Abstract
To determine the median effective dose (ED50) and the 95% effective dose (ED95) of ciprofol combined with sufentanil for inhibiting the tracheal intubation response in female patients and to evaluate the hemodynamic stability and adverse events associated with this drug combination. This was a prospective dose-finding study using an up-and-down sequential allocation method. A total of 30 female patients undergoing general anesthesia surgeries between November 2024 and December 2024 were enrolled. Patients were administered ciprofol for induction, with an initial dose of 0.4 mg/kg and subsequent doses adjusted based on the presence or absence of an intubation response. The primary outcome was the ED50 of ciprofol, with secondary outcomes including patient baseline characteristics, hemodynamic parameters, and adverse events. The ED50 and ED95 of ciprofol for inhibiting tracheal intubation response were 0.318 mg/kg and 0.496 mg/kg, respectively. Patients with a positive tracheal intubation response had significantly higher heart rates and mean arterial pressures, as well as a higher incidence of hypertension and body movement. The ED50 and ED95 of ciprofol combined with sufentanil for suppressing the response to tracheal intubation in female patients were 0.318 mg/kg and 0.496 mg/kg, respectively.
Similar content being viewed by others
Introduction
Intubation of the trachea is a crucial step in general anesthesia to ensure patent airway and effective ventilation1,2. However, this procedure often elicits robust stress responses in patients, including but not limited to coughing, elevated blood pressure, and tachycardia. These stress responses complicate anesthetic management and may adversely affect patients3,4. In female patients, due to possible physiological peculiarities, they may show a different sensitivity to stress associated with tracheal intubation than males5. The study by Laborde et al. concluded that in the pathways regulating the sympathetic nervous system, in women sensitivity to excitatory stimuli is lower and sensitivity to inhibitory stimuli is higher6. Therefore, exploring anesthesia strategies tailored to this specific population is of paramount importance.
Ciprofol, a novel non-barbiturate intravenous anesthetic, has garnered attention for its multiple advantages in clinical anesthesia. As a structural analogue of propofol, ciprofol demonstrates rapid onset, swift recovery, reduced injection pain, and hemodynamic stability7,8. A meta-analysis by Guilherme et al., involving 1225 patients, found that ciprofol had lower incidences of adverse reactions such as injection pain, respiratory depression, and hypotension compared to propofol9. Ciprofol exhibits approximately 4 to 5 times greater affinity for the GABAA receptor than propofol, suggesting that it can achieve comparable sedative effects at lower doses, thereby showing unique potential in inhibiting stress responses triggered by tracheal intubation10.
Sufentanil, a potent opioid analgesic, significantly alleviates the stress response induced by tracheal intubation11,12. When combined with ciprofol, sufentanil not only effectively mitigates the stress response associated with tracheal intubation but also reduces adverse reactions during anesthesia induction. A randomized controlled trial by Lan et al., involving 149 patients, found that sufentanil combined with ciprofol resulted in a lower incidence of injection pain, more stable hemodynamics, and less respiratory depression compared to propofol during general anesthesia induction for hysteroscopic surgery13. 1104 study of hysteroscopic general anaesthesia also found that ciprofol combined with sufentanil had advantages over propofol combined with sufentanil in terms of hypotension, injection pain and respiratory depression14. However, there is a paucity of research on the median effective dose (ED50) of this drug combination for inhibiting the stress response to tracheal intubation in female patients. Therefore, the aim of this study was to explore the ED50 and 95% effective dose (ED95) of ciprofol combined with sufentanil in inhibiting the response to tracheal intubation in female patients through a prospective sequential trial design.
Methods
Participants
This study enrolled a total of 30 female patients who underwent general anesthesia surgeries between November 2024 and December 2024. The inclusion criteria were: age 18–65 years; body mass index (BMI) 19–28 kg/m²; ASA (American Society of Anesthesiologists) physical status I-II. The exclusion criteria encompassed: severe cardiopulmonary diseases; baseline systolic blood pressure > 160 mmHg or heart rate > 100 beats per minute; abnormalities in liver or kidney function; history of allergy to study medications; and difficult airway.
Anesthesia
All patients were fasted for 8 h and abstained from fluids for 6 h preoperatively. Upon arrival in the operating room, intravenous access was established for the infusion of compound sodium chloride, and oxygen was administered via a facemask at 3 L/min. Continuous monitoring was maintained for electrocardiogram (ECG), pulse oximetry (SpO2), heart rate (HR), blood pressure, and bispectral index (BIS). HR and blood pressure were measured three times consecutively after the patient entered the operating theatre for a 10-minute rest and averaged as baseline values. Anaesthesia was induced with a slow intravenous injection of ciprofol. After BIS ≤ 60 and MOAA/S (Modified Observer’s Assessment of Alertness/Sedation) ≤ 1, sufentanil 0.4ug/kg and cisatracurium 0.2 mg/kg were administered intravenously. Following muscle relaxation, tracheal intubation was using a video laryngoscope and a single-use endotracheal tube of size 6.5–7.5, with the tip of the tube coated with lidocaine gel. After successful intubation, mechanical ventilation was initiated. The initial dose of ciprofol was set at 0.4 mg/kg for the first patient, with subsequent doses adjusted based on the presence or absence of an intubation response. In this study, positive tracheal intubation reaction was defined as an increase in mean arterial pressure (MAP) or HR of more than 20% from baseline or MOAA/S > 1 during intubation and within 2 min after intubation15,16,17,18,19. If no intubation response occurred, the ciprofol dose was decreased by 0.05 mg/kg for the next patient. If the intubation response was observed, the ciprofol dose was increased by 0.05 mg/kg for the next patient. If MOAA/S > 1 during intubation, additional sufentanil or ciprofol was administered as required. Throughout the surgery, sevoflurane was used to maintain the depth of anesthesia, with intermittent boluses of sufentanil and cisatracurium given to maintain analgesia and muscle relaxation. After surgery, the endotracheal tube was removed, and the patient was transferred to the Post-Anesthesia Care Unit.
Determination of ED50
The ED50 of ciprofol for inhibiting tracheal intubation response in female patients was determined using the up-and-down sequential allocation method. The initial dose of ciprofol was set at 0.4 mg/kg, with a dose gradient of 0.05 mg/kg. Sample size calculations were based on the principles of the sequential allocation method, and the ED50 was calculated by completing the study when 7 crossings between successes and failures were reached20,21.
Study outcomes
The primary outcome was the ED50 of ciprofol combined with sufentanil in inhibiting tracheal intubation response in female patients. The secondary outcomes included the following. Patient baseline characteristics (age, gender, height, weight, BMI, ASA classification, etc.). In addition, hemodynamic parameters were recorded at baseline (T1), 1 min after induction (T2), immediately after tracheal intubation (T3), and 2 min after tracheal intubation (T4). Furthermore, adverse events such as increased blood pressure, decreased blood pressure (exceeding 30% of baseline value), bradycardia and tachycardia (HR < 50 or > 100 per minute), hypoxemia (SpO2 < 90%), intraoperative awareness, injection pain, and myoclonus were recorded.
Statistical analysis
The sample size for this study was based on the sequential allocation method, which ended the trial when 7 crossover points were reached20,21. ED50 and ED95 were estimated using probabilistic regression analysis. Normally distributed data were expressed using mean and standard deviation, and independent sample t-test was used for comparison. Non-normally distributed data were expressed as median and interquartile spacing. Categorical data were compared using the χ2 test or Fisher’s exact probability method. P < 0.05 was considered a statistically significant difference.
Results
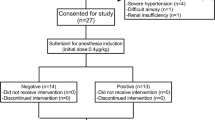
The flow chart of this study is shown in Fig. 1. We assessed the eligibility of 30 patients between November and December 2024. Five patients were excluded, four of whom did not meet the eligibility criteria and one of whom presented with an unanticipated difficult airway. A total of 25 patients were included in the final analysis, of which 13 had a negative tracheal intubation reaction and 12 had a positive tracheal intubation reaction. The baseline profile of the patients is shown in Table 1.
Figure 2 shows the up-and-down sequential response to induction of intubation with ciprofol in 25 female patients. The number of positive and negative tracheal intubations induced by different doses of ciprofol is shown in Table 2. At 0.45 mg/kg of ciprofol induction the tracheal intubation response was negative in all patients. Tracheal intubation response was positive in all patients at a dose of 0.25 mg/kg of ciprofol. Our study ended the trial after seven positive-negative crossovers occurred.
Table 3 shows that ED50, ED95 and 95% confidence intervals (CI) of ciprofol inhibiting endotracheal intubation in female patients are 0.318 (0.284–0.349) and 0.496 (0.435–0.662) mg/kg, respectively.
The results of HR and MAP in the negative and positive groups are shown in Fig. 3. Comparison between groups showed that both HR and MAP at T3 were significantly higher in the positive group than in the negative group (A:P = 0.03,B:P = 0.004).
The incidence of adverse events in this study is shown in Table 4. In the comparison of the incidence of hypertension (P = 0.01) and body movement (P = 0.001), the positive group was significantly higher than the negative group. In this study, no patients experienced hypoxemia, injection pain, intraoperative awareness, or myoclonus.
Discussion
In this study, we determined the median effective dose (ED50) and the 95% effective dose (ED95) of ciprofol combined with sufentanil for inhibiting the tracheal intubation response in female patients using the up-and-down sequential allocation method. The results indicated that the ED50 and ED95 of ciprofol were 0.318 mg/kg (95% CI: 0.284–0.349 mg/kg) and 0.496 mg/kg (95% CI: 0.435–0.662 mg/kg), respectively.
Propofol, as a widely used clinical intravenous anaesthetic, has advantages similar to those of ciprofol, including rapid onset of action, rapid awakening, and precise anaesthesia22,23. However, compared with propofol, ciprofol performs better in terms of circulatory suppression, respiratory depression, injection pain, and drug efficacy24. Currently, the clinical use of ciprofol has shown promising results, with a growing body of evidence supporting its efficacy and safety in a variety of surgical settings. Therefore, it is likely that ciprofol will replace propofol as the anaesthetic of choice in the future25,26. The ED50 of ciprofol combined with sufentanil for tracheal intubation was found to be 0.326 mg/kg in the study of liao et al. The ED50 of our study was 0.318 mg/kg, which was slightly lower than the study of liao et al. The possible reason for this is that our study focused only on female patients, which may have contributed to the decrease in ED5027.
The sequential allocation method is effective for assessing the ED50 of drugs in small sample sizes, making it widely utilized in anesthesia study design. Dilireba et al.‘s meta-analysis found that using 0.4 mg/kg of ciprofol for anesthesia induction achieves good induction effects with lower adverse reactions. Therefore, the initial dose of ciprofol in this study was set at 0.4 mg/kg28.
The hemodynamic findings of our study underscore the clinical significance of effectively managing the tracheal intubation response. Notably, patients in the positive intubation response group exhibited marked elevations in HR and MAP during intubation (at T3), accompanied by a higher incidence of hypertension and body movement, as compared to those in the negative group. In the positive group, the patients’ induction dose of ciprofol was insufficient to inhibit the response to tracheal intubation, which led to higher catecholamine release causing an increase in heart rate and blood pressure (> 20% of baseline values). The results of our study contribute to the clinical understanding of the optimal dose of ciprofol combined with sufentanil for induction of general anesthesia. The ED50 and ED95 values obtained provide a scientific basis for the use of these drugs in a manner that balances their efficacy and safety. The significance of these results is that they have the potential to guide clinical practice in minimizing hemodynamic responses during tracheal intubation, thereby reducing the risk of adverse outcomes29,30,31.
Furthermore, the low incidence of adverse events observed in our study, such as injection pain, hypoxemia, and intraoperative awareness, highlights the favorable safety profile of ciprofol when used in combination with sufentanil. This is particularly important given the concerns about propofol-related side effects, such as injection pain and hemodynamic instability. Yang et al.‘s mete-analysis, which included 2002 patients, found that injection pain occurred in 60 (1,102 total, 5.4%) patients in the ciprofol group, compared with 406 (931 total, 43.6%) in the propofol group32. The lower incidence of adverse events with ciprofol suggests that it may offer a safer alternative to propofol for anesthesia induction, especially in patients with pre-existing cardiovascular or respiratory conditions33,34. In the study by Yang et al. it was shown that ciprofol may have cardioprotective effects by attenuating isoproterenol-induced oxidative damage, inflammatory response and cardiomyocyte apoptosis35. The incidence of body movement and hypertension was higher in the positive group compared to the negative group. This may be attributed to body movements due to shallow depth of anaesthesia caused by inadequate dose of ciprofol. Whereas hypertension may be due to strong tracheal intubation response. These findings highlight the importance of accurately determining the ED50 and ED95 of ciprofol in combination with sufentanil to minimise adverse events during tracheal intubation.
There are some limitations of this study. Firstly, the sample size calculated based on Dixon’s up-and-down sequential allocation method can satisfy the calculation of ED50, however, it may be insufficient for evaluating secondary outcome indicators. Second, our study focused solely on young female patients with ASA I-II, which may restrict the applicability of the results to other demographic groups, such as males or older patients. In addition, the ED50 for ciprofol in this study was only applicable at the 0.4ug/kg sufentanil induction dose. Lastly, future studies are needed to further validate the ED50 and ED95 of ciprofol combined with sufentanil in larger and more diverse patient populations.
Conclusion
The median effective dose and the 95% effective dose of ciprofol in combination with sufentanil for suppressing the response to tracheal intubation in female patients are 0.318 mg/kg and 0.496 mg/kg, respectively. Patients with a positive tracheal intubation response have a higher incidence of body movement and hypertension during intubation.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
References
Weller, J. M. Failed intubation: anaesthesia’s Achilles’ heel. Br. J. Anaesth. 133, 1126–1128. https://doi.org/10.1016/j.bja.2024.08.023 (2024).
Sparling, J. L., Chitilian, H. V., Korn, E., Alfille, P. H. & Bao, X. Induction of anaesthesia and airway management in patients with severe tracheal stenosis: a single-centre retrospective study. Br. J. Anaesth. https://doi.org/10.1016/j.bja.2024.10.027 (2024).
Zhao, L. et al. Efficacy and safety of remimazolam versus etomidate for induction of general anesthesia: protocol for a systematic review and meta-analysis. Jmir Res. Protoc. 13, e55948. https://doi.org/10.2196/55948 (2024).
Grover, N., Taneja, R., Rashid, Y. & Shrivastava, N. Nebulised Fentanyl, Dexmedetomidine and magnesium sulphate for Attenuation of haemodynamic response to laryngoscopy and tracheal intubation: a double-blinded, randomised comparative study. Indian J. Anaesth. 67, 730–735. https://doi.org/10.4103/ija.ija_397_22 (2023).
Jaensson, M., Gupta, A. & Nilsson, U. G. Gender differences in risk factors for airway symptoms following tracheal intubation. Acta Anaesthesiol. Scand. 56, 1306–1313. https://doi.org/10.1111/j.1399-6576.2012.02771.x (2012).
Hinojosa-Laborde, C., Chapa, I., Lange, D. & Haywood, J. R. Gender differences in sympathetic nervous system regulation. Clin. Exp. Pharmacol. Physiol. 26, 122–126 (1999).
Liang, Z. et al. Postoperative quality of recovery comparison between Ciprofol and Propofol in total intravenous anesthesia for elderly patients undergoing laparoscopic major abdominal surgery: a randomized, controlled, double-blind, non-inferiority trial. J. Clin. Anesth. 99, 111660. https://doi.org/10.1016/j.jclinane.2024.111660 (2024).
Ding, G., Wang, L., Zhao, W., Diao, Y. & Song, D. Comparison of the efficacy and safety of Ciprofol and Propofol for Ercp anesthesia in older patients: a single-center randomized controlled clinical study. J. Clin. Anesth. 99, 111609. https://doi.org/10.1016/j.jclinane.2024.111609 (2024).
Ortegal, G. H. et al. Ciprofol versus Propofol for adult sedation in Gastrointestinal endoscopic procedures: a systematic review and meta-analysis. Minerva Anestesiol. 90, 1013–1021. https://doi.org/10.23736/S0375-9393.24.18203-X (2024).
Liu, L. et al. Population Pharmacokinetic/pharmacodynamic modeling and exposure-response analysis of Ciprofol in the induction and maintenance of general anesthesia in patients undergoing elective surgery: a prospective dose optimization study. J. Clin. Anesth. 92, 111317. https://doi.org/10.1016/j.jclinane.2023.111317 (2024).
Jia, D., Yuan, X., He, C. & Tu, F. Intravenous Lidocaine decreased the median effective concentration of sufentanil for tracheal intubation in obese patients. Drug. Des. Devel. Ther. 17, 2431–2439. https://doi.org/10.2147/DDDT.S415872 (2023).
Su, P. et al. A response surface analysis of the combination of Dexmedetomidine and sufentanil for attenuating the haemodynamic response to endotracheal intubation. Dose-Response 20, 1495842319. https://doi.org/10.1177/15593258221092367 (2022).
Lan, H. et al. Efficacy and safety of Ciprofol for sedation/anesthesia in patients undergoing hysteroscopy: a randomized, parallel-group, controlled trial. Drug. Des. Devel. Ther. 17, 1707–1717. https://doi.org/10.2147/DDDT.S414243 (2023).
Zhang, H. et al. Comparison of the effects of Ciprofol and Propofol on postoperative nausea and vomiting in patients undergoing outpatient hysteroscopy. Drug. Des. Devel. Ther. 18, 5701–5707. https://doi.org/10.2147/DDDT.S489223 (2024).
Pastis, N. J. et al. Correlation of vital signs and depth of sedation by modified observer’s assessment of alertness and sedation (moaa/s) scale in bronchoscopy. J. Bronchol. Interv Pulmonol. 29, 54–61. https://doi.org/10.1097/LBR.0000000000000784 (2022).
Panda, N. B., Bharti, N. & Prasad, S. Minimal effective dose of magnesium sulfate for Attenuation of intubation response in hypertensive patients. J. Clin. Anesth. 25, 92–97. https://doi.org/10.1016/j.jclinane.2012.06.016 (2013).
Puri, G. D., Marudhachalam, K. S., Chari, P. & Suri, R. K. The effect of magnesium sulphate on hemodynamics and its efficacy in attenuating the response to endotracheal intubation in patients with coronary artery disease. Anesth. Analg. 87, 808–811 (1998).
Kakkar, A., Tyagi, A., Nabi, N., Sethi, A. K. & Verma, U. C. Comparision of clonidine and Dexmedetomidine for Attenuation of laryngoscopy and intubation response - a randomized controlled trial. J. Clin. Anesth. 33, 283–288. https://doi.org/10.1016/j.jclinane.2016.04.026 (2016).
Qu, L. et al. Determination of the 95% effective dose of remimazolam Tosylate in anesthesia induction inhibits endotracheal intubation response in senile patients. Front. Pharmacol. 14, 1136003. https://doi.org/10.3389/fphar.2023.1136003 (2023).
Görges, M., Zhou, G., Brant, R. & Ansermino, J. M. Sequential allocation trial design in anesthesia: an introduction to methods, modeling, and clinical applications. Paediatr Anaesth. 27, 240–247. https://doi.org/10.1111/pan.13088 (2017).
Yu, W. et al. The median effective dose of one intravenous bolus of oxycodone for postoperative analgesia after myomectomy and hysterectomy with local ropivacaine wound infiltration: an up-down dose-finding study. Anesth. Analg. 131, 1599–1606. https://doi.org/10.1213/ANE.0000000000005011 (2020).
Liang, P. et al. Efficacy and safety of Ciprofol vs. Propofol for the induction and maintenance of general anaesthesia: a multicentre, single-blind, randomised, parallel-group, phase 3 clinical trial. Eur. J. Anaesthesiol. 40, 399–406. https://doi.org/10.1097/EJA.0000000000001799 (2023).
Gan, T. J. et al. Comparison of the efficacy of hsk3486 and Propofol for induction of general anesthesia in adults: a multicenter, randomized, double-blind, controlled, phase 3 noninferiority trial. Anesthesiology 140, 690–700. https://doi.org/10.1097/ALN.0000000000004886 (2024).
Liu, Y. et al. Safety and efficacy of Ciprofol vs. Propofol for sedation in intensive care unit patients with mechanical ventilation: a multi-center, open label, randomized, phase 2 trial. Chin. Med. J. (Engl). 135, 1043–1051. https://doi.org/10.1097/CM9.0000000000001912 (2022).
Durai Samy, N. K. & Taksande, K. Exploring Ciprofol alternatives: a comprehensive review of intravenous anesthesia options. Cureus 16, e57581. https://doi.org/10.7759/cureus.57581 (2024).
Lu, M., Liu, J., Wu, X. & Zhang, Z. Ciprofol: a novel alternative to Propofol in clinical intravenous anesthesia? Biomed. Res. Int. 2023, 7443226. https://doi.org/10.1155/2023/7443226 (2023).
Liao, M. et al. Comparative effective dose of Ciprofol and Propofol in suppressing cardiovascular responses to tracheal intubation. Sci. Rep. 15, 1822. https://doi.org/10.1038/s41598-025-85968-2 (2025).
Ainiwaer, D. & Jiang, W. Efficacy and safety of Ciprofol versus Propofol for anesthesia induction in adult patients received elective surgeries: a meta–analysis. Bmc Anesthesiol. 24, 93. https://doi.org/10.1186/s12871-024-02479-9 (2024).
Veith, S. B. et al. Hemodynamics and cutaneous microcirculation during induction of general anesthesia with and without Esketamine. Clin. Hemorheol Microcirc. 84, 385–398. https://doi.org/10.3233/CH-231711 (2023).
Yuan, J. et al. Exploring the median effective dose of ciprofol for anesthesia induction in elderly patients: impact of frailty on ed(50). Drug Des. Dev. Ther. 18, 1025–1034. https://doi.org/10.2147/DDDT.S453486 (2024).
Zhou, X. et al. The median effective dose of Ciprofol combined with sufentanil in suppressing the laryngeal mask airway insertion response in both young and older adult patients. Bmc Anesthesiol. 24, 464. https://doi.org/10.1186/s12871-024-02855-5 (2024).
Yang, Y. et al. Comparison of the efficacy and safety of Ciprofol and Propofol in sedating patients in the operating room and outside the operating room: a meta-analysis and systematic review. Bmc Anesthesiol. 24, 218. https://doi.org/10.1186/s12871-024-02609-3 (2024).
Yu, L. et al. Ciprofol versus Propofol for anesthesia induction in cardiac surgery: a randomized double-blind controlled clinical trial. Bmc Anesthesiol. 24, 412. https://doi.org/10.1186/s12871-024-02795-0 (2024).
Yu, L., Bischof, E. & Lu, H. Anesthesia with Ciprofol in cardiac surgery with cardiopulmonary bypass: a case report. World J. Clin. Cases. 11, 157–163. https://doi.org/10.12998/wjcc.v11.i1.157 (2023).
Yang, Y. et al. Ciprofol attenuates the isoproterenol-induced oxidative damage, inflammatory response and cardiomyocyte apoptosis. Front. Pharmacol. 13, 1037151. https://doi.org/10.3389/fphar.2022.1037151 (2022).
Funding
This study was supported by research funds from the Nanchong Science and Technology Bureau (22SXQT0293).
Author information
Authors and Affiliations
Contributions
LZ, XZ, and TZ were responsible for the conception and design of the article, the collection and organization of research data, and the writing of the paper responsible for the article as a whole. YG and LC was responsible for data analysis and processing. WM, XL, and LZ were responsible for editing and organizing the tables. LL was responsible for the revision of the paper, quality control and proofreading of the article, supervision, and management.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This was an effective dose-finding study based on Dixon’s up-and-down method. The study was approved by the Ethics Committee of Nanchong Central Hospital (2024, trial (003) No.) and registered in the China Clinical Trial Registry (Registration time: 15/01/2025; Available at ; Registration Number: ChiCTR2500095959 ). We confirm that our research is consistent with the Declaration of Helsinki.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhao, L., Zhou, X., Zhang, T. et al. Determination of the Median Effective Dose of Ciprofol Combined with Sufentanil in Inhibiting Tracheal Intubation Response in Female Patients. Sci Rep 15, 11864 (2025). https://doi.org/10.1038/s41598-025-95135-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-95135-2