Abstract
The demand for sustainable energy storage has driven advancements in supercapacitors, known for their high-power density and rapid charge cycles. However, challenges like limited energy density and material stability must be addressed for practical applications. In this study, VSe2/CuS nanocomposites were synthesized using a simple wet chemical method and investigated as electrode materials for supercapacitors. X-ray diffraction (XRD) analysis confirmed the phase purity of the materials while scanning electron microscopy (SEM) revealed spherical and flake-like morphology. The synergy between VSe2’s high electrical conductivity and CuS’s pseudocapacitive properties enhances charge storage and electrochemical performance. The VSe2/CuS electrode exhibited a high specific capacitance of 853.9 F/g at 1 A/g, outperforming individual VSe2 (395.6 F/g) and CuS (471.6 F/g). The VSe2/CuS||AC device demonstrated a specific capacitance of 147.6 F/g, excellent rate capability, and 88.3% capacitance retention over 10,000 cycles at 10 A/g. These findings highlight the potential of VSe2/CuS nanocomposites as high-performance electrode materials, advancing the development of next-generation energy storage technologies.
Similar content being viewed by others
Introduction
Recently, the significance of clean and renewable energy sources has grown considerably, driven by the diminishing availability of fossil fuels and increasing concerns over environmental pollution1,2. Non-renewable sources, such as coal, natural gas, and petroleum remain vital for various sectors, including agriculture, transportation, and industries2,3. On the other hand, these non-renewable sources are finite and emit greenhouse gases, contributing to global warming. To mitigate this issue, renewable energy sources such as wind power, biofuels, hydropower, solar power, and tidal power have garnered increasing interest. Moreover, they have a negligible impact on the environment4. To meet the escalating energy demands essential for human advancement and development in the modern era, energy storage technologies such as solar cells, fuel cells, lithium-ion batteries, and supercapacitors can be utilized5. Economic instability and significant population growth have resulted in a shortage of environmentally friendly energy resources. Flexible, lightweight, and wearable or portable electronic devices are attracting global interest due to the increasing demand for cost-effective, durable, and sustainable energy solutions. Supercapacitors (SCs) have gained increasing attention as a unique energy storage technology due to their exceptional properties, including rapid charge/discharge cycles, high power density, and excellent cycle stability. The pursuit of clean and renewable energy sources has gained prominence due to imminent energy challenges and environmental concerns6,7. However, their lower energy density remains a critical challenge for widespread applications8. The performance of SCs is largely determined by the electrode materials, which play a key role in addressing this limitation. Based on their electrochemical storage mechanisms, SC electrodes are classified into two categories: Electric double-layer capacitors (EDLCs), which store energy through electrostatic charge separation at the electrode–electrolyte interface, and Pseudocapacitors, which utilize electroactive materials to undergo fast redox reactions, enabling rapid charge and discharge rates9,10. The performance of EDLCs and pseudocapacitors is greatly influenced by factors such as electrolyte type, electrode surface shape, surface area, and overall structure. EDLCs are typically made using high-surface-area carbon materials, while pseudocapacitors rely on conducting polymers and transition metal oxides or sulfides. Although pseudocapacitors offer much higher specific capacitance and energy density than EDLCs, their cycle life is often limited due to the irreversibility of faradic reactions11,12. SCs are highly promising as electrochemical energy storage devices, attributed to their superior power density, minimal environmental impact, and exceptional long-term cycling performance13. SCs still have the disadvantage of having a lower energy density than batteries, even if they have somewhat outperformed conventional rechargeable batteries in terms of useful uses in electric cars and electronic gadgets like mobile phones14. Improvements must therefore be made to the supercapacitor characteristics of the electrode materials that control the electrochemical performance. The capacitance of a supercapacitor is influenced by the electrode material, whether it operates through an electric double-layer mechanism or a redox faradaic process. The most effective materials for active electrodes in supercapacitors include carbon-based materials, metal sulfides, transition metals, and their composites15. Transition metal oxides are frequently utilized as electrode materials in SCs; however, their restricted ion transport properties limit their effectiveness in high-power applications16. In contrast, transition metal sulfides and selenides offer improved ion transport pathways compared to their oxide counterparts, resulting in enhanced electrochemical reaction kinetics17. The lower electronegativity and faster charge transfer characteristics of transition metal selenides, relative to sulfides and oxides, make them particularly promising candidates for energy storage applications18. Vanadium diselenide (VSe2), a prominent member of the two-dimensional transition metal dichalcogenide (TMD) family, has shown promising energy storage performance when utilized as a supercapacitor electrode. VSe2 exhibits metallic characteristics in its steady state when compared to other TMDs. Layered VSe2 consists of monolayer VSe2 units separated by van der Waals gaps. In each unit cell, a vanadium atom is positioned between two selenium atoms. The remarkable intercalation activity within the van der Waals gaps, combined with its diverse electronic properties, contributes to the enhanced energy storage performance of VSe2. VSe2 demonstrates promising electrochemical performance, largely due to its high electrical conductivity, approximately 1000 S/m at 300 K. However, its high Gibbs free energy impedes its ability to achieve optimal electrochemical efficiency19,20,21,22.
Conversely, studies have indicated that metal sulfides, such as NiS, WS2, CuS, CoS, Ag2S, Ni3S2, and MnS, exhibit superior electrochemical activities and enhanced electrical conductivity. Copper sulfides, in particular, represent a class of functional semiconductors with various phases (Cu2S, CuS, Cu1.9S, and Cu1.75S). These materials are widely used in various applications, including gas sensors, photocatalysts, solar cells, and lithium-ion batteries. CuS is an abundant and cost-effective material, commonly employed as a cathode in lithium-ion batteries because of its elevated theoretical capacity and substantial energy storage capabilities23,24,25,26. Only a limited number of studies have explored the use of CuS as an electrode material for supercapacitors. These studies have generally reported relatively low capacitance and cycling stability, primarily due to inadequate interfacial charge-transfer kinetics at the liquid/solid interface and the material’s low surface area25,27,28. While many studies have investigated VSe2 and CuS-based materials for supercapacitor applications29,30,31,32. In the present work, integrating them into a nanocomposite designed for high-performance supercapacitor applications. Hydrothermal synthesis method was used due to its enclosed reaction environment and elevated pressure, this method offers precise control over phase purity, crystallinity, and morphology, in comparison to other chemical methods33. In this study, CuS was integrated with the VSe2 composite, enhancing the electrochemical properties of VSe2. The combination of VSe2, known for its excellent electrical conductivity, and CuS, valued for its pseudocapacitive properties, significantly enhances electrochemical performance. This composite structure improves charge storage by enabling rapid electron transport through VSe2 while utilizing the fast redox activity of CuS. The strong interfacial interaction between these materials reduces internal resistance and promotes efficient ion diffusion, resulting in higher specific capacitance, improved rate capability, and long-term cycling stability. Furthermore, the nanocomposite structure prevents VSe2 layer restacking and minimizes volume expansion during charge/discharge cycles, further enhancing durability. These advantages make the VSe2/CuS composite a highly promising electrode material for next-generation supercapacitors.
Experimental section
Chemicals
In the present study, all chemicals were sourced from Sigma-Aldrich with a purity of 99.9%. They were of analytical grade, including selenium powder (Se), vanadium pentoxide (V2O5), sodium thiosulfate pentahydrate (Na2S2O3·5H2O), copper nitrate hexahydrate (Cu(NO3)2·6H2O), and oxalic acid dihydrate (C2H2O4·2H2O). All aqueous solutions were prepared using deionized (DI) water, and all chemicals were utilized as received without any additional purification34.
Synthesis of VSe2
The hydrothermal synthesis was performed using a high-pressure Teflon-lined stainless steel autoclave to ensure controlled reaction conditions. A mixture of 10 mmol oxalic acid dihydrate (C2H2O4·2H2O), 3.5 mmol vanadium pentoxide (V2O5), and 6 mmol selenium dioxide (SeO2) was prepared in a 100 mL Teflon-lined autoclave. Subsequently, 100 mL of deionized water was added to the mixture. The autoclave was then heated to 200 °C in a muffle furnace (VF-14MA) for 24 h. After the reaction, the resulting product was collected and centrifuged multiple times, washing it with ethanol and deionized water. The residual washing solvent was removed using a rotary evaporator (SARET43), yielding VSe2 powder. The reaction was maintained at 200 °C for 24 h to ensure uniform heat distribution. High-purity argon gas was used as a shielding medium to prevent oxidation and undesired side reactions during the process.
Synthesis of CuS
Copper sulfide (CuS) was synthesized hydrothermally by dissolving 250 mg of copper nitrate and 150 mg of sodium thiosulfate in 50 mL of deionized (DI) water. The solution was continuously stirred for 40 min before being transferred to a 100 mL Teflon-lined stainless-steel autoclave. The autoclave was heated to 180 °C in a furnace for 24 h. After natural cooling to ambient temperature, the solid product was collected by centrifugation, rinsed sequentially with DI water and ethanol, and dried at 70 °C for 8 h.
Synthesis of VSe2/CuS
To fabricate VSe2/CuS nanocomposites with uniform composition, a wet chemical technique was employed. The components were dissolved in 40 mL of ethanol, stirred magnetically for 1 h, and then treated ultrasonically for an additional hour to ensure homogeneity. The solvent was eliminated by drying the mixture at 80 °C for 12 h. A schematic representation of the synthesis process is shown in Fig. 1.
Preparation of electrodes
To prepare the active electrodes, the composite powder, PVDF, and carbon black were mixed in a weight ratio of 80:10:10 and ground in a cylindrical mortar for 30 min. The addition of N-methyl-N-pyrrolidone (NMP) produced a viscous slurry, which was applied onto a cleaned nickel foam substrate with a makeup brush. The coated electrodes were dried in a vacuum oven at 80 °C for 10 h, resulting in active material weights of approximately 2.1 mg (VSe2), 2.3 mg (CuS), and 2.0 mg (VSe2/CuS), with an uncertainty of ± 0.002 mg.
Material characterization
The crystallinity and phase composition of the synthesized electrode materials, including VSe2, CuS, and the VSe2/CuS composite, were characterized using X-ray diffraction (XRD). The analysis was carried out using a Bruker-D8 Advanced spectrometer; employing high-intensity CuKα radiation with a monochromatic wavelength of 0.1514 nm. Laser Raman spectroscopy, with an excitation wavelength of 532 nm, was conducted on the prepared electrode materials using a spectrometer at room temperature. Morphological investigations were carried out via scanning electron microscopy (SEM), which also provided energy-dispersive X-ray (EDX) analysis.
Electrochemical measurements
Standard electrochemical techniques, including Cyclic Voltammetry (CV), Galvanostatic Charge/Discharge (GCD), and Electrochemical Impedance Spectroscopy (EIS), were employed using a Gamry potentiostat to assess the electrochemical efficiency of the synthesized electrode materials. Electrochemical measurements are crucial for analyzing the electrical response of materials to an applied voltage or current. These techniques are essential for evaluating the performance of materials in energy storage technologies, including batteries, supercapacitors, and fuel cells. Electrochemical experiments were conducted using a three-electrode configuration with 2 M KOH as the electrolyte. The cyclic voltammetry (CV) profiles provided data for assessing the specific capacity of the samples. The specific capacitance of the synthesized active materials was calculated from the galvanostatic charge/discharge (GCD) curves, employing the corresponding formula outlined below35.
where “I” represents the discharge current, “Δt” is the discharge time, and “m” is the mass of the electroactive substance.
Specific capacitance (Fg−1) is denoted by Cs, current density (I) is measured in milliamperes (mA), discharge duration (s) is denoted by Δt, active material mass (m) is denoted by mg, and potential range (V) is denoted by ΔV. Using the following formulas, the device’s energy density (E) and power density (P) were determined36:
Specific capacitance is represented by “Cs” and discharge voltage by “ΔV” in Eq. (3), and energy density and discharge duration are represented by “E” and “Δt” respectively in Eq. (4).
Results and discussion
Structural investigation of VSe2/CuS nanocomposites
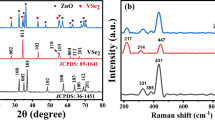
Figure 2a displays the XRD patterns of the synthesized VSe2/CuS nanocomposites, as well as those of pure VSe2 and CuS. The measurements were performed across a 2θ range of 20° to 80°, with a step increment of 0.02°. The XRD profile of pure VSe2 aligns with the hexagonal crystal structure, as identified by JCPDS card number 89-164137. The XRD pattern of VSe2 reveals intense peaks at planes (001), (002), (011), (102), (110), (004), (112), (201), and (022), corresponding to 2θ values of 14.8°, 83.4°, 32.4°, 44.2°, 56.1°, 57.1°, 62.4°, 66.1°, 69.1°, and 72.5°38. In the synthesized VSe2, the intensity of XRD peaks declines, which may be affected by the crystallographic orientation of the sample. When a material exhibits preferential growth along a specific direction, certain peaks may appear more or less intense than the standard JCPDS reference pattern. Furthermore, the crystallite size plays a crucial role in influencing peak intensities, as variations in size can impact the diffraction pattern. The XRD pattern of VSe2 reveals a hexagonal crystal structure, demonstrating strong agreement with the previously reported data39.The hexagonal phase of CuS is identified by planes located at (101), (102), (103), (006), (110), (108), and (116)40. The absence of additional diffraction peaks in the VSe2/CuS composite compared to pure VSe2 and CuS, demonstrating the composite’s high level of purity. Crystallite size estimations were made using the Scherrer’s equation41.
The parameters include k as a constant, λ as the CuKα wavelength, β as the FWHM, and θ as the diffraction angle. Crystallite size calculations for VSe2, CuS, and VSe2/CuS nanoparticles were performed, and the results are summarized in Table 1.
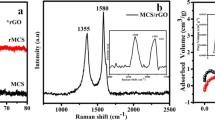
To gain deeper insight into the phase formation and structural features, the samples were subjected to Raman spectroscopy. The corresponding Raman spectra for VSe2, CuS, and VSe2/CuS nanocomposites are depicted in Fig. 2b. The Raman spectrum of VSe2 typically exhibits vibrational peaks at 160 cm−1 and 261 cm−1, corresponding to the D and G bands, respectively42. The D band arises from the A1g symmetry of k-point phonons, while the G band is associated with the E2g phonon of sp2-bonded atoms. The D band is often linked to defects and disorder in the hexagonal lattice, while the G band corresponds to the vibrations of sp2-bonded atoms within a 2D hexagonal lattice. Additionally, prominent and sharp peaks at 256 cm−1 and 469 cm−1, corresponding to the bending and stretching of Cu–S bonds and stretching of the Cu–S bonds respectively are observed43. This indicates that the lattice atoms of CuS are arranged in a periodic array, consistent with the covellite structure of CuS possessing a hexagonal crystal lattice. The Raman spectrum of the VSe2/CuS nanocomposites shows distinct peaks from both VSe2 and CuS nanoplatelets, providing evidence for the successful formation of the composite with high purity.
The nitrogen adsorption–desorption method was used to measure the surface area and of synthesized samples. Figure 2c,d show the BET isotherms for VSe2, CuS and VSe2/CuS nanocomposites, respectively. The specific surface areas, determined using the multi-point BET method, were 30, 38, and 54 m2/g, respectively. A noticeable hysteresis loop appeared in the BET isotherms within the relative pressure range of 0.1 < P/P₀ < 1.0, indicating irreversible nitrogen desorption. This suggests that the materials have mesoporous structures34,44.
Morphology and elemental analysis
SEM image of VSe2 (Fig. 3a) provides a high-magnification view of its surface morphology and structure. The image highlights particle clusters with a rough, textured surface, along with the presence of elongated, needle-like structures interspersed within the clusters45. The material’s intricate surface morphology is captured at high magnification in the CuS SEM image (Fig. 3b). It exhibits a complex structure with a range of textures and shapes, such as lengthy nanoflakes-like structures and spherical formations with rough surfaces. These characteristics provide important information about the morphology and microstructure of CuS, which are important in defining its chemical and physical characteristics46. The coexistence of both the VSe2 and CuS phases is suggested by the combination of spherical and irregularly shaped particles seen in Fig. 3c. Furthermore, features that resemble needles or rods are seen, which probably indicate that one of the components VSe2 is crystalline.
The elemental composition of materials was ascertained by electron microscopy using the analytical method known as Energy Dispersive X-ray Spectroscopy (EDX). As illustrated in Fig. 4a, the existence of both vanadium and selenium in the sample is confirmed by the distinctive X-ray peaks of vanadium at roughly 4.9 keV and 5.4 keV, as well as the peaks of selenium at 1.4 keV, 1.3 keV, and higher energy peaks around 11.2 keV and 12.5 keV. Likewise, copper is indicated by peaks at 1 keV, 8 keV, and 9 keV, but sulfur is suggested by a peak at 2.3 keV (Fig. 4b). Vanadium is represented by peaks in Fig. 4c that are located between 4.5 and 5.2 keV. Copper is represented by peaks close to 1 keV, 8 keV, and 9 keV. Sulfur is present at about 2.3 keV, while selenium is expected to show up between 1.3 keV and 11.2 keV. The ultra-high purity of the produced nanocomposites is indicated by the absence of impurity peaks in the composite. The values of atomic and weight percentages of VSe2, CuS, and VSe2/CuS are shown in Table 2.
Electrochemical studies
The cyclic voltammogram of VSe2, CuS and VSe2 /CuS electrodes, shown in Fig. 5a–c, were recorded in a potential range of 0–0.6 V at scan rates from 10–50 mV/s. Clearly, visible oxidation and reduction peaks confirm the pseudocapacitive nature of the materials. Additionally, the unchanged CV curve shapes at higher scan rates highlight the electrodes’ electrochemical stability, improved mass transport, and superior rate capability. Figure 5d presents a comparison of the CV profiles for VSe2, CuS, and VSe2/CuS nanocomposites recorded at a scan rate of 20 mV/s. The larger integrated area of the CV curves for VSe2/CuS nanocomposites indicates an enhanced charge storage capacity relative to VSe2 and CuS, which can be assigned to the significant surface area of the composite. The layered structure of VSe2 enables it to accommodate multiple oxidation states, such as V3⁺ and V4⁺, which are crucial for charge storage. Throughout the electrochemical process, VSe2 undergoes redox transitions, including the V4⁺/V3⁺ couple47. Additionally, selenium (Se) actively participates in redox reactions, cycling between Se2− and Se4⁺ states. These redox activities enhance charge storage capacity, thereby improving overall energy storage performance. During charging, K⁺ ions from the electrolyte, along with OH⁻ diffusion, gradually occupy available sites, particularly at lower scan rates and currents, leading to an increase in capacity30. The relatively weak bonding of Se within the V–Se framework, both chemically and electronically, varies with oxidation states, further enhancing the material’s electrochemical reactivity. Meanwhile, CuS undergoes redox transitions between Cu2⁺/Cu⁺ and Cu⁺/Cu⁰, contributing to the overall charge storage process. The electrochemical interaction between VSe2 and CuS allows their redox mechanisms to work harmoniously, which may bring their peak potential values closer together. The charging and discharging processes of the VSe2/CuS electrode involve the following electrochemical reactions48,49.
Enhancing the interaction between active electrodes and the electrolyte optimizes the utilization of active materials, leading to improved supercapacitor performance. During the discharge process, host ions migrate back into the electrolyte, while external electrons facilitate charge balance, ensuring charge neutrality. The preservation of the electrode’s shape throughout all scan rates underscores its remarkable reversibility.
To evaluate the rate capability and electrochemical capacitive properties of VSe2, CuS, and VSe2/CuS nanocomposite electrodes, GCD measurements were performed at various current densities (1–5 Ag−1). The GCD profile lacks a potential-independent region, highlighting the electrode’s predominant capacitive behavior. Figure 6a–c reveals that discharge times decrease as current density increases, with VSe2/CuS electrodes displaying longer discharge times than VSe2 and CuS signifying enhanced specific capacitance. The GCD profile aligns with the CV performance, emphasizing the improved electrochemical activity of the VSe2/CuS electrode (Fig. 6c). Figure 6d shows the relative GCD profiles of the three electrodes. Additionally, the electrode demonstrated outstanding rate capability, retaining approximately 88.3% of its capacitance at the highest current density. Demonstrating superior performance, the VSe2/CuS nanocomposites achieved the highest specific capacitance, recording 853.9 Fg−1 at 1 Ag−1 and 566.6 Fg−1 at 5 Ag−1, exceeding that of individual VSe2 and CuS electrodes. Equation (1) was applied to calculate the specific capacitance of the synthesized specimens, and Retention percentages were determined using Eq. (6), and the corresponding data are compiled in Table 350.
Figure 7a highlights the variation of specific capacitance with current density for VSe2, CuS, and VSe2/CuS nanomaterials. EIS measurements were carried out to explore the electrochemical kinetics of the electrodes, with the Nyquist plots presented in Fig. 7b. The experiments, conducted under the same conditions as CV and GCD, covered a frequency range from 100 kHz to 0.01 Hz. The VSe2/CuS electrodes exhibited enhanced capacitive behavior compared to pristine VSe2 and CuS. The Nyquist plot revealed that the equivalent series resistance (Rs) is represented on the x-axis, while the charge transfer resistance (Rct) is identified by the low-frequency slope, indicating improved ion and electron transport. Rs and Rct values in Table 4 suggest variations in electrochemical series resistance (ESR) arise from differences in internal resistance, despite uniform fabrication of all samples. The VSe2/CuS electrode displayed linear capacitive characteristics in the low-frequency region, indicative of limited ion transport.
Two electrode analysis
To evaluate the practical applicability of the VSe2/CuS electrode material, an asymmetric supercapacitor (ASC) was constructed, with VSe2/CuS serving as the positive electrode and commercially available porous activated carbon (AC) with a high surface area as the negative electrode. The elevated surface area and specific energy of AC make it ideal for use as the negative electrode in HASC applications. The CV curves of the VSe2/CuS||AC based ASC at scan rates of 10–50 mV/s are displayed in Fig. 8a. It displays a quasi-rectangular shape, indicating capacitance contributions from both battery-type and electric double-layer capacitor (EDLC)-like performance. The CV curve profile remained consistent across different scan rates, while the peak currents increased with higher scan rates. Based on the initial results from the CV experiments, a GCD test was performed on the VSe2/CuS||AC-based ASC within a potential window of 0–1.6 V and a current density range of 1–5 Ag−1 (Fig. 8b). At a current density of 1 Ag−1, the symmetrical GCD profile observed at low current density indicates a well-balanced mass ratio between the positive and negative electrodes. Furthermore, the GCD curve demonstrates nearly identical charge and discharge durations, highlighting the exceptional reversibility of the cell. Figure 8c illustrates the relationship between specific capacitance and current density, highlighting their variation. Figure 8d presents the Ragone plot, a significant performance indicator for ASCs. The VSe2/CuS||AC ASC delivered a maximum energy density of 52.4 Wh/kg at a power density of 825 W/kg, aligning well with recent findings (Table 5). Even at a high-power density of 3998.5 W/kg, the device retained an energy density of 28.3 Wh/kg, indicating superior rate performance.
Furthermore, the ASC exhibited outstanding stability with 88.3% and nearly 100% coulombic efficiency after 10,000 GCD cycles, as illustrated in Fig. 9a. Cycling stability was further analyzed by performing GCD tests on the as-prepared VSe2/CuS||AC electrodes at a current density of 10 Ag−1 for 10,000 cycles. Figure 9a includes an insert highlighting the charge–discharge performance of VSe2/CuS||AC electrodes, with the first 8 cycles represented in black and the last 8 cycles in red, tested at a current density of 5 Ag−1. Both electrodes maintain a stable voltage of 1.6 V (± 0.005%) after 10,000 cycles, highlighting their durability under high current conditions.
The impedance spectrum of the VSe2/CuS||AC ASC device, depicted in Fig. 9b, exhibits a minor semicircular feature, signifying a Rct of 2.3 Ω and a Rs of 1.45 Ω. These values indicate efficient charge transport, minimal electrolyte resistance, and strong electrochemical interactions between the active material, electrolyte ions, and the current collector. Furthermore, the nearly vertical trend observed in the high-frequency region of the Nyquist plot suggests negligible diffusion impedance and excellent capacitive performance of the VSe2/CuS||AC ASC device.
Summary
The synthesis of VSe2, CuS, and VSe2/CuS nanocomposites was successfully carried out using a facile hydrothermal and wet chemical method, respectively. X-ray diffraction (XRD) analysis confirmed the phase purity of the materials while scanning electron microscopy (SEM) revealed spherical and flake-like morphology. The fabricated VSe2/CuS electrode demonstrates exceptional capacitance, achieving 853.9 F/g at a current density of 1 A/g, significantly surpassing the individual capacitance values of VSe2 (395.6 F/g) and CuS (471.6 F/g) in a three-electrode configuration. At a current density of 5 A/g, the VSe2/CuS||AC nanocomposites achieved a specific capacitance of 147.6 F/g, along with a high energy density of 52.5 Wh/kg at a power density of 3998.5 W/kg, exhibiting exceptional cyclic stability. Cycling performance tests showed an 88.3% retention rate after 10,000 cycles, confirming their potential for supercapacitor applications. This study highlights the promising capabilities of combining metal selenides and sulfides for advanced supercapacitor applications used in energy storage devices.
Data availability
The data presented in this study are included in the manuscript.
References
Deshmukh, M. K. G. et al. Renewable energy in the 21st century: A review. Mater. Today: Proc. 80, 1756–1759 (2023).
Han, S. et al. Harnessing technological innovation and renewable energy and their impact on environmental pollution in G-20 countries. Sci. Rep. 15(1), 2236 (2025).
Flórez-Orrego, D. et al. Renewable and non-renewable exergy costs and CO2 emissions in the production of fuels for Brazilian transportation sector. Energy 88, 18–36 (2015).
Al-Shetwi, A. Q. Sustainable development of renewable energy integrated power sector: Trends, environmental impacts, and recent challenges. Sci. Total Environ. 822, 153645 (2022).
Sonkar, P. K. & Ganesan, V. Nanomaterials for Sustainable Energy Applications (CRC Press, 2023).
Sen, S. & Ganguly, S. Opportunities, barriers and issues with renewable energy development: A discussion. Renew. Sustain. Energy Rev. 69, 1170–1181 (2017).
Yedluri, A. K. & Kim, H.-J. Wearable super-high specific performance supercapacitors using a honeycomb with folded silk-like composite of NiCo2O4 nanoplates decorated with NiMoO 4 honeycombs on nickel foam. Dalton Trans. 47(43), 15545–15554 (2018).
Kumar, K. D. et al. Cactus-like Ni-Co/CoMn2O4 composites on Ni foam: Unveiling the potential for advanced electrochemical materials for pseudocapacitors. Mater. Sci. Eng.: B 296, 116715 (2023).
Kumar, K. D. et al. Hierarchically fabricated nano flakes-rod-like CoMoO–S supported Ni-foam for high-performance supercapacitor electrode material. J. Phys. Chem. Solids 185, 111735 (2024).
Kumar, Y. A., Kumar, K. D. & Kim, H.-J. Reagents assisted ZnCo2O4 nanomaterial for supercapacitor application. Electrochim. Acta 330, 135261 (2020).
Béguin, F., Raymundo-Piñero, E. & Frackowiak, E. Electrical Double-Layer Capacitors and Pseudocapacitors. Carbons for electrochemical energy storage and conversion systems 329–375 (2010).
Kumar, Y. A. & Kim, H.-J. Preparation and electrochemical performance of NiCo2O4@ NiCo2O4 composite nanoplates for high performance supercapacitor applications. N. J. Chem. 42(24), 19971–19978 (2018).
Zhou, G. et al. Nanowires for electrochemical energy storage. Chem. Rev. 119(20), 11042–11109 (2019).
Yadlapalli, R. T. et al. Super capacitors for energy storage: Progress, applications and challenges. J. Energy Stor. 49, 104194 (2022).
Melkiyur, I. et al. A comprehensive review on novel quaternary metal oxide and sulphide electrode materials for supercapacitor: Origin, fundamentals, present perspectives and future aspects. Renew. Sustain. Energy Rev. 173, 113106 (2023).
Liang, R. et al. Transition metal oxide electrode materials for supercapacitors: a review of recent developments. Nanomaterials 11(5), 1248 (2021).
Wu, Z. Transition metal selenides for oxygen evolution reaction. Energy Technol. 12(6), 2301574 (2024).
Lu, T. et al. Fabrication of transition metal selenides and their applications in energy storage. Coord. Chem. Rev. 332, 75–99 (2017).
Papavasileiou, A. V. et al. Ferromagnetic elements in two-dimensional materials: 2D magnets and beyond. Adv. Funct. Mater. 34(2), 2309046 (2024).
Xu, K. et al. Ultrathin nanosheets of vanadium diselenide: A metallic two-dimensional material with ferromagnetic charge-density-wave behavior. Angewandte Chemie 125(40), 10671–10675 (2013).
Kaura, D. & Bansalb, S. 2D Magnetic Systems Magnetic Properties. Fundamentals of Low Dimensional Magnets 73 (2022).
Ma, G. et al. Flower-branch-like carbon microtube/vanadium selenide: A highly efficient electromagnetic wave absorbing composite. Chem. Eng. J. 495, 152904 (2024).
Lu, Y. et al. Syntheses and energy storage applications of MxSy (M= Cu, Ag, Au) and their composites: Rechargeable batteries and supercapacitors. Adv. Funct. Mater. 27(44), 1703949 (2017).
Dutta, D. P. et al. An insight into the sodium-ion and lithium-ion storage properties of CuS/graphitic carbon nitride nanocomposite. RSC Adv. 12(20), 12383–12395 (2022).
Wang, Z. et al. Microwave-assisted synthesis of CuS hierarchical nanosheets as the cathode material for high-capacity rechargeable magnesium batteries. ACS Appl. Mater. Interfaces 11(7), 7046–7054 (2019).
Kumar, Y. A. et al. A MoNiO 4 flower-like electrode material for enhanced electrochemical properties via a facile chemical bath deposition method for supercapacitor applications. N. J. Chem. 44(2), 522–529 (2020).
Krishnamoorthy, K. et al. One-pot hydrothermal synthesis, characterization and electrochemical properties of CuS nanoparticles towards supercapacitor applications. Mater. Res. Expr. 1(3), 035006 (2014).
Liu, Q., Zhang, S. & Xu, Y. Two-step synthesis of CuS/C@ PANI nanocomposite as advanced electrode materials for supercapacitor applications. Nanomaterials 10(6), 1034 (2020).
Wang, C. et al. VSe2/carbon-nanotube compound for all solid-state flexible in-plane supercapacitor. Appl. Phys. Lett. https://doi.org/10.1063/1.5078555 (2019).
Raj, K. S. & Rout, C. S. Facile synthesis of manganese-doped 2D vanadium diselenide nanosheets for high-performance supercapacitor applications. Emergent Mater. 4(4), 1037–1046 (2021).
El-Hout, S. I. et al. High electrochemical performance of rGO anchored CuS nanospheres for supercapacitor applications. J. Energy Stor. 34, 102001 (2021).
Raghavendra, K. V. G., Rao, K. M. & Kumar, N. U. Hydrothermal synthesis of CuS/CoS nano composite as an efficient electrode for the supercapattery applications. J. Energy Stor. 40, 102749 (2021).
Shandilya, M., Rai, R. & Singh, J. Hydrothermal technology for smart materials. Adv. Appl. Ceram. 115(6), 354–376 (2016).
Hira, S. A. et al. NO2-functionalized metal–organic framework incorporating bimetallic alloy nanoparticles as a sensor for efficient electrochemical detection of dopamine. Electrochem. Commun. 125, 107012 (2021).
Mohammed, A. A., Chen, C. & Zhu, Z. Low-cost, high-performance supercapacitor based on activated carbon electrode materials derived from baobab fruit shells. J. Colloid Interface Sci. 538, 308–319 (2019).
Zhang, F. et al. A high-performance supercapacitor-battery hybrid energy storage device based on graphene-enhanced electrode materials with ultrahigh energy density. Energy Environ. Sci. 6(5), 1623–1632 (2013).
Raj, K. A. S. et al. Heterostructured metallic 1T-VSe2/Ti3C2T x MXene nanosheets for energy storage. ACS Appl. Nano Mater. 5(3), 4423–4436 (2022).
Yang, C. et al. Metallic graphene-like VSe2 ultrathin nanosheets: Superior potassium-ion storage and their working mechanism. Adv. Mater. 30(27), 1800036 (2018).
Kuo, F.-Y. et al. Synthesis of surfactant-free and morphology-controllable vanadium diselenide for efficient counter electrodes in dye-sensitized solar cells. ACS Appl. Mater. Interfaces 11(28), 25090–25099 (2019).
Du, W. et al. Shape-controlled synthesis and self-assembly of hexagonal covellite (CuS) nanoplatelets. Chem.: Eur. J. 13(11), 3241–3247 (2007).
Burton, A. W. et al. On the estimation of average crystallite size of zeolites from the Scherrer equation: A critical evaluation of its application to zeolites with one-dimensional pore systems. Microporous Mesoporous Mater. 117(1–2), 75–90 (2009).
Zhang, H. et al. A simple synthesis of VSe2/B4C@ HCG composite as high-performance anodes for sodium-ion batteries. J. Alloys Compounds 935, 168111 (2023).
Tao, F. et al. Copper sulfide-based plasmonic photothermal membrane for high-efficiency solar vapor generation. ACS Appl. Mater. Interfaces 10(41), 35154–35163 (2018).
Kumar, Y. A., Kumar, K. D. & Kim, H.-J. A novel electrode for supercapacitors: Efficient PVP-assisted synthesis of Ni3S2 nanostructures grown on Ni foam for energy storage. Dalton Trans. 49(13), 4050–4059 (2020).
Al-Farraj, E. S. et al. Development of VSe2@ Cu2Se nano-composites via facile one-pot hydrothermal method for pharmaceutical applications. Phys. Script. 98(9), 095004 (2023).
Li, F. et al. Controllable synthesis, optical and photocatalytic properties of CuS nanomaterials with hierarchical structures. Powder Technol. 198(2), 267–274 (2010).
Wu, X. et al. Boosting energy and power performance of aqueous energy storage by engineering ultra-fine metallic VSe2 nanoparticles anchored reduced graphene oxide. J. Power Sources 448, 227399 (2020).
Blanc, C. & Rufer, A. Understanding the vanadium redox flow batteries. Paths Sustain. Energy 18(2), 334–336 (2010).
Wang, W. et al. Recent progress in redox flow battery research and development. Adv. Funct. Mater. 23(8), 970–986 (2013).
Yang, J. & Gunasekaran, S. Electrochemically reduced graphene oxide sheets for use in high performance supercapacitors. Carbon 51, 36–44 (2013).
Ashok Kumar, K. et al. Effect of bi-functional hierarchical flower-like CoS nanostructure on its interfacial charge transport kinetics, magnetic and electrochemical behaviors for supercapacitor and DSSC applications. Sci. Rep. 9(1), 1228 (2019).
Miao, Y. et al. Hierarchical NiS@ CoS with controllable core-shell structure by two-step strategy for supercapacitor electrodes. Adv. Mater. Interfaces 7(3), 1901618 (2020).
Liu, G. et al. 3D self-supported hierarchical core/shell structured MnCo2O4@ CoS arrays for high-energy supercapacitors. J. Mater. Chem. A 6(4), 1822–1831 (2018).
Subramani, K. et al. All-solid-state asymmetric supercapacitors based on cobalt hexacyanoferrate-derived CoS and activated carbon. RSC Adv. 7(11), 6648–6659 (2017).
Wang, S. et al. Facile synthesis of truncated cube-like NiSe2 single crystals for high-performance asymmetric supercapacitors. Chem. Eng. J. 330, 1334–1341 (2017).
Chauhan, H. et al. Development of SnS2/RGO nanosheet composite for cost-effective aqueous hybrid supercapacitors. Nanotechnology 28(2), 025401 (2016).
Marri, S. R. et al. 3D cuboidal vanadium diselenide embedded reduced graphene oxide hybrid structures with enhanced supercapacitor properties. Chem. Commun. 53(1), 228–231 (2017).
Thangappan, R. et al. Graphene decorated with MoS2 nanosheets: A synergetic energy storage composite electrode for supercapacitor applications. Dalton Trans. 45(6), 2637–2646 (2016).
Pandit, B., Karade, S. S. & Sankapal, B. R. Hexagonal VS2 anchored MWCNTs: First approach to design flexible solid-state symmetric supercapacitor device. ACS Appl. Mater. Interfaces 9(51), 44880–44891 (2017).
Gopi, C. V. M. et al. One-pot hydrothermal synthesis of tungsten diselenide/reduced graphene oxide composite as advanced electrode materials for supercapacitors. Mater. Lett. 223, 57–60 (2018).
Acknowledgements
The authors would like to acknowledge the Higher Education Commission of Pakistan (HEC) via the National Research Program for Universities (NRPU) Project No. 20-16971/NRPU/R&D/HEC/2021 for funding this study. The authors also acknowledge the financial support extended by the Researchers Supporting Project number (RSP2025R242), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
I.K, A.U.S and D.A: data curation; D.A, A.S, and A.U.S: formal analysis; A.S, B.A, and K.M.A: methodology; I.K and S.A: project administration; B.A and K.S: resources; A.S, W.M.G, and D.A: supervision; A.U.S, D.A, I.K, and A.S: writing-original draft; W.M.G, B.A, K.M.A and K.S: writing-review and editing. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Khan, I., Arif, D., Shah, A.U. et al. Synergistic integration of VSe2 and CuS nanostructures for advanced energy storage applications. Sci Rep 15, 19761 (2025). https://doi.org/10.1038/s41598-025-95088-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-95088-6