, with a sensitivity of 55.8% and specificity of 71.4%, yielding an area under the curve (AUC) of 0.650.
-
Venous phase: CT attenuation cutoff value of 39.63, with a sensitivity of 76.7% and specificity of 59.2%, yielding an AUC of 0.700.
-
Delayed phase: CT attenuation cutoff value of 27.80, with a sensitivity of 90.7% and specificity of 46.9%, yielding an AUC of 0.717.
These findings indicate that CT attenuation, particularly in the non-contrast phase, exhibits high accuracy for preoperative diagnosis of LNM. The venous and delayed phases also demonstrate moderate predictive value, further supporting the utility of CT imaging in LNM assessment.
Nomogram for predicting LNM
To address potential multicollinearity among variables, multicollinearity diagnostics were conducted for CT attenuation in different phases. The results indicated significant collinearity for venous phase CT attenuation (variance inflation factor, VIF = 10.8). Consequently, venous phase CT attenuation was excluded from further analysis.
Multivariate logistic regression analysis was subsequently performed, and the results are summarized in Table 4. Three independent predictors of LNM were identified:
-
Preoperative SCC levels
-
Maximum short axis of lymph nodes
-
Non-contrast CT Attenuation
Based on these factors, a preoperative nomogram for predicting the risk of LNM was constructed (see Fig. 3). The nomogram assigns points to each predictive variable based on its relative contribution to the likelihood of LNM. By summing the total points for a given patient, the corresponding probability of LNM can be estimated.
This nomogram provides an intuitive and individualized tool for clinicians to assess the risk of LNM, facilitating more accurate preoperative decision-making for patients with cervical cancer.
Validation of the nomogram for predicting LNM
Discrimination
ROC curves were plotted using data from both the training and validation sets based on the results of the regression equations, as shown in Fig. 4. The AUC for the training set was 0.843 (95% CI 0.764–0.933), while the AUC for the validation set was 0.907 (95% CI 0.818–0.996). These results indicate that the nomogram prediction model exhibits excellent discrimination.
Calibration
Figure 5 displays the calibration curves for the training and validation sets. The x-axis represents the predicted probability of LNM, while the y-axis represents the actual incidence rate. The dashed line indicates the ideal performance of the nomogram, whereas the solid line represents the actual performance. The calibration curves closely follow the dashed line, indicating that there is no significant difference between the predicted probabilities and the actual incidence rates (P > 0.05). The Hosmer–Lemeshow goodness-of-fit test was conducted to assess the calibration ability of the model, yielding P values of 0.183 for the training set and 0.826 for the validation set, both of which suggest a good model fit.
Clinical utility
Figure 6 shows the clinical decision curves for the training and validation sets. The x-axis represents threshold probabilities, while the y-axis represents the corresponding net benefit values. The black solid line represents the net benefit assuming all patients have no LNM, while the gray solid line represents the net benefit assuming all patients have LNM. The red curve indicates the net benefit from using the nomogram model to guide patient treatment decisions. When the threshold probability is between 0.1 and 0.8, the decision curve lies above both the “None” and “All” lines, indicating that using the nomogram model provides clinical benefits for patients regarding lymphadenectomy decisions, thereby demonstrating good clinical utility.
Discussion
Cervical cancer is one of the most common malignant tumors among women globally, and lymph node metastasis (LNM) is a critical factor influencing prognosis. Accurate assessment of lymph node status is essential for formulating appropriate treatment plans. While pathological examination remains the gold standard for evaluating lymph node status, controversy persists regarding the necessity of traditional pelvic lymphadenectomy in all patients due to its associated high risk of postoperative complications8,19,20. To avoid unnecessary lymphadenectomy in patients without LNM, preoperative non-invasive imaging assessments have garnered significant attention.
This study conducted a retrospective analysis and found that CT attenuation during the non-contrast phase, Arterial phase, venous phase, and delayed phase has high diagnostic accuracy for assessing LNM in cervical cancer, with AUCs of 0.803, 0.650, 0.700, and 0.717, respectively (Table 3, Fig. 2). These results indicate that CT attenuation can serve as a potentially useful tool for predicting LNM in cervical cancer. Additionally, we developed a nomogram prediction model that integrates preoperative SCC levels, the maximum short axis of lymph nodes, and CT attenuation from the non-contrast phase. The AUCs for the training and validation sets were 0.843 and 0.907, respectively, demonstrating good discrimination and calibration.
Despite the high sensitivity and detection rate of sentinel lymph node biopsy (SLNB), its invasiveness restricts its widespread clinical application21,22,23. Currently, imaging modalities such as ultrasound (US), magnetic resonance imaging (MRI), computed tomography (CT), and positron emission tomography-computed tomography (PET-CT) are the primary methods for evaluating pelvic and abdominal LNM. Studies have demonstrated that diffusion-weighted MRI (DW-MRI) achieves the highest sensitivity (87%), while [18F] FDG-PET/CT exhibits the highest specificity (97%)11. However, the high costs and technical demands limit the broader adoption of these techniques. A meta-analysis conducted by Choi et al. reviewed 41 articles on imaging methods for predicting lymph node status. They found that the sensitivity and specificity of CT, MRI, and PET/PET-CT were 52% and 92%, 38% and 97%, and 54% and 97%, respectively. The study indicated that technological advancements have not significantly improved the diagnostic performance of CT; although image quality has improved, relying solely on lymph node diameter does not enhance staging accuracy24. Similarly, a meta-analysis by Liu et al., which included 61 articles, demonstrated that CT has high specificity (92%) but limited sensitivity (below 60%) for diagnosing lymph node metastasis (LNM) in cervical cancer11. In contrast, our study demonstrated that using CT attenuation values during the non-contrast scan, venous phase, and delayed phase to predict LNM in cervical cancer achieved sensitivities of 65.1%, 76.7%, and 90.7%, all exceeding 60%. The corresponding specificities were 87.8%, 59.2%, and 46.9%, with AUC values of 0.803, 0.700, and 0.717 (Table 3, Fig. 2). These findings indicate a significant improvement in CT sensitivity across different scanning phases, representing an encouraging development.
In recent years, CT attenuation values have increasingly been utilized to evaluate lymph node metastasis (LNM), particularly in surgical applications. However, the literature remains limited, and no universally accepted critical thresholds have been established, especially for cervical cancer. A study on colon cancer found that the average CT attenuation value of non-metastatic lymph nodes during the plain scan phase was 14.92 HU, while that of metastatic lymph nodes was 18.75 HU, consistent with our findings. This study demonstrated that tumor cell infiltration increases blood supply in metastatic lymph nodes, leading to more pronounced enhancement and higher CT attenuation. In contrast, inflammatory or reactive hyperplastic lymph nodes exhibit fewer new blood vessels and reduced blood supply, resulting in weaker enhancement. Additionally, the study indicated that combining the short-to-long axis ratio with the relative enhancement CT attenuation (the difference between venous and plain scan phases) yields the best diagnostic performance (sensitivity 86.7%, specificity 85.0%)15. In our study, however, the relative enhancement value did not show significant diagnostic value, possibly due to the smaller sample size.
Another study on lymph nodes around the liver found that those with a CT attenuation value below 38 HU were more likely to be metastatic, contrasting with our findings and without providing an explanation. The study noted that unenhanced CT values best reflect the true density of the tissue. After contrast agent injection, necrosis or vascular proliferation in malignant tissues can lead to heterogeneous enhancement of the lymph nodes14. Currently, it remains unclear whether factors such as tumor type, tissue characteristics, tumor stage, and the number of metastatic lymph nodes influence CT attenuation, necessitating further research. As highlighted in studies on hepatocellular carcinoma and colon cancer, the variation in HU cutoff values across different cancers underscores the need for standardized scanning protocols and larger, multicenter studies to establish more universally applicable thresholds.
CT imaging is cost-effective and widely utilized, providing gynecologic oncologists with a valuable tool. By reviewing extensive image datasets, oncologists can enhance their imaging interpretation skills and accurately obtain CT attenuation through simple lymph node delineation. Utilizing CT attenuation or nomograms that incorporate CT attenuation for predicting cervical cancer LNM is both straightforward and readily implementable. Future research should focus on optimizing CT scanning protocols to standardize CT attenuation acquisition. In clinical practice, meticulous attention to detail is essential when delineating CT attenuation, such as precisely defining the measurement area; for larger or heterogeneous lymph nodes, it is advisable to have multiple clinicians measure and average the results to improve accuracy. These approaches position CT attenuation as a promising quantitative imaging parameter for aiding in the diagnosis of cervical cancer LNM.
It is noteworthy that the ESUR guidelines for cervical cancer suggest a size threshold of 7–8 mm for lymph node metastasis in the pelvis25. In contrast, Yamanoi et al. reported a cut-off value of 5 mm26. Although our study focused on lymph nodes with a short-axis diameter greater than 1 cm, we acknowledge that future research should consider including smaller lymph nodes (e.g., 5 mm or larger) to provide a more comprehensive assessment of lymph node metastasis in early-stage cervical cancer.
This study has certain limitations. First, the relatively small sample size may limit the external validation capacity of the model. Additionally, only the largest suspicious lymph node was selected for each patient, and information on other lymph nodes was not included. The impact of the number of metastatic lymph nodes on CT attenuation values remains unclear, potentially introducing heterogeneity. To reduce sample bias, we employed methods such as multiple collinearity diagnostics; however, due to the single-source data, selection bias may still exist.
Future studies should prioritize expanding the sample size and conducting multicenter research. Comprehensive baseline data, including disease stage and grade, as well as the number and size of suspicious lymph nodes, should be collected to more accurately assess lymph node status and reduce study heterogeneity. Furthermore, combining CT attenuation with other imaging modalities, such as MRI and PET-CT, could enhance the precision of multimodal imaging evaluation systems. Prospective studies are also crucial to confirm the clinical applicability of the nomogram in real-world settings.
Conclusion
This study underscores the clinical significance of CT attenuation as a reliable imaging parameter for assessing LNM in cervical cancer. The nomogram, which integrates CT attenuation with key clinical parameters, exhibits robust predictive performance and serves as a practical tool for preoperative evaluation. By enhancing the accuracy of lymph node status assessment, this model can optimize treatment strategies and support personalized care for cervical cancer patients. Further research and external validation are essential to confirm its generalizability and maximize its impact on patient outcomes.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN Estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Bhatla, N. et al. Revised FIGO staging for carcinoma of the cervix uteri. J. [published correction appears in Int J Gynaecol Obstet.147, 279–280 (2019).] Int. J. Gynaecol. Obstet. 145, 129–135 (2019).
Cheng, X. et al. The prognosis of women with stage IB1-IIB node-positive cervical carcinoma after radical surgery. World J. Surg. Oncol. 2, 47 (2004).
Jeong, S. et al. Pretreatment lymph node metastasis as a prognostic significance in cervical cancer: Comparison between disease status. Cancer Res. Treat. 52, 516–523 (2020).
Li, J. et al. Interpretation of the 2018 FIGO cancer report: New staging and treatment guidelines for cervical cancer. Chin. J. Clin. 47, 646–649 (2019).
Schmeler, K. M. et al. ConCerv: A prospective trial of conservative surgery for low-risk early-stage cervical cancer. Int. J. Gynecol. Cancer 31, 1317–1325 (2021).
Cao, L. et al. Analysis of lymph node metastasis and risk factors in 975 patients with FIGO 2009 stage IA-IIA cervical cancer. Gynecol. Obstet. Invest. 88, 30–36 (2023).
Togami, S. et al. Risk factors for lymphatic complications following lymphadenectomy in patients with cervical cancer. Jpn. J. Clin. Oncol. 48, 1036–1040 (2018).
Marnitz, S. et al. Surgical versus clinical staging prior to primary chemoradiation in patients with cervical cancer FIGO stages IIB-IVA: oncologic results of a prospective randomized international multicenter (Uterus-11) intergroup study. Int. J. Gynecol. Cancer 30, 1855–1861 (2020).
Paño, B. et al. Pathways of lymphatic spread in gynecologic malignancies. Radiographics 35, 916–945 (2015).
Liu, B., Gao, S. & Li, S. A comprehensive comparison of CT, MRI, positron emission tomography or positron emission tomography/CT, and diffusion weighted imaging-MRI for detecting the lymph nodes metastases in patients with cervical cancer: A meta-analysis based on 67 studies. Gynecol. Obstet. Invest. 82, 209–222 (2017).
Li, Z. Y., Zhu, D. & Xia, C. H. Clinical application of CT value and IC value of spectral CT scan in qualitative assessment of lymph nodes in gastric adenocarcinoma. China Med. Herald. 18, 147–150+183 (2021).
Zhang, W. et al. The study of relationship between CT value of lung cancer with its differenciation, pathological type and lymph node metastasis. Clin. Pulm. Med. 16, 1554–1555 (2011).
Wang, X. Y. et al. Diagnostic value of CT value for perihepatic lymph node metastasis of primary liver cancer. Chin. J. Hepat. Surg. 5, 168–172 (2016).
Feng, L. et al. The diagnostic value of multislice spiral CT in lymph node metastasis of colon cancer. CT Theory Appl. 30, 124–130 (2021).
Wang, Y. Z. et al. Comparative analysis of CT signs of colonic lymphoma and colon cancer. Med Imaging. 30, 260–263 (2020).
van Timmerson, J. E. et al. Radiomics in medical imaging-"how-to" guide and critical reflection. Insights Imaging 11, 91 (2020).
Balachandran, V. P. et al. Nomograms in oncology: more than meets the eye. Lancet Oncol. 16, 173–180 (2015).
Huang, B. X. & Fang, F. Progress in the study of lymph node metastasis in early-stage cervical cancer. Curr. Med. Sci. 38, 567–574 (2018).
Bhatla, N. et al. Cancer of the cervix uteri. J. [published correction appears in Int J Gynaecol Obstet. 164, 1229–1230(2024).] Int. J. Gynaecol. Obstet. Suppl 2, 22–36 (2018).
Salvo, G. et al. Sensitivity and negative predictive value for sentinel lymph node biopsy in women with early-stage Cervical cancer. Gynecol. Oncol. 145, 96–101 (2017).
Kinkel, K. Pitfalls in staging uterine neoplasm with imaging: A review. Abdom. Imaging. 31, 164–173 (2006).
Liu, J. et al. Ultrasound-guided fine needle aspiration cytology of para-aortic lymph node metastasis in uterine cervical cancer: diagnostic accuracy and impact on clinical decision making. BMC Cancer 21, 964–974 (2021).
Choi, H. J., Ju, W., Myung, S. K. & Kim, Y. Diagnostic performance of computer tomography, magnetic resonance imaging, and positron emission tomography or positron emission tomography/computer tomography for detection of metastatic lymph nodes in patients with cervical cancer: meta-analysis. Cancer Sci. 101, 1471–1479 (2010).
Manganaro, L. et al. Staging, recurrence and follow-up of uterine cervical cancer using MRI: Updated Guidelines of the European Society of Urogenital Radiology after revised FIGO staging 2018. Eur. Radiol. 31, 7802–7816 (2021).
Yamanoi, K. et al. A novel diagnostic criterion for lymph node metastasis in cervical cancer using multi-detector computed tomography. Gynecol. Oncol. 131, 701–707 (2013).
Author information
Authors and Affiliations
Contributions
X.M.: Conceptualization; Methodology; Writing—Original Draft. S.S.: Conceptualization; Formal analysis. K.L.: Investigation; Validation; Project administration. Y.D.: Formal analysis. J. Z.: Data Curation. J. W. : Software; Visualization. S.H.; Conceptualization; Writing—Review & Editing; Supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All the methods and procedures carried out in this study were by the guidelines of the Declaration of Helsinki. The aims of the study were explained in detail, and written informed consent was obtained from all participants. They were allowed to withdraw from the study. The study was approved by the ethics committee of the Second Affiliated Hospital of Dalian Medical University (ethical approval number: KY2025-038-01).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Meng, X., Song, S., Li, K. et al. Application of CT in predicting lymph node metastasis in cervical cancer and construction of a preoperative nomogram. Sci Rep 15, 11674 (2025). https://doi.org/10.1038/s41598-025-94999-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-94999-8