Abstract
Astragalus species, members of the Fabaceae family, have been used in traditional medicine for centuries. In addition to their traditional applications, many subspecies are now utilized in modern medicine. This study investigated the antioxidative, phenolic, and mineralogical properties of Astragalus chamaephaca Freyn for the first time. Antioxidant capacities were evaluated using the ABTS+ free cation radical scavenging activity, FRAP, and DPPH methods. The total phenolic content was determined using the Folin-Ciocalteu method, while quantification was performed via the LC-MS method. Substantial phenolic compounds, such as p-coumaric acid, gallic acid, rutin, quercetin, and naringenin—known for their diverse biological activities—were identified. In addition, mineralogical analyses have shown the presence of essential elements, such as Ca, Mg, and K, which play crucial roles in human health.
Similar content being viewed by others
Introduction
Free radicals are constantly formed in the human body through natural processes. When their levels rise above a certain threshold, they can damage healthy cells due to their unstable chemical structure, increasing the risk of diseases such as cancer, diabetes, Alzheimer’s, and heart disease. This condition, which is characterized by elevated free radical levels, is known as oxidative stress. Antioxidants are substances that cation and neutralize free radicals, thereby reducing oxidative stress1. Phenolic and flavonoids are increasingly recognized as major bioactive components that contribute to the antioxidant potential of many plants2. These antioxidants can be derived from plants, animals, and microorganisms. Medicinal and aromatic plants have historically played a crucial role in treating a wide range of diseases. Phenolic compounds are the main contributors to the therapeutic properties of plants. Numerous studies have highlighted the protective role of plant phenolics against cancer, inflammation, and degenerative diseases, including cardiovascular problems, diabetes, and more3. Plants are fascinating for their ability to produce raw materials or preparations containing phytochemicals with significant antioxidant capacities and health benefits4. The World Health Organization’s 2014–2023 strategy seeks to promote the use of traditional medicines, including herbal medicines, to keep people healthy by providing access to adequate and affordable alternatives to medicines and offering health choices that align with people’s cultural practices5.
Astragalus L. is the largest genus in the family Fabaceae (subfamily Papilionideae, tribe Galegeae). It is widely distributed worldwide, with many species found across continents such as Asia, Europe, North America, and Africa. Astragalusspp. has been used in traditional medicine for centuries6. There are many studies on extracts obtained from the parts of these commercially valuable plants and their antioxidant properties, such as Astragalus cicer and A. glycyphyllos7, A. tribuloides8, A. membranaceus9, A. flavescens10, A. armatus11, A. sinicus12. The studies, which examined different parts of the plant, reported that Astragalus L. and its subspecies are essential sources of antioxidants and beneficial against many diseases. Tan et al. found that the extracts obtained from A. membranaceusare an effective source of antioxidants for the food and medical industries13. Muhammed et al. Reported Astragalusspp. provides substantial defense and protection against damage to the heart, brain, kidneys, intestines, liver, and lungs in various disease models caused by oxidative stress14. Li et al. speculated that, based on standard criteria for antitumor activity, AstragalusL. polysaccharides exhibit moderate to high antitumor activity in tumor cells15. Wei et al., in their in vitro tests, stated that polysaccharides obtained from Astragalus L.have anti-inflammatory effects16.
Astragalus chamaephacaFreyn was first described in Turkey in 1911 by the Hungarian botanist Andrasovszky17. Türkiye is home to 481 taxa belonging to 64 sections, 44.9% of which are endemic to the country18,19,20,21. Although studies have identified varying distributions of these taxa in Anatolia, their antioxidant activities and mineralogical analyses have not been carried out until now22,23,24,25,26,27.
In this study, 2,2-diphenyl-1-picrylhydrazil (DPPH) radical scavenging activity, ABTS+, Ferric Ions (Fe3+) Reducing Antioxidant Power (FRAP), Total Phenolic Content, and mineralogical analysis were carried out to investigate the antioxidant activity of A. chamaephaca’s leaf and flower extracts obtained from different solvents. Phenolic compounds were identified and quantified using UHPLC-HESI-MS/MS.
Materials and methods
Reagents and plant materials
Potassium ferricyanide [K3Fe(CN)6], DPPH, Trichloroacetic acid (TCA), FeCl3, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), Folin-Ciocalteu reagent, Na2SO4 (anhydrous sodium sulfate), Na2CO3 (sodium carbonate), ABTS+, Potassium persulfate, phosphate buffer, nitric acid and phenolic compounds were obtained from Sigma (Sigma-Aldrich Chemie GmbH, Sternheim, Germany) and used without any purification. Ethanol, methanol, dichloromethane, and Hydrogen peroxide (H2O2) were purchased from Merck. In May 2023, A. chamaephaca was collected in the region of Çorum (40.660, 34.797) with the approval of the Republic of Türkiye Ministry of Agriculture and Forestry. The collected herbs were identified by Ömer Koray Yaylacı, PhD., who is affiliated with Anadolu University, Medicinal Plants, Drugs, and Scientific Research Centre.
Extraction
A. chamaephacacollected from the Çorum-Laçin /TÜRKİYE region was divided into two parts: flowers and leaves. The samples were dried in the shade and pulverized using a grinder. A total of 500 mg of each part was weighed and placed into tubes. Each sample was combined with 25 mL of one of three solvents, water, methanol, or ethanol, and mixed. The mixtures were kept in an ultrasonic bath at a fixed temperature of 25 °C and a frequency of 53 kHz for 60 min. The mixture was then vortexed thoroughly. All extracts were prepared using the same method and subsequently analyzed for the quantification of phenolic compounds and antioxidant capacities27.
Phenolic compounds identification and quantitation
This study conducted the LC-MS/MS phenolic compound analysis using a Thermo Scientific Dionex Ultimate 3000 UHPLC system coupled with a TSQ Quantum Access Max tandem mass spectrometer. The liquid chromatography system comprised binary pumps, a degasser, a column compartment, and an autosampler. Chromatographic separation was achieved using a C18 reversed-phase Inertsil ODS HYPERSIL analytical column (250 mm × 4.6 mm, 5 μm), with the column temperature maintained at 30 °C. Mobile phase A (water with 0.1% formic acid) and mobile phase B (methanol) were used for the elution gradient. The gradient program was set as follows 0–1 min, 0% B; 1–22 min, 95% B; 22–25 min, 95% B; 25–30 min, 100% B. The total evaluation time, including the conditioning period, was 34 min. The injection volume and solvent flow rate were adjusted to 20 µL and 0.7 mL/min, respectively, to achieve the desired outcome. After a series of trials to optimize ionization and molecule separation conditions, the aforementioned mobile phase was selected for use in this study. The conditions for the analysis were determined based on the study by Kayir et al.27. Limit of Detection (LOD) and Limit of Quantification (LOQ) values were calculated by using the signal-to-noise (S/N) method. The calculations were performed according to the following equations; LOD = 3*S/N, LOQ = 10*S/N28. The compounds listed in Table 1 were analyzed using LC-MS/MS and chromatograms of the phenolic compound standards are given as supplementary files (S1-S5). Total Ion Chromatogram (TIC) is a chromatogram created by summing up intensities of all mass spectral peaks belonging to the same scan. Chromatograms containing peaks of 29 phenolic compounds used as standards are shown separately in the total ion chromatogram (TIC) between S1 and S5 in the supplementary material. Since the phenolic standards were prepared as a mixture, there is a single total ion chromatogram (TIC) and this TIC is located on the standard chromatogram figures. For the calibration graph, phenolic compound standard mixtures at six different concentrations were prepared and analyzed in LC-MS/MS. The calibration graph was obtained using the peak area versus concentration of standards. In addition, the total ion chromatograms (TIC) of leaf and flower extracts extracted in different solvents are shown between S6 and S11 in the supplementary material. In the total ion chromatograms (TIC) given between S6 and S11, it was observed that the intensities of the ion peaks changed in proportion to the concentrations of phenolic compounds detected in the plant extracts. The amounts of phenolic compounds detected in the plant extracts were calculated quantitatively with the obtained calibration graphs.
Total phenolic content determination
The total phenolic content of A. chamaephaca extracts was determined using the method described by Kayir et al.27, which is a modified version of the Singleton and Slinkard method29. The total phenolic content was calculated using the equation of the standard curve, which was prepared with the standard compound gallic acid equation (GAE/kg dried plant).
Method of free radical scavenging activity (DPPH.)
The DPPH free radical scavenging activity of A. chamaephacaextracts was determined using the Blois method30. Briefly, 1 mL of 0.26 mM DPPH solution was added to 40 µL of extract solution, and the final volume was adjusted to 4 mL with ethyl alcohol. The mixture was then shaken at room temperature and left to stand for 30 min. The same DPPH solution was used for all solvent extracts. The absorbance was measured on a spectrophotometer at 517 nm. Calculations were performed based on the study by Kayir et al. The results have been calculated as the IC50.
Method of ferric ions (Fe3+) reducing antioxidant power (FRAP)
The FRAP antioxidant power of A. chamaephacaextracts was measured by the pristine method31. Potassium ferricyanide and phosphate buffer were added to the A. chamaephaca extract solutions. TCA and FeCl3 were added after the incubation of the mixture for 20 min at 50 oC. The mixture was then vortexed vigorously. The absorbance of the resulting solution was recorded at 700 nm using a spectrophotometer, as described in the Oyaizu method. A higher absorbance of the reaction mixture indicated a stronger reducing power. The reduction power results were calculated from the equation of the standard curve, which was prepared using the standard compound Trolox (µmol TE g−1).
Method of free cation radical scavenging activity (ABTS+)
The ABTS⁺ (2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)) activity of A. chamaephacaextracts was determined using the Re method32. Briefly, 40 µL of ABTS+- K2S2O8 solution (1 mL) was added to the extract solution. Phosphate buffer was then added until the volume of the solution reached 4 mL. The mixture was vortexed at room temperature and left to stand for 30 min. The absorbance was measured on a spectrophotometer at 734 nm. A decrease in absorbance indicated a higher ability to scavenge free cation radicals. Calculations were performed according to the study by Kayir et al.27. The results were calculated as IC50.
Mineral analysis
Samples for ICP-OES mineral analysis were prepared using a Berghof Instruments Speedwave system (Germany). The preparation involved weighing 3 g of sample into Teflon digestion vessels, followed by the careful addition of 2 mL of 30% (w/v) H₂O₂ solution and 5 mL of 65% (w/v) HNO₃ solution using a clean glass pipette. The mixture was thoroughly mixed and allowed to stand for 10 min before the vessels were sealed. The samples then underwent a three-step microwave digestion process: 170 °C for 5 min, 190 °C for 15 min, and 50 °C for 10 min. After cooling, the resulting colorless solutions were quantitatively transferred into 10 mL volumetric flasks and diluted to volume with deionized water33.
Standard solutions for Ca, Cu, Fe, Na, K, Mg, Mn, P and Zn were prepared by diluting a multi-element ICP QC standard solution (100 mg L-1) obtained from Chem-Lab. (Zedelgem, Belgium). The total concentrations of the elements were determined using inductively coupled plasma optical emission spectrometry (ICP-OES) with a Thermo Scientific iCap 6000 Dual view instrument.
Statistical analysis
The experimental data were analyzed using SPSS Statistics Version 22.0. We utilized the ANOVA test to identify any statistical differences in the results (p < 0.05). The results are presented as the mean ± standard deviation (n = 3).
Results and discussion
Total phenolic content
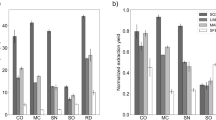
The Folin-Ciocalteu method was employed to determine the total phenolic content of the A. chamaephaca. The results are expressed as gallic acid equivalent (GAE). Among the extracts, the methanol extract of the leaf part exhibited the highest total phenolic content, while the methanol extract of the floral part showed the lowest. For the aqueous extracts, the total phenolic content was 713 mg GAE/kg of dried plant for the floral part and 971 mg GAE/kg of dried plant for the leaf part. The values were 632 mg GAE/kg dried plant and 961 mg GAE/kg dried plant, respectively, in ethanolic extracts. The values were 580 mg GAE/kg dried plant and 1109 mg GAE/kg dried plant in the methanol extracts. Phenolic compounds were found to be the lowest in the flower part of the plant in all extracts (Fig. 1).
DPPH. Free radical scavenging activity
The result of the DPPH radical scavenging test to determine the IC50 value of two parts of A. chamaephaca water, methanol and ethanol extracts are shown in Figure. 2. Antiradical effectiveness refers to the quantity required to reduce DPPH levels by 50%. A high IC50 value, calculated based on DPPH antioxidant activity, indicates poor antioxidant activity. According to Fig. 2, the water extract of the plant’s leaf showed the highest DPPH antioxidant activity, while ethanol extracts showed the lowest.
IC50 values (mg/mL) for different extract solutions of A. chamaephaca parts, obtained using the DPPH assay, are presented in Fig. 2. The water extract of the leaf part showed an IC50 of 0.007 mg/mL, while the flower part exhibited and IC50 of 0.42 mg/mL. When The DPPH antioxidant capacities of the leaf part of the plant in methanol and ethanol extracts were compared with water extracts, it was found that the water extract was approximately 33 times more effective. Additionally, The water extract of A. chamaephaca leaves demonstrated a higher antioxidant capacity than BHT (0.021 mg/mL), based on their IC50 values.
ABTS+ free cation radical scavenging activity
Figure 3 presents the IC50 values obtained from ABTS+ free cation radical scavenging assays for water, methanol, and ethanol extracts of two parts of A. chamaephaca. Anti-radical activity refers to the amount of antioxidants required to reduce the ABTS+ concentration by 50%. A high IC50 level, calculated based on ABTS+ antioxidant activity, signifies lower antioxidant activity. Among the extracts, the water extract of the leaf part exhibited the highest ABTS+ antioxidant activity, whereas the ethanol extract showed the lowest antioxidant activity, as shown in Fig. 3.
Ferric ions (Fe 3+) reducing antioxidant power (FRAP)
The antioxidant activities of the leaf and flower parts of the A. chamaephaca extracts, expressed as µM trolox equivalents (TEAC value) using the FRAP method, are shown in Fig. 4. The methanol extract of the leaf part of the plant exhibited the highest antioxidant capacity. Antioxidant capacity values of 93, 207, and 241 µM Trolox equivalents were recorded for the flower part in ethanol, methanol and water extracts, respectively. Among all extracts, the ethanolic extract of the flower part showed the lowest FRAP activity.
In literature, studies on different Astragalus species have reported varying results. Albayrak et al. conducted a study to determine the total phenolic content in methanol extracts of four different Astragalus: A. gummifer (8.47 mg GAE/g extract), A. microcephalus (10.84 mg GAE/g extract), A. thalassus (9.78 mg GAE/g extract), and A. acmophyllus (16.38 mg GAE/g extract). They found that each species exhibited a different total phenolic content. The DPPH activity efficiency of Astragalus species was determined as follows: A. gummifer (IC50 = 195.19 µg/mL), A. microcephalus (IC50 = 86.67 µg/mL), A. thalasseus (IC50 = 211.66 µg/mL), and A. acmophyllus (IC50 = 253.88 µg/mL). In addition, the antioxidant capacities of these species were determined in FRAP studies: A. gummifer (0.76 mM/L), A. microcephalus (0.97 mM/L), A. thalasseus (0.66 mM/L), and A. acmophyllus(0.60 mM/L)32.
Jaradat et al. determined the total phenolic content in methanol extracts from A. aleppicus, A. angustifolius, A. annularis, and A. boeticus, reporting values of 35.14 mg GAE/g, 60.14 mg GAE/g, 75.34 mg GAE/g, and 35.54 mg GAE/g, respectively. They concluded that A. boeticus has a moderate phenolic content. In their DPPH studies with extracts obtained from water, acetone, methanol, and DCM from Astragalus species, they found that methanol extracts exhibited the best antioxidant activity. Specifically, they determined that A. boeticushad an IC50 ± SD value of 3.91 ± 0.34 µg/mL for radical scavenging activity34.
Zhang et al., in their study wit32 A. complanatususing methanol by ultrasonic extraction, determined the optimal extraction conditions as 50 °C, 30 min, and 15.1 mL/g. They reported the total phenolic content as 40.12 ± 1.10 mg GAE/g. In the same study, they found a free radical scavenging capacity of 0.85 ± 0.13 mg dry extract/mg DPPH35 .
Nayeem et al. determined the total phenolic content in extracts obtained from the stem of A. spinosus using methanol and chloroform, with values of 420 and 265 mg GAE/g, respectively. They concluded that the methanol extract of A. spinosusexhibited significant dose-dependent analgesic and anti-inflammatory activity36 .
Pu et al. conducted studies on total phenols, ABTS+, FRAP, and DPPH assays using ethanol, petroleum ether-water, ethyl acetate-water, and n-BuOH-water extractions of A. taipaishanensis. Their results indicated that the ethyl acetate-water extraction yielded the best outcomes. They determined the total phenolic content to be 185.45 ± 1.04 mmol equivalent QUE/100 g. The ethyl acetate-water extract demonstrated the highest radical scavenging activity and reducing ability, with ABTS+ and FRAP values of 994.50 ± 4.21 µmol Trolox/g and 685.67 ± 3.21 µmol Trolox/g, respectively. Additionally, the DPPH IC50 value was found to be 0.059 ± 0.002 mg/mL. They noted that the main chemical components and bioactivity of A. taipaishanensis are similar to those of A. membranaceus, a plant recognized as both a medicinal and food source in the pharmacopoeia37.
Naghiloo et al. conducted studies on total phenolic and antioxidant activities in methanol extracts obtained from roots, leaves, and flowers of A. compactus. Their results indicated that the extracts from the leaves exhibited the best outcomes, consistent with previous studies on other Astragalusspecies. They determined the total phenol content and DPPH radical scavenging activity in the leaves to be 8.25 µg GAE/mg and 280.5 ± 3.15 g/mL, respectively38.
Arumugam et al. investigated the phenolic profile and antioxidant activity potential of methanol extracts obtained from different parts (flower, stem, leaf and root) of A. ponticus. Their findings revealed that the extracts from the leaf showed the best results. They reported that the leaf part of the plant contained a phenolic content of 26.34 ± 0.50 mg GAE/g extract. The DPPH and ABTS+radical scavenging activities from leaf extracts were determined as 43.64 ± 0.94 mg TEs/g extract and 98.79 ± 1.95 mg TEs/g extract, respectively. Also, FRAP reducing power was found to be 56.79 ± 4.34 mg TEs/g extract39.
When comparing the total phenol content, DPPH, FRAP, and ABTS+ results from our study with those of different Astragalus species in the literature, it was observed that our plant exhibits a medium-level phenol content. The methanol extracts from the leaf part of the plant showed superior DPPH and ABTS+ radical scavenging activities, as well as reducing abilities, compared to many species reported in the literature. However, according to the study by Arumugam et al. on the leaf part of A. ponticus, this species demonstrated significantly higher radical scavenging activity and reducing ability than our plant.
Mineral analysis
Mineral analysis of the A. chamaephaca flower (ACF) and leaf (ACL) parts was conducted using the ICP-OES device, with the results presented in mg/L. The analysis findings are shown in Table 2.
According to the analysis results, the minerals present in the highest concentrations in both parts of the plant are K, Ca, Mg, Fe, and Al. Both parts of the plant are particularly rich in K and Ca. Humans require more than 22 mineral elements, some of which are required in large quantities, while others, such as Se, Cu, Zn, I, and Fe, are required in trace amounts because excessive concentrations can be harmful. Calcium is essential for human health, playing a role in the biological functions of various tissues, including the skeletal system, nervous and heart systems, bones, teeth, and parathyroid glands. Magnesium contributes to muscle and nerve stimulability as the cofactor of up to 300 enzymes. Recent findings suggest that increased magnesium intake can help protect against chronic diseases such as metabolic syndrome, diabetes, cardiovascular diseases, and hypertension. Potassium helps maintain the balance of bodily fluids by transmitting nerve signals and supporting nerve function. The primary function of Fe which is also necessary for energy production is related to myoglobin and hemoglobin synthesis40.
In a study conducted by Bronislava et al., the mineral content of A. glycyphyllos and A. cicer plants was investigated. According to this study; the mineral content in the leaves and flowers of A. glycyphyllos was as follows: K (2.22; 2.94 g/100 g), Ca (1.91; 0.463 g/100 g), Mg (0.596; 0.341 g/100 g), P (0.256; 0.428 g/100 g), Zn (2.7; 4.76 mg/100 g), Fe (22.66; 14.10 mg/100 g), respectively. In the mineral analysis of A. cicer, the values were: K (2.38; 3.02 g/100 g), Ca (2.09; 0.643 g/100 g), Mg (0.545; 0.328 g/100 g), P (0.288; 0.473 g/100 g), Zn (2.69; 4.61 mg/100 g), Fe (14.52; 13.13 mg/100 g)7.
Determination of some phenolic compounds of A. chamaephaca
The tendency of each phenol to solubilize, transfer or diffuse into a given solvent is governed by thermodynamics. One of the primary thermodynamic factors describing this tendency is the activity coefficient, which generally affects reversibly the solubility of phenols in non polar solvents (smaller coefficient corresponds to better solubility). For example, in a study by Galanakis et al., ethanol was found to be the most efficient solvent for recovering caffeic acid and its ester derivative, rosmarinic acid. Other phenolic acids, such as ferulic, sinapic, vanillic, and syringic acids, are well soluble in methanol, while gallic acid, cinnamic acid, and coumaric acids are better soluble in water, dichloromethane and acetone, respectively. Many studies have confirmed these differences. Based on this, extractions were carried out using water, methanol and ethanol solvents, which have distinct properties, and the results were evaluated41.
Twenty-nine different compound screenings were performed to detect phenolic compounds in the plant. The flower part of A. chamaephaca was evaluated independently of the solvents; p-coumaric acid, gallic acid, rutin, quercetin and Naringenin were detected, respectively (Table 3). In the leaf part of the plant, p-coumaric acid, rutin, quercetin, and gallic acid were identified, with p-coumaric acid and rutin being the most abundant. Gallic acid, p-coumaric acid, and rutin, which were the most abundant compounds detected.
Among these, p-coumaric acid, found in the highest amounts in both the flower and leaf parts of A. chamaephaca, was evaluated across different solvents. It was primarily found in water (flower = 233.452 µg/g, leaf = 77.361 µg/g dried plant), followed by methanol (flower = 106.291 µg/g, leaf = 16.439 µg/g dried plant) and ethanol (flower = 100.306 µg/g, leaf = 11.744 µg/g dried plant). Numerous studies have focused on p-coumaric acid due to its low toxicity, wide natural distribution, and pharmacological effects. It has been shown to act as a neuroprotective agent through its potent antioxidant and anti-apoptotic properties. p-coumaric acid can alleviate diabetes symptoms by improving beta-cell function, enhancing glucose transporter (GLUT) expression, increasing antioxidant and anti-inflammatory activities, and modulating enzymes involved in glucose metabolism. Moreover, treatment with p-coumaric acid has been found to improve histopathological parameters and reduce kidney damage. In summary, p-coumaric acid holds promise as a therapeutic agent with potential applications in the treatment of various diseases42.
Gallic acid was detected at a concentration of 204.309 µg/g in the aqueous extract of the flower part of the plant, but it was not found in the other solvent extractions. In the leaf extracts, gallic acid was detected in methanol (4.113 µg/g dried plant) and ethanol (10.857 µg/g dried plant) extracts, but not in the water extract. Gallic acid is known for its wide range of bioactivities, including antioxidant, antimicrobial, anti-inflammatory, and anti-cancer properties. Additionally, anti-HIV, anti-ulcerogenic, and antifungal activities have been reported. Gallic acid is used in various industries, such as a chelating agent in the skin and leather industry first time. It is also employed as a preservative in food and beverages due to its antimicrobial effect43.
Rutin, one of the phenolic compounds, was found in the highest amounts in methanol extracts (flower = 99.917 µg/g dried plant, leaf = 76.595 µg/g dried plant), followed by ethanol (flower = 70.420 µg/g dried plant, leaf = 72.002 µg/g dried plant) and water (flower = 24.811 µg/g dried plant, leaf = 42.346 µg/g dried plant) extracts from both the flower and leaf parts. Due to its strong antioxidant properties, rutin exhibits various biological activities, including anti-inflammatory, antimicrobial, anti-tumor, and anti-asthmatic effects, and serves as an excellent free radical scavenger. Additionally, rutin is widely used as a stabilizer, preservative, and natural colorant in the pharmaceutical, nutraceutical, and cosmetic industries due to its antioxidative capacity44.
While the phenolic compound of Naringenin was detected in the flower part of the plant, it was not detected in any solvent extract of the leaf part. The highest level of Naringenin was found in the ethanol extract (16.484 µg/g dried plant) of the flower part, followed by methanol (12.001 µg/g dried plant) and water (1.126 µg/g dried plant) extracts, respectively. Naringenin has been extensively studied for its anti-diabetic activity, where it has been shown to reduce blood glucose levels. Naringenin also inhibits the proliferation of cancer cells across a broad range of cancer types, including colon, breast, stomach, prostate, liver, cervix, pancreas, uterus, and leukemia. The antioxidant and anti-inflammatory mechanisms of Naringenin contribute to its neuroprotective effects. Beyond hyperlipidemia and atherosclerosis, numerous studies have highlighted its protective role in various cardiovascular diseases45.
Quercetin was found in the flower part of the plant in ethanol (7.490 µg/g dried plant), methanol (6.255 µg/g dried plant) and water (0.540 µg/g dried plant) extracts, and in the leaf part in water (0.678 µg/g dried plant), ethanol (0.160 µg/g dried plant) and methanol (0.105 µg/g dried plant) extracts, respectively. Kaempferol, another phenolic compound, was detected in the flower part but not in any solvent extract from the leaf part. The highest concentration of kaempferol was detected in the ethanol extract (6.007 µg/g dried plant) in the flower part, followed by methanol (5.937 µg/g dried plant), with no detection in the water extract. Flavonoids, particularly kaempferol and quercetin, are among the bioactive compounds that contribute significantly to human health. Both kaempferol and quercetin offer heart-protective and antihypertensive benefits. In addition, they exhibit antifungal and bacterial properties. In recent years, the significance of these flavonoids has increased due to their wide range of beneficial bioactive effects, such as anti-viral, anti-bacterial, antifungal, anti-inflammatory and cardioprotective properties46.
In the study conducted by Albayrak et al., the phenolic compositions of the Astragalusextracts were analyzed using LC-MS. They identified chlorogenic acid, epicatechin, catechin hydrate, rutin, quercetin, kaempferol, syringic acid, cinnamic acid and ferulic acid. Among these compounds, ferulic acid was found to be the most abundant, with a concentration of 1123.9 µg/g dried plant, followed by syringic acid (735.18 µg/g dried plant) and cinnamic acid (558 µg/g dried plant). The compound detected in the least amount was quercetin (293.5 µg/g dried plant)47.
Phenolic acids generally have one aromatic ring, along with carboxyl and hydroxyl groups in their structure. These groups increase the polarity of the molecule, making phenolic acids highly soluble in water28. In contrast, although flavones contain hydroxyl groups, the increased number of aromatic rings with apolar properties reduces the overall polarity of the molecules28. This results in lower solubility in highly polar solvents like water28. Methanol and ethanol solvents have been reported to dissolve flavone molecules better because they are less polar than water28. According to the findings of this study, Gallic acid, Caffeic acid, Protocatechuic acid phenolic compounds were detected in higher amounts in water solvent, while Rutin, Naringenin, Quercetin and Kaempferol flavonoid compounds were detected in higher amounts in methanol / ethanol solvents.
Free radicals are atoms, molecules or ions with unpaired electrons that are highly unstable and active towards chemical reactions with other molecules. They derive from three elements: oxygen, nitrogen and sulfur. For example, oxygen-centered free radicals are known as reactive oxygen species (ROS) and include superoxide (O2·−), hydroxyl (HO·), peroxyl (ROO·), alkoxyl (RO·) and nitric oxide (NO·). The hydroxyl (half-life of 10–9s) and the alkoxyl (half-life of seconds) free radicals are very reactive and rapidly attack the molecules in nearby cells, and probably the damage caused by them is unavoidable and is dealt with by repair processes48,49,50.
Antioxidants play a vital role in both food systems as well as in the human body to reduce oxidative processes and harmful effects of ROS51,52.
The antioxidant potential of phenolic compounds depends on the number and arrangement of the hydroxyl groups in the molecules of interest. The antioxidant activity of phenolic acids and their derivatives depends on the number and position of the hydroxyl groups bound to the aromatic ring, the binding site and mutual position of hydroxyl groups in the aromatic ring, and the type of substituents53,54,55. Substitution in phenolic compounds at the meta-position has a rather limited effect. Steric and electronic effects are responsible for the antioxidant activities and stoichiometric factors of the chain-breaking phenolic antioxidants56. For elucidation of the hydrogen abstraction mechanism of phenolic antioxidants in the chain process of autoxidation, molecular orbital theory has been applied55,57.
In radical scavenging studies, the antioxidant effects of phenolic compounds (Ar–OH) generally occur by two mechanisms, including hydrogen atom transfer (HAT) or single-electron transfer followed by proton transfer (SET-PT). However, in some cases, it may not be possible to separate these two mechanisms with clear boundaries58. In a HAT-based assay, an antioxidant molecule can quench free radicals through H-donation, while in a SET-based method, a potential antioxidant agent exhibits antioxidant ability by transferring an electron (e-) to reduce any compound, including radicals, metals, and carbonyls59. Recently, in addition to these two mechanisms, a third mechanism called the sequential proton loss electron transfer (SPLET) mechanism has been developed.
The -OH group in the 7th position of flavonoids had great importance as the site of ionization and electron transfer, according to SPLET. This mechanism has been discovered recently60,61. In the first step, the reaction enthalpy corresponds to the proton affinity of the phenoxide anion (ArO−), while in the second step, the phenoxy radical is formed by electron transfer from the phenoxide anion to ROO·. In terms of antioxidant effect, SPLET is similar to free radicals in the HAT mechanism.
In addition, since the SET-PT and SPLET mechanisms in the solvent environment are important, the effect of water on the three mechanisms also has significance. Additionally, Litwinienko and Ingold (2005) proposed a different mechanism for SPLET. Among organic solvents, methanol is the leading solvent that supports ionization. This mechanism is preferred in that phenols with low pKa react with electron-deficient radicals with relatively lower HAT activities and yield product molecules with low pKa60. In addition, SPLET formation in methanol and ethanol solutions was also reported by Foti et al.62. Many studies have shown that DPPH· reacts with phenolic acids. As a result of the suppression of the ionization of the phenolic hydroxyl group by the free carboxylic acid, the rate constants of the reactions for methyl esters of these acids are several times higher than for free acids. These experiments nicely confirm the effective role of ionization of phenolic compounds in the reaction of phenols with DPPH·in solvents that can promote ionization53,61.
The findings obtained from the study suggest that the identification and isolation of specific compounds with high antioxidant activity, particularly from methanol and water extracts, could contribute to research on the mechanisms of underlying antioxidant activity.
Conclusion
In the study, the leaf and flower parts of A. chamaephaca were separated and extracted using water, methanol, and ethanol. The total phenolic content, measured using the Folin-Ciocalteu method, showed the highest value in the methanol extracts from the leaf part. The flower part exhibited the lowest phenolic compound levels across all extracts.
The water extract from the leaf of A. chamaephaca demonstrated the highest DPPH antioxidant activity, while the ethanol extract showed the lowest activity. When comparing the IC50 values of BHT and the plant extracts, the water extract of the leaf exhibited a higher antioxidant capacity than BHT. In the FRAP method, the highest antioxidant capacity was observed in the methanol extract of the leaf, while the lowest FRAP activity was found in the ethanol extract of the flower. In the ABTS+ free cation radical scavenging method, the highest activity in the leaf was detected in the water extract, whereas the highest activity in the flower was found in the methanol extract. The lowest ABTS+ values were determined in the ethanol extracts of the plant.
LC-MS/MS was employed to screen 29 different compounds for the detection and determination of phenolic compounds in all extracts. High levels of p-coumaric acid, gallic acid, rutin, quercetin, and naringenin were detected in the flower and leaf parts of A. chamaephaca, irrespective of the solvent used. The highest phenolic components in the leaf part were p-coumaric acid, rutin, and gallic acid. p-Coumaric acid and gallic acid, known for their numerous pharmacological effects, were predominantly found in the water extract of the flower part. Naringenin was detected only in the flower part and was absent in any solvent extract of the leaf part. Rutin was found in the highest amounts in the methanol extracts, while kaempferol was predominantly detected in the ethanol extract of the flower part. Quercetin was identified in all plant extracts through LC-MS/MS analysis.
According to the ICP-OES analyses, K, Ca, B, Zn, Mg, Fe and Al were detected in both parts of the plant, with K and Ca being the most abundant. These detected elements include both the major and minor minerals essential for human health.
In general, the potential health-promoting properties of A. chamaephaca, especially the presence of antioxidant-rich leaf extracts and bioactive phenolic compounds with various biological activities were highlighted by comprehensive analysis. These findings offer valuable insights for further investigation of A. chamaephaca as a potential source of natural antioxidants and bioactive compounds with therapeutic applications in various health conditions. However, further research is required to explore the underlying mechanisms of action and confirm the therapeutic efficacy of these bioactive components in vivo.
Phenolic substances identified in this study are compounds of significant industrial value across various fields, such as food, cosmetics and medicine. For this reason, the Astragalus genus also has the potential to be used in various industrial areas.
Data availability
The datasets used and analyzed during the current study available from the corresponding author on reasonable request.
References
Munteanu, I. G. & Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 22 https://doi.org/10.3390/ijms22073380 (2021).
Xu, X. et al. Vitro synergistic antioxidant activity and identification of antioxidant components from astragalus Membranaceus and paeonia lactiflora. PLoS One. 9, e96780. https://doi.org/10.1371/journal.pone.0096780 (2014).
Zeljkovıć, S. Ć. et al. Exploring the Pharmacological potential of Onosma Riedliana: phenolic compounds and their biological activities, plant foods Hum. Nutr 79, 106–112. https://doi.org/10.1007/s11130-023-01131-0 (2024).
VeliogluY.S. & G. Mazza, L. Gao, B.D. Oomah Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J. Agric. Food Chem. 46, 4113–4117. https://doi.org/10.1021/jf9801973 (1998).
Poswal, F. S. et al. Herbal teas and their health benefits: A scoping review, plant foods Hum. Nutr 74, 266–276. https://doi.org/10.1007/s11130-019-00750-w (2019).
Wang, H. F. et al. Effects of astragalus Membranaceus on growth performance, carcass characteristics, and antioxidant status of broiler chickens, acta agric. Scand. Sect. - Anim. Sci. 60, 151–158. https://doi.org/10.1080/09064702.2010.511255 (2010).
B. Butkutė, A. Dagilytė, R. Benetis, A. Padarauskas, J. Cesevičienė, V. Olšauskaitė, N. Lemežienė, Mineral and Phytochemical Profiles and Antioxidant Activity of Herbal Material from Two Temperate Astragalus Species, Biomed Res. Int. 2018 (2018) 6318630. https://doi.org/10.1155/2018/6318630.
Sharifi-Rad, M., Pohl, P., Epifano, F. & Álvarez-Suarez, J. M. Green synthesis of silver nanoparticles using astragalus tribuloides delile. Root extract: characterization, antioxidant, antibacterial, and Anti-Inflammatory activities. Nanomaterials 10, 2383. https://doi.org/10.3390/nano10122383 (2020).
Khan, F. U. et al. An astragalus Membranaceus based eco-friendly biomimetic synthesis approach of ZnO nanoflowers with an excellent antibacterial, antioxidant and electrochemical sensing effect. Mater. Sci. Eng. C. 118, 111432. https://doi.org/10.1016/j.msec.2020.111432 (2021).
Yaglioglu, A. S., Erenler, R., Gecer, E. N. & Genc, N. Biosynthesis of silver nanoparticles using astragalus flavesces leaf: identification, antioxidant activity, and catalytic degradation of methylene blue. J. Inorg. Organomet. Polym. Mater. 32, 3700–3707. https://doi.org/10.1007/s10904-022-02362-5 (2022).
Labed, A. et al. Compounds from the pods of astragalus armatus with antioxidant, anticholinesterase, antibacterial and phagocytic activities. Pharm. Biol. 54, 3026–3032. https://doi.org/10.1080/13880209.2016.1200632 (2016).
Liu, S. et al. Weed suppression and antioxidant activity of astragalus sinicus L. decomposition leachates, front. Plant. Sci. 13. https://doi.org/10.3389/fpls.2022.1013443 (2022).
Lim, D. H. et al. Effect of astragalus sinicus L. seed extract on antioxidant activity. J. Ind. Eng. Chem. 17, 510–516. https://doi.org/10.1016/j.jiec.2011.02.040 (2011).
Shahzad, M., Shabbir, A., Wojcikowski, K., Wohlmuth, H. & Gobe, G. C. The antioxidant effects of radix astragali (Astragalus Membranaceus and related Species) in protecting tissues from injury and disease. Curr. Drug Targets. 17, 1331–1340. https://doi.org/10.2174/1389450116666150907104742 (2016).
Li, R., Chen, W., Wang, W., Tian, W. & Zhang, X. Antioxidant activity of astragalus polysaccharides and antitumour activity of the polysaccharides and SiRNA. Carbohydr. Polym. 82, 240–244. https://doi.org/10.1016/j.carbpol.2010.02.048 (2010).
HUANG, W. M., LIANG, Y. Q., TANG, L. J., DING, Y. & WANG, X. H. Antioxidant and anti-inflammatory effects of astragalus polysaccharide on EA.hy926 cells, exp. Ther. Med. 6, 199–203. https://doi.org/10.3892/etm.2013.1074 (2013).
Çeçen, Ö., Aytaç, Z., Mısırdalı, H. & Ünal, A. József Andrasovszky’nin 1889–1943 Türkiye’deki astragalus L. Fabaceae türlerine Ait Kayıtları. Bağbahçe Bilim Derg. 5, 1–9. https://doi.org/10.30796/ANGV.2018.8 (2018).
Güner, A., Aslan, S., Ekim, T., Vural, M. & Babaç, M. T. Türkiye Bitkileri Listesi (Damarlı Bitkiler), (2012).
KARAMAN ERKUL, S., AYTAÇ, Z. & EKİCİ, M. Synopsis of the sect. Hymenocoleus, sect. Hymenostegis, and sect. Macrophyllium belonging to astragalus (Fabaceae) in Turkey. Turk. J. Bot. 40, 412–418. https://doi.org/10.3906/bot-1501-55 (2016).
A.Z.K.E.S. ÇETER, T. & Türkiye’ nin Astragalus, L. Leguminosae) Cinsine Ait poterion bunge Seksiyonunun Revizyonu, Ot Sist. Bot. Derg. 24, 1–20 (2017).
Adıgüzel, N. A new species of astragalus (Fabaceae) from East Anatolia, Turkey. Ann. Bot. Fenn. 36, 231–233 (1999). http://www.jstor.org/stable/23726580
Cengiz, H. & Behçet, L. The flora of Göynük Township and its surroundings (Karlıova/Bingöl). Commagene J. Biol. 7, 79–85. https://doi.org/10.31594/commagene.1272931 (2023).
Ertuğrul, T. & Aytaç, Z. Delihöbek Dağı Florası (Göksun/ Kahramanmaraş). Bağbahçe Bilim Derg. 10, 45–98. https://doi.org/10.35163/bagbahce.1263401 (2023).
Erkul, S. K., Aytaç, Z., Hamzaoğlu, E., Ekici, M. & Ertuğrul, T. Contributions to genus astragalus L. (Fabaceae) in Turkey. Commagene J. Biol. 4, 110–114. https://doi.org/10.31594/commagene.798800 (2020).
Uzun, A., Palabaş Uzun, S., Durmaz, A., SPATIAL ANALYSES OF ASTRAGALUS SPECIES DISTRIBUTION & AND RICHNESS IN KAHRAMANMARAŞ (TURKEY) BY GEOGRAPHICAL INFORMATION SYSTEMS (GIS). Turkish J. Sci. 3 37–59. https://doi.org/10.32328/turkjforsci.553375. (2019).
Yapar, Y. & Behçet, L. Hiro Yaylası Adaklı-Bingöl/Türkiye ve Çevresinin Florası. Biol. Divers. Conserv. 11, 126–140 (2018). https://dergipark.org.tr/tr/pub/biodicon/issue/55721/761918
Kayir, Ö., Doğan, H., Alver, E. & Bilici, İ. Quantification of phenolic component by LC-HESI‐MS/MS and evaluation of antioxidant activities of crocus ancyrensis (Ankara Çiğdemi) extracts obtained with different solvents. Chem. Biodivers. 20 https://doi.org/10.1002/cbdv.202201186 (2023).
Erenler, R. et al. Quantification of flavonoids isolated from \textit{Mentha spicata} in selected clones of Turkish mint landraces. Turkish J. Chem. 42, 1695–1705. https://doi.org/10.3906/kim-1712-3 (2018).
Slinkard, K. & Singleton, V. L. Total phenol analysis: automation and comparison with manual methods. Am. J. Enol. Vitic. 28, 49–55. https://doi.org/10.5344/AJEV.1977.28.1.49 (1977).
Blois, M. S. Antioxidant determinations by the use of a stable free radical [10]. Nature 181, 1199–1200. https://doi.org/10.1038/1811199A0 (1958).
Oyaizu, M. Studies on products of Browning reaction. Antioxidative activities of products of Browning reaction prepared from glucosamine. Japanese J. Nutr. Diet. 44, 307–315. https://doi.org/10.5264/EIYOGAKUZASHI.44.307 (1986).
Re, R. et al. Antioxidant activity applying an improved ABTS radical cation decolorization assay, free Radic. Biol. Med. 26, 1231–1237. https://doi.org/10.1016/S0891-5849(98)00315-3 (1999).
Sezer, B., Ozturk, M., Ayvaz, H., Apaydın, H. & Boyaci, I. H. Laser-induced breakdown spectroscopy as a reliable analytical method for classifying commercial cheese samples based on their cooking/stretching process. Food Chem. 390, 132946. https://doi.org/10.1016/j.foodchem.2022.132946 (2022).
Jaradat, N. A., Zaid, A. N., Abuzant, A., Khalaf, S. & Abu-Hassan, N. Phytochemical and biological properties of four astragalus species commonly used in traditional Palestinian medicine. Eur. J. Integr. Med. 9, 1–8. https://doi.org/10.1016/J.EUJIM.2017.01.008 (2017).
Zhang, Q. A. et al. Extraction, antioxidant capacity and identification of semen astragali complanati (Astragalus complanatus R. Br.) phenolics. Food Chem. 141, 1295–1300. https://doi.org/10.1016/J.FOODCHEM.2013.04.014 (2013).
Nayeem, N. et al. Total phenolic, flavonoid contents, and biological activities of stem extracts of astragalus spinosus (Forssk.) muschl. Grown in Northern border Province, Saudi Arabia. Saudi J. Biol. Sci. 29, 1277–1282. https://doi.org/10.1016/J.SJBS.2021.12.029 (2022).
Pu, W., Wang, D. & Zhou, D. Structural characterization and evaluation of the antioxidant activity of phenolic compounds from astragalus taipaishanensis and their Structure-Activity relationship. Sci. Rep. 2015. 51 5, 1–11. https://doi.org/10.1038/srep13914 (2015).
Naghiloo, S. et al. Ontogenetic variation of total phenolics and antioxidant activity in roots, leaves and flowers of astragalus compactus lam. (Fabaceae). Bioimpacts 2, 105. https://doi.org/10.5681/BI.2012.015 (2012).
Arumugam, R., Kirkan, B. & Sarikurkcu, C. Phenolic profile, antioxidant and enzyme inhibitory potential of methanolic extracts from different parts of astragalus Ponticus pall. South. Afr. J. Bot. 120, 268–273. https://doi.org/10.1016/J.SAJB.2018.07.002 (2019).
Martínez-Ballesta, M. C. et al. Minerals in Plant Food: Effect of Agricultural Practices and Role in Human Health, in: Sustain. Agric Vol. 2, 111–128 (Springer Netherlands, 2011). https://doi.org/10.1007/978-94-007-0394-0_8.
Galanakis, C. M., Goulas, V., Tsakona, S., Manganaris, G. A. & Gekas, V. A knowledge base for the recovery of natural phenols with different solvents. Int. J. Food Prop. 16, 382–396. https://doi.org/10.1080/10942912.2010.522750 (2013).
Ferreira, P. S., Victorelli, F. D., Fonseca-Santos, B. & Chorilli, M. A review of analytical methods for p-Coumaric acid in Plant-Based products, beverages, and biological matrices. Crit. Rev. Anal. Chem. 49, 21–31. https://doi.org/10.1080/10408347.2018.1459173 (2019).
Fernandes, F. H. A. & Salgado, H. R. N. Gallic acid: review of the methods of determination and quantification. Crit. Rev. Anal. Chem. 46, 257–265. https://doi.org/10.1080/10408347.2015.1095064 (2016).
Chua, L. S. A review on plant-based Rutin extraction methods and its Pharmacological activities. J. Ethnopharmacol. 150, 805–817. https://doi.org/10.1016/j.jep.2013.10.036 (2013).
Thirumalaisamy, R. et al. Curcumin, naringenin and Resveratrol from natural plant products hold promising solutions for modern world Diseases – A recent review. South. Afr. J. Bot. 151, 567–580. https://doi.org/10.1016/j.sajb.2022.06.027 (2022).
Jan, R. et al. Bioactivity and therapeutic potential of Kaempferol and Quercetin: new insights for plant and human health. Plants 11 https://doi.org/10.3390/plants11192623 (2022).
Albayrak, S. & Kaya, O. Antioxidant and antimicrobial activities of four astragalus species growing wild in Turkey. Turkish J. Biochem. 43, 425–434. https://doi.org/10.1515/tjb-2017-0241 (2018).
B.N. Ames, M.K. Shigenaga, T.M. Hagen, Oxidants, antioxidants, and the degenerative diseases of aging., Proc. Natl. Acad. Sci. 90 (1993) 7915–7922. https://doi.org/10.1073/PNAS.90.17.7915.
Han, H., Yılmaz, H. & Gülçin, İ. Antioxidant activity of flaxseed (Linum usitatissimum L.) shell and analysis of its polyphenol contents by LC-MS/MS, Nat. Prod 12, 397–402. https://doi.org/10.25135/rnp.46.17.09.155 (2018).
Bulut, N. et al. Synthesis of some novel pyridine compounds containing bis-1,2,4-triazole/thiosemicarbazide moiety and investigation of their antioxidant properties, carbonic anhydrase, and acetylcholinesterase enzymes Inhibition profiles. J. Biochem. Mol. Toxicol. 32, e22006. https://doi.org/10.1002/JBT.22006 (2018).
Çakmakçi, S. et al. Antioxidant capacity and functionality of oleaster (Elaeagnus angustifolia L.) flour and crust in a new kind of fruity ice cream. Int. J. Food Sci. Technol. 50, 472–481. https://doi.org/10.1111/IJFS.12637 (2015).
Göçer, H., Akincioǧlu, A., Öztaşkin, N., Göksu, S. & Gülçin, I. Synthesis, antioxidant, and antiacetylcholinesterase activities of sulfonamide derivatives of Dopamine-Related compounds. Arch. Pharm. (Weinheim). 346, 783–792. https://doi.org/10.1002/ARDP.201300228 (2013).
Diplock, A. T. et al. Viña-Ribes, functional food science and defence against reactive oxidative species. Br. J. Nutr. 80, S77–S112. https://doi.org/10.1079/BJN19980106 (1998).
Sroka, Z. & Cisowski, W. Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chem. Toxicol. 41, 753–758. https://doi.org/10.1016/S0278-6915(02)00329-0 (2003).
I. Gülçin, Antioxidant activity of food constituents: an overview, Arch. Toxicol. 2011 863 86 (2011) 345–391. https://doi.org/10.1007/S00204-011-0774-2.
Barclay, L. R. C. et al. Chain-Breaking Phenolic Antioxidants: Steric and Electronic Effects in Polyalkylchromanols, Tocopherol Analogs, Hydroquinones, and Superior Antioxidants of the Polyalkylbenzochromanol and Naphthofuran J. Org. Chem. 58 7416–7420. https://doi.org/10.1021/JO00078A020/ASSET/JO00078A020.FP.PNG_V03. (1993).
Tomiyama, S., Sakai, S., Nishiyama, T. & Yamada, F. Factors influencing the antioxidant activities of phenols by an Ab initio study. Bull. Chem. Soc. Jpn. 66, 299–304. https://doi.org/10.1246/BCSJ.66.299 (1993).
ApakR. et al. Methods to evaluate the scavenging activity of antioxidants toward reactive oxygen and nitrogen species (IUPAC technical Report). Pure Appl. Chem. 94, 87–144. https://doi.org/10.1515/PAC-2020-0902/ASSET/GRAPHIC/J_PAC-2020-0902_INL_030.JPG (2022).
Shahidi, F. & Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: antioxidant activity and health effects – A review. J. Funct. Foods. 18, 820–897. https://doi.org/10.1016/J.JFF.2015.06.018 (2015).
Litwinienko, G. & Ingold, K. U. Abnormal solvent effects on hydrogen atom abstraction. 3. Novel kinetics in sequential proton loss electron transfer chemistry. J. Org. Chem. 70, 8982–8990. https://doi.org/10.1021/JO051474P/SUPPL_FILE/JO051474PSI20050818_113607.PDF (2005).
Musialik, M., Kuzmicz, R., Pawlowski, T. S. & Litwinienko, G. Acidity of hydroxyl groups: an overlooked influence on antiradical properties of flavonoids. J. Org. Chem. 74, 2699–2709. https://doi.org/10.1021/JO802716V/SUPPL_FILE/JO802716V_SI_001.PDF (2009).
Foti, M. C., Daquino, C. & Geraci, C. Electron-Transfer reaction of cinnamic acids and their Methyl esters with the DPPH. Radical in alcoholic solutions. J. Org. Chem. 69, 2309–2314. https://doi.org/10.1021/JO035758Q/SUPPL_FILE/JO035758QSI20040113_051425.PDF (2004).
Acknowledgements
This study has been supported by Anadolu University Scientific Research Projects Coordination Unit under grant number 2206S046 and y Hitit University with project number of MUH19002.19.001. All experiments were carried out by Hitit University Scientific Research and Application Center (HUBTUAM).
Author information
Authors and Affiliations
Contributions
Hacer Doğan: Analysis, Investigation, Resources, Writing -review & editing. Ömer Kayır: Analysis, Investigation, Resources, Writing -review & editing.Ömer Koray Yaylacı: The collected herbs were identified by him. İbrahim Bilici: Supervision, collecting, Methodology, Investigation, Resources, Writing -review & editing. Erol Alver: Supervision, collecting, Methodology, Investigation, Resources, Writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Doğan, H., Kayir, Ö., Yaylaci, Ö.K. et al. Determination of phytochemical content, antioxidant and mineral properties of Astragalus chamaephaca Freyn in different solvents. Sci Rep 15, 20886 (2025). https://doi.org/10.1038/s41598-025-95203-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-95203-7