Abstract
Previous studies have explored the role of the immune system in osteoporosis. This Mendelian randomization (MR) study further evaluated the causal relationship between 731 immune cell phenotypes and osteoporosis, exploring the mediating role of serum metabolites based on genetic prediction. Bidirectional two-sample Mendelian randomization was used to investigate the relationship between 731 immune cell phenotypes and osteoporosis. The 731 immune cell phenotypes, serum metabolites, and osteoporosis data used in this study were obtained from respective genome-wide association studies (GWAS). Inverse variable weighting (IVW) was used as the primary analysis method, and the P values were further corrected for false discovery rate (FDR). In addition, MR-Egger, weighted mode, simple mode, and weighted median analyses were performed to demonstrate the robustness of the results. In sensitivity analysis, the potential impact of horizontal pleiotropy was assessed by examining the intercept in MR-Egger regression, and the heterogeneity between SNPs was evaluated using Cochran’s Q test, which was further supplemented with MR PRESSO analysis to detect pleiotropy. The analysis revealed significant associations between seven immune cell phenotypes and osteoporosis (OP). Furthermore, we identified several serum metabolites mediating immune cell phenotypes’ effects on osteoporosis. For example, HLA DR++ monocyte %leukocyte promotes the occurrence of osteoporosis through the ratio of citrulline/dimethylarginine (SDMA + ADMA). Mediating effect ratios further elucidate the complex dynamics between immune cell phenotypic exposure, serum metabolites, and their combined effects on OP. We demonstrated the causal relationship between multiple immune cell phenotypes and osteoporosis through a bidirectional two-sample MR analysis. This study further elucidated the mediation effect of serum metabolites through mediation analysis, providing important insights into further understanding the pathogenesis of osteoporosis.
Similar content being viewed by others
Introduction
Osteoporosis (OP) is the most common bone disease worldwide, characterized by the destruction of bone tissue microstructure and decreased bone density1,2,3. According to statistics, over 10.2 million individuals in the United States aged 50 and above suffer from osteoporosis, while the prevalence in those aged 65 and above in China is 32.0%4. Osteoporosis can lead to serious consequences such as fractures, chronic pain, decreased mobility, and decreased quality of life. Orthopedic patients requiring joint replacement are also at high risk of osteoporosis. Statistics show that among osteoarthritis patients over 70 years old preparing for total hip arthroplasty (THA), 18% have osteoporosis, and 41% have low bone mass5. Osteoporosis is also one of the main causes of periprosthetic fractures and reduced prosthetic stability after surgery6,7. Therefore, an in-depth understanding of the mechanisms underlying the occurrence and development of osteoporosis, along with targeted prevention, is crucial for surgical treatment and improving patient prognosis.
The pathogenesis of osteoporosis is closely associated with immune system dysregulation8. 2000 the bone immunology theory was published, elucidating the intricate interplay between the immune and skeletal systems. The theory particularly highlighted the direct regulatory role of T and B lymphocytes, along with related cytokines, in bone remodeling via the RANKL/RANK/OPG signaling pathway9. Since the advent of the “bone immunology” theory, studying the interplay between the skeletal and immune systems has become a cutting-edge focus in the scientific community. The disruption of bone immune regulation may be a significant cause of the onset and progression of osteoporosis, where immune cells play a crucial role in this regulatory process10. In 2018, the concept of “immune osteoporosis” was introduced, underscoring the pivotal role of various T-lymphocyte subpopulations in the pathophysiology of osteoporosis11. This concept highlights the strong correlation between the immune and skeletal systems, focusing on the mechanisms of action of immune cells in the pathogenesis of osteoporosis12. Pan et al. conducted a transcriptome sequencing analysis on the skeletal tissues of patients with postmenopausal osteoporosis (PMOP), revealing an enhanced immune-inflammatory response pathway in PMOP, providing significant insights into the potential relationship between the immune system and osteoporosis13. Wang et al. discovered that early alterations in metabolites and bone marrow lymphocyte subpopulations may play a pivotal role in the onset of osteoporosis14. Yang et al. developed a nano-engineered multifunctional extracellular vesicle that mitigates bone loss in osteoporosis by binding to RANKL and rebalancing the ratio of Treg to Th17 cells15. Cai et al. discovered that increasing the frequency of circulating regulatory T cells (Tregs) can promote the osteogenic differentiation of bone marrow mesenchymal stem cells (BM-MSCs), thereby alleviating the symptoms of osteoporosis16. All these findings suggest that there may be a direct link between immune cells and osteoporosis.
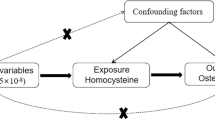
Osteoimmunology is an interdisciplinary field that explores the shared mechanisms and the complexities of interactions between osteocytes and immune cells8. The exploration of the specific relationship between immune cells and osteoporosis remains insufficient, and their interconnection remains to be fully elucidated. Mendelian randomization (MR) is a data analysis technique used in epidemiological research to infer causality17. It utilizes genetic variants strongly correlated with the exposure as instrumental variables (IVs), evaluating the causal relationship between the exposure and the outcome18. Therefore, we conducted a Mendelian randomization analysis in this study, exploring the complex causal relationships between 731 immune cell phenotypes and seven specific immune cells in osteoporosis.
Emerging Mendelian randomization studies have delineated causal links between immunophenotypic profiles and osteoporosis pathogenesis, with quantitative cellular metrics (absolute counts, fluorescence intensities) demonstrating capacity to reflect osteoporotic pathophenotypes through cellular enumeration and morphometric profiling19. Furthermore, metabolites interact with other cells through mechanisms of material exchange, such as permeation, transport, and extracellular vesicles, playing a key role in cutting-edge research areas like immunotherapy and essential for intercellular communication20. Analyzing 1400 metabolites allows us to identify specific metabolic pathways that may act as communication mediators between immune cells and the occurrence and progression of osteoporosis.
Notably, serum metabolite ratio analysis exhibits unique capacity for interrogating dynamic biological processes, motivating the prioritized inclusion of signature ratio parameters. These ratios serve as sensitive indices of metabolic states, capturing kinetic interplay between immune cell activation states and cytokine secretory dynamics that modulate the equilibrium between osteogenic-anabolic and osteoclastic-catabolic activities21,22. For instance, while citrulline emerges as a nitric oxide synthase (NOS)-dependent byproduct of arginine catabolism, asymmetric/symmetric dimethylarginines (ADMA/SDMA) act as endogenous NOS inhibitors. Individual quantification of arginine or ADMA proves confounded by dietary fluctuations and renal clearance efficiency, whereas the citrulline/(ADMA + SDMA) ratio directly reflects net NOS activity by integrating substrate-product-inhibitor relationships, thereby serving as a vascular endothelial dysfunction biomarker. However, further validation and functional studies are needed to explain the effects of proportional changes in different cell types on bone metabolism23.
In summary, this study aims to bridge the knowledge gap between immune cells, metabolites, and the pathogenesis of osteoporosis. Employing advanced genetic techniques and a comprehensive analysis of immune cell phenotypes, it aims to uncover potential therapeutic targets and further deepen our understanding of the intricate etiology of osteoporosis.
Materials and methods
Study design
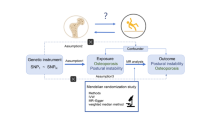
Our study employed a two-sample Mendelian randomization approach to investigate the potential causal relationship between immune cell phenotypes and osteoporosis. Mendelian randomization (MR) is based on three key assumptions: (1) The relevance assumption: IVs are strongly associated with the exposure factor; (2) The independence assumption: IVs are independent of known or unknown confounding factors; (3) The exclusion restriction assumption: Instrumental variables influence the outcome solely through the exposure factor24. The data derived in this study were from the public databases of Finngen and OPEN GWAS, and have been anonymized, thus eliminating the need for additional ethical review25. The design of the study is depicted in Fig. 1.
Data acquisition
Immune cell phenotypes
The aggregated data of immune cell phenotypes is publicly accessible through the GWAS Catalog database, with catalog identifiers ranging from GCST90001391 to GCST90002121. This study included a total of 731 immune cell phenotypes, which specifically encompass: absolute cell counts (AC, n = 118), median fluorescence intensities (MFI) indicative of surface antigen levels (n = 389), morphological parameters (MP, n = 32), and relative cell counts (RC, n = 192). Absolute cell counts (AC) quantify tissue-resident cellular populations within bone marrow niches, while relative counts (RC) delineate subset proportions within hematopoietic hierarchies—both mechanistically linked to osteogenic capacity and volumetric bone mineral density (vBMD). Median fluorescence intensity (MFI), a quantitative flow cytometry parameter, evaluates surface marker density reflecting cellular activation states. Morphometric parameters (MP) capture cytostructural features including cellular geometry and nuclear-cytoplasmic ratios, which correlate with osteoclast activation states in osteoporosis19,22. The metrics of MFI, AC, and RC comprehensively cover a variety of immune cell types, including B cells, conventional dendritic cells (CDCs), T cells at various stages of maturation, monocytes, myeloid cells, as well as the TBNK population and regulatory T cells (Treg). The MP metric specifically refers to the morphological characteristics of CDCs and TBNK.
The original GWAS for immune traits was conducted using data from a population cohort of 3757 Sardinians, without overlapping participant cohorts26.
Osteoporosis
The osteoporosis data used in this study were derived from the FinnGen database, encompassing 8017 cases and 391,037 controls from Europe. The FinnGen study is a large-scale genomics initiative that analyzed over 500,000 Finnish biobank samples and correlated genetic variation with health data to understand disease mechanisms and predispositions. The project is a collaboration between research organizations and biobanks within Finland and international industry partners27.
Serum metabolites
Serum metabolite GWAS data were obtained from the Canadian Longitudinal Study on Aging (CLSA) cohort, encompassing 1091 metabolites and 309 metabolite ratios from 8299 individuals28. These data are publicly accessible through the GWAS Catalog database, with catalog identifiers for the 1400 serum metabolites ranging from GCST90199621 to GCS90201020.
Selection of IVs
Initially, we filtered the GWAS data, selecting only the single nucleotide polymorphisms (SNPs) that were significantly associated with the exposure data at a threshold of 1 × 10–5, using these as IVs29. Furthermore, we established the threshold for the parameter r2 to 0.001 to prevent linkage disequilibrium and limited the distance between SNPs to 10,000 Kb30. Parameter selection for r2 thresholds and SNP proximity in linkage disequilibrium analyses may vary across studies depending on genomic architecture, linkage disequilibrium (LD) patterns, or specific research objectives. In this work, the r2 metric was employed to quantify pairwise SNP correlation strength, with a lower threshold (0.001) selected to maximize sensitivity for detecting subtle yet biologically relevant associations while minimizing type II error. The extended genomic window (10,000 kb) was implemented to account for potential long-range cis-regulatory interactions and population-specific recombination hotspots. This dual-strategy approach systematically mitigates confounding from regional linkage effects and genetic redundancy, thereby enhancing analytical resolution for robust causal inference19,22.
Finally, we utilized the F-statistic for screening to mitigate the influence of weak instrumental variables on SNPs. Specifically, we employed the formula F = R2 × [(N − 1 − k)/k] × (1 − R2) to calculate the F-statistic, with R2 representing the degree to which the instrumental variables explain the exposure, N denotes the sample size, while k indicates the number of instrumental variables. A low F-value, such as below 10, suggests the possibility of weak instrumental variable bias. Therefore, we chose instrumental variables with F-values greater than 10 to ensure the reliability of the results.
MR analysis
In this study, we assessed the following key metrics: (1) the association between immune cell phenotypes and osteoporosis, to identify a greater number of statistically significant immune cell phenotypes, a significance threshold of P < 0.01 was applied; (2) the link between serum metabolites and osteoporosis, with the corresponding statistical significance threshold set at P < 0.01; (3) the interrelationship between immune cell phenotypes and serum metabolites, where the threshold for statistical significance was set at P < 0.05.
The Mendelian randomization framework incorporates three complementary analytical approaches: Inverse-Variance Weighted (IVW), Weighted Median, and MR-Egger methods. The IVW method operates under the assumption of balanced pleiotropy across valid instrumental variables, aggregating SNP-specific causal estimates through inverse-variance weighting to maximize efficiency in null violation scenarios. While demonstrating superior statistical efficiency under valid instrument assumptions, this approach exhibits sensitivity to violations of the exclusion restriction criterion, where directional pleiotropy among instrumental variants may produce biased estimates. Weighted Median provides robustness against invalid instruments by requiring causal consistency in only > 50% of genetic variants, utilizing a weighted quantile approach to mitigate outlier influence. Though inherently conservative, this method sacrifices precision compared to IVW when all instruments satisfy validity assumptions. MR-Egger regression introduces an intercept term to explicitly model pleiotropic effects, relaxing instrument validity requirements through the InSIDE assumption. While enabling systematic detection of horizontal pleiotropy, this approach reduced statistical power for causal detection and requires stronger assumptions relative to IVW31. The primary analysis of this study employed the inverse-variance weighted (IVW) method. To improve the reliability of the results, we also performed supplementary analyses using the weighted median method, MR-Egger analysis, simple mode method, and weighted mode method. The potential impact of horizontal pleiotropy was assessed by examining the intercept in the MR-Egger regression, with a P-value greater than 0.05 indicating no significant pleiotropy. The MR PRESSO was utilized to detect pleiotropy and outliers32. Furthermore, Cochran’s Q test was used to evaluate heterogeneity among SNPs, with a P-value greater than 0.05 suggesting no significant heterogeneity33. The "leave-one-out" method is a frequently used technique in MR studies, employed to evaluate the impact of individual genetic loci on overall results, thereby testing the robustness and reliability of the model34. All statistical analyses in this study were conducted using R software version 4.3.3. For the execution of Mendelian randomization analyses.
Result
Immune cell phenotype instrumental variables (IVs)
In this study, we identified the IVs from the GWAS data of 731 immune cell phenotypes. The F-statistics for all selected IVs were above a value of 10, indicating no bias from weak instrumental variables (see Supplementary Table S1).
The impact of immune cell phenotypes on osteoporosis
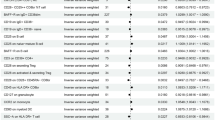
Utilizing Mendelian randomization analysis, we have successfully identified seven immune cell phenotypes that exhibit a causal association with osteoporosis. Figure 2 presents a forest plot of the IVW method results for the genetic prediction of four groups of immune cells regarding osteoporosis. Of these, five immune cell phenotypes showed a positive causal association with osteoporosis development (IVW: OR > 1, P-value < 0.01). B cell Panel: IgD- CD38dim %B cell; Monocyte Panel: HLA DR on CD14 + CD16- monocyte, HLA DR on CD14 + monocyte; TBNK Panel: HLA DR++ monocyte %leukocyte, CD8br %leukocyte. Two immune cell phenotypes were found to be negatively correlated with the development of osteoporosis (IVW: OR < 1, P-value < 0.01). cDC Panel: CD11c + CD62L- monocyte %monocyte; TBNK Panel: CD16-CD56 on NK.
Mendelian randomization analysis between immune cell phenotypes and osteoporosis. The forest plot displays the results of Mendelian randomization analysis between seven immune cell phenotypes and osteoporosis. Each point in the plot represents the odds ratio (OR) of the exposure factor, with the horizontal line indicating the 95% confidence interval. The vertical dashed line, positioned at an OR of 1, denotes the null effect reference point. By examining the position of each point to this reference line, one can visually assess the impact of various immune cell phenotypes on the risk of osteoporosis.
After the FDR (False Discovery Rate) adjustment of the P-values, we identified that four immune cell phenotypes still exhibit a correlation with osteoporosis. IgD- CD38dim %B cell (P-FDR = 0.0127), HLA DR on CD14 + CD16- monocyte (P-FDR = 0.0002), HLA DR on CD14 + monocyte (P-FDR = 0.0002), HLA DR++ monocyte %leukocyte (P-FDR = 0.0147). In addition, additional analyses were performed, including MR Egger, weighted median, Simple mode, and Weighted mode methods. These analyses largely supported the main findings and demonstrated a consistent direction of effect. None of the seven immune cell phenotypes showed significant heterogeneity in the heterogeneity test (Q-test, with all P-values exceeding 0.05), and no significant horizontal pleiotropy was found in the MR-Egger intercept method (P > 0.05). MR PRESSO analysis showed no evidence of outliers or pleiotropy (see Supplementary Table S2). Furthermore, “leave-one-out” analyses demonstrated that no specific IV significantly influenced causal inferences (Supplementary Figure S1). These findings further confirm the robustness and reliability of the Mendelian randomization results.
Reverse Mendelian randomization analysis of immune cell phenotypes
Initially, we screened IVs from the genome-wide association study (GWAS) data on osteoporosis, ensuring that all IVs had an F-statistic greater than 10, thereby excluding the influence of weak instruments (see Supplementary Table S3). Osteoporosis as an exposure factor was causally associated with four immune cell phenotypes. Im MDSC %CD33dim HLA DR- CD66b-(IVW: OR = 0.76, P-value = 0.0009), CD33- HLA DR + AC (IVW: OR = 1.38, P-value = 0.0002), HLA DR + CD4 + %T cell (IVW: OR = 1.14, P-value = 0.0094), CD38 on CD3- CD19-(IVW: OR = 1.28, P-value = 0.0015) (Fig. 3). Additionally, sensitivity analyses were conducted (see Supplementary Table S4), the four immune cell phenotypes showed no significant heterogeneity in the heterogeneity test (Q-test, with P-values greater than 0.05), and no significant horizontal pleiotropy was found using the MR-Egger intercept method (P > 0.05). Furthermore, “leave-one-out” analyses demonstrated that no specific IV significantly influenced causal inferences (Supplementary Figure S2). These findings further confirm the robustness and reliability of the Mendelian randomization results. Ultimately, no reverse causality was observed for the same type of immune cells.
Role of serum metabolites in osteoporosis
In the study aimed to identify potential mediators, 1,400 serum metabolites were initially selected to investigate their impact on osteoporosis. Upon analyzing the associations between these serum metabolites and osteoporosis, several significant associations were discovered. Thirteen serum metabolites demonstrate a causal relationship with an increased risk of developing osteoporosis. Five of these metabolites are positively correlated with an increased risk of osteoporosis, including 3-(3-amino-3-carboxypropyl)uridine levels, Heneicosapentaenoate (21:5n3) levels, 12,13-DiHOME levels, Arachidonoylcholine levels, X-18345 levels. Eight metabolites are negatively correlated with an increased risk of osteoporosis, including N6-methyllysine levels, Glycosyl ceramide (d18:1/20:0, d16:1/22:0) levels, Citrulline to dimethylarginine (SDMA + ADMA) ratio, (S)-3-hydroxybutyrylcarnitine levels, Sulfate of piperine metabolite C16H19NO3 (3) levels, 2,4-di-tert-butylphenol levels, 8-methoxykynurenate levels, Cholesterol to benzoate ratio (Fig. 4). These findings highlight the complex relationship between specific serum metabolites and osteoporosis and lay the groundwork for further mediation analyses. The results of the serum metabolite instrumental variable selection are shown in Supplementary Table S5. The results of the other four Mendelian randomization analysis methods and the sensitivity analyses are shown in Supplementary Table S6 and Supplementary Figure S3.
Effects of immune cell phenotype on serum metabolites
In the preceding sections of this study, seven immune cell phenotypes and thirteen serum metabolites were identified as having a causal relationship with osteoporosis. Therefore, Mendelian randomization analysis was subsequently conducted to examine the potential causal relationships between the seven immune cell phenotypes and the thirteen serum metabolites. The Mendelian randomization results (IVW) revealed a positive association between IgD- CD38dim %B cells and 8-methoxykynurenate levels (OR = 1.03, 95% CI 1.00–1.06), a negative correlation between CD11c + CD62L-monocyte %monocyte and 3-(3-amino-3-carboxypropyl)uridine levels (OR = 0.94, 95% CI 0.90–0.99), and a negative correlation between HLA DR + + monocyte %leukocyte and the citrulline to dimethylarginine (SDMA + ADMA) ratio (OR = 0.94, 95% CI 0.88–1.00) (Fig. 5). Other methods, such as MR Egger, weighted median, simple mode, and weighted mode, show consistent trends with the IVW method, with no evidence of heterogeneity, or horizontal pleiotropy (Supplementary Table S7 and Supplementary Figure S4).
Mediation effect
Further investigation was conducted on the potential mediating effects of immune cell phenotypic exposure on these important mediators. As shown in Fig. 6, CD11c + CD62L- monocyte %monocyte exerted its effect on osteoporosis via 3-(3-amino-3-carboxypropyl) uridine levels, with a mediating ratio of 10.7%. HLA DR + + monocyte %leukocyte mediated its effect on osteoporosis through Citrulline to dimethylarginine (SDMA + ADMA) ratio, with a mediating ratio of 4.64%. The mediating effect values are presented in Supplementary Table S8. The CD11c + CD62L- monocyte subset demonstrated statistically significant negative total effect on osteoporosis risk (β = −0.0850), mediated through 3-(3-carboxypropyl)uracil levels with direct suppression of metabolite production (direct effect β = − 0.0618) . Conversely, the HLA DR++ monocyte population exhibited non-significant positive total effect (β = 0.1339) via the citrulline/dimethylarginine ratio.
Discussion
In recent years, advancements in immunology have provided new insights into the study of orthopedic diseases, revealing the intricate connections between immune cells and osteoporosis. Immune cells, commonly referred to as white blood cells, include lymphocytes and various phagocytes. These cells specifically include lymphocytes capable of recognizing antigens and generating antigen-specific immune responses11. The skeletal system and the immune system share numerous cytokines and signaling molecules1. Immune cells may influence bone regeneration, osteoclastogenesis, and the regulation of osteoblast function through paracrine effects, and are involved in bone homeostasis35. For instance, T cells play a dual role in bone regulation. In a resting state, they can inhibit osteoclastic bone resorption; however, when activated and expressing RANKL, T cells mediate the generation of osteoclasts, thereby enhancing bone resorption11. Dendritic cells also act as precursors of osteoclasts, promoting inflammation-induced osteoclastogenesis and enhancing bone loss36. Regulatory T cells directly inhibit osteoclastogenesis by suppressing the production of RANKL and M-CSF, which leads to increased bone volume11.
Current osteoimmunology frameworks substantiate the central regulatory role of innate and adaptive immune in sustaining chronic inflammatory states underlying osteoporosis. In theory, immune cell subsets (e.g., IgD⁻ CD38dim B cells, CD8bright T lymphocytes) dynamically coordinate bone metabolic equilibrium through cytokine networks that bidirectionally modulate osteoclastogenesis and osteoblast functionality. Notably, lymphocyte-derived signaling molecules such as RANKL serve as critical mediators of this immunometabolic crosstalk, as evidenced by their direct involvement in osteoclast precursor differentiation pathways19,22. This study conducted a comprehensive analysis of the association between immune cell phenotypes and osteoporosis, yielding compelling insights into this intricate interplay. To begin with, the causal relationship between 731 immune cell phenotypes and osteoporosis was examined using a two-sample bidirectional Mendelian randomization (MR) analysis. We observed causal relationships among four types of immune cells—B cells, conventional dendritic cells (cDCs), monocytes, and TBNK—as well as seven phenotypes, and osteoporosis. Among these, five immune cell phenotypes were positively associated with the risk of osteoporosis, specifically implicating B cells (IgD- CD38dim %B cell), monocytes (HLA DR on CD14 + CD16- monocyte, HLA DR on CD14 + monocyte), and TBNK (HLA DR++ monocyte %leukocyte, CD8br %leukocyte). B lymphocytes serve a pivotal function in various bone homeostasis disorders, with their effects being predominantly pathological or protective, contingent upon the specific context. Within the context of fractures, B cell-derived OPG changes the cytokine environment for RANKL, reducing osteoclast activity and favoring bone formation in the middle and late stages of fracture healing37,38. In inflammatory arthritis, inflammatory B cells produce CCL3, RANKL, and TNF, which stimulates osteoclast activity and inhibits osteoblast function37,39. In osteoporosis, B cell-derived RANKL mediates cancellous bone loss after estrogen withdrawal, corroborating the study’s findings that the B cell phenotype (IgD-CD38dim%B cell) increases the risk of osteoporosis37,40. HLA DR is a class II molecule of the major histocompatibility complex (MHC) that plays a role in the regulation of immune responses41. Evidence indicates that HLA DR mismatch increases the risk of osteoporosis and hip fracture in renal transplant patients, potentially due to the inhibition of bone matrix synthesis and increased bone turnover42. Monocytes are early progenitor cells of osteoclasts43. Evidence suggests that attenuated monocyte apoptosis is crucial for osteoporosis, and a higher proportion of monocytes may be a potential predictor of osteoporosis44. Therefore, this study demonstrated the promoting effect of IgD- CD38dim %B cell, HLA DR on CD14 + CD16-monocyte, HLA DR on CD14 + monocyte, HLA DR + + monocyte %leukocyte, CD8br %leukocyte on the occurrence of osteoporosis. Additionally, two immune cell phenotypes were negatively associated with the risk of osteoporosis, including cDCs (CD11c + CD62L- monocyte %monocyte), and TBNK (CD16-CD56 on NK). For CD11c + cells, research indicates that targeting CD11c + cells in vivo in an inflammatory arthritis model can reduce inflammatory bone destruction and osteoclast (OC) production, thereby improving arthritis45. Nevertheless, no direct study has confirmed this phenomenon in osteoporosis.
The findings reveal a pathological dominance of active osteoimmunological regulation over passive feedback mechanisms in bone homeostasis dysregulation. While bone-derived factors (e.g., TGF-β cleavage products, calcium flux) may modulate immune surveillance under physiological conditions, the RANKL/RANK axis-driven proactive immunostromal crosstalk emerges as the principal driver of osteolytic cascades. In particular, this also reflects that its active driving role dominates in the pathological state8. Immune-related genes operate through cell-autonomous regulatory programs, wherein classical monocytes serve as the principal osteoclast precursors. While their differentiation into osteoclasts strictly requires RANKL and M-CSF signaling, their population dynamics are governed by hematopoietic stem cell-derived transcriptional networks rather than bone resorption byproducts. In addition, key molecules (RANKL, IL-17) have actively-secreted properties, and HLA DR + + monocytes and IgD-CD38dim B cells may actively participate in bone remodeling through direct interaction with osteoblasts or cytokine signaling19,22,46. Some immune-related phenotypes have demonstrated a protective effect against osteoporosis. CD11C + CD62L- monocytes belong to the classical pro-inflammatory monocytes (e.g., human CD14 + CD16-), but downregulation of CD62L (L-selectin) suggests that they are in an activated state and may have the potential to migrate to tissues and differentiate into anti-inflammatory macrophages. In addition, the proportion of CD11C + CD62L- monocytes in the peripheral blood of postmenopausal osteoporosis patients was significantly reduced and was positively correlated with lumbar BMD. This may imply a close relationship between immune cells and osteoporosis47,48.
The dynamic changes in serum metabolites can reveal the metabolic activities of bone and its constituent cells, suggesting a potential role as promising biomarkers. They can be used to identify and monitor the status of diseases such as osteoporosis, assess treatment effectiveness, and potentially provide predictive information on disease progression and ultimate outcomes20. To investigate whether these seven immune cell phenotypes modulate serum metabolites affecting osteoporosis, we conducted a two-step MR analysis. Initially, we found causal relationships between 13 serum metabolites and osteoporosis. Among these, 8 serum metabolites were negatively correlated with osteoporosis, while 5 serum metabolites were positively correlated with osteoporosis. Subsequently, we analyzed the mediating role of serum metabolites.
While the pathobiological mechanisms linking most identified metabolites to osteoporosis remain uncharacterized, emerging evidence suggests plausible osteoimmunological interactions. Heneicosapentaenoate (21:5n3), an omega-3 polyunsaturated fatty acid structurally analogous to vitamin D metabolites49, shows potential associations with calcium homeostasis requiring mechanistic validation. The 12,13-DiHOME may exacerbate osteoclastogenesis via mitochondrial oxidative stress potentiation50, contrasting with 2,4-di-tert-butylphenol’s synthetic antioxidant capacity to mitigate redox imbalance51. Arachidonoylcholine appears to engage inflammatory cascades through RANKL pathway potentiation52. N6-methyllysine demonstrates insulin-mimetic properties that enhance osteoblastic differentiation through lysine metabolism rewiring53,54. Glycosylceramides (d18:1/20:0, d16:1/22:0) likely modulate skeletal cell fate via sphingolipid rheostat regulation55. (S)-3-hydroxybutyrylcarnitine sustains osteocyte bioenergetics through mitochondrial β-oxidation intermediates56,57. 8-methoxykynurenine implicates tryptophan catabolism dysregulation in bone loss, potentially via kynurenine pathway-mediated immunometabolic crosstalk58. Structural elucidation of X-18345 and related unannotated metabolites remains prerequisite for functional interrogation.
The results revealed that two serum metabolites mediate the influence of two immune cell phenotypes on osteoporosis. CD11c + CD62L- monocyte %monocyte exerts a protective influence on osteoporosis by negatively regulating the 3-(3-amino-3-carboxypropyl) uridine level, with the mediation ratio being 10.7%0.3-(3-amino-3-carboxypropyl) uridine plays a key role in the stability and function of tRNA. Its biosynthesis is tightly controlled and influenced by multiple factors59. As terminal differentiation subpopulations (the lack of CD62L indicates that tissue homing has been completed), are closely related to the inflammatory activity of the disease60. This novel RNA modification metabolite, though lacking direct evidence of immunomodulatory function, may mechanistically link to mitochondrial bioenergetics regulation in osteoclast precursors via tRNA stability modulation57,59. Currently, there is a lack of relevant research on its role in osteoporosis. HLA DR + + monocyte %leukocyte promotes osteoporosis by negatively regulating the citrulline/dimethylarginine (SDMA + ADMA) ratio, with a mediating ratio of 4.64%. Elevated HLA-DR + + subsets showed metabolic coupling to citrulline flux, potentially influencing nitric oxide synthesis through arginine metabolic rewiring48,61,62.
Current studies have confirmed that appropriate concentrations of nitric oxide (NO) in the body can support angiogenesis and remodeling, thereby enhancing bone regeneration in osteoporotic conditions63. The citrulline to dimethylarginine (SDMA + ADMA) ratio is an indicator for evaluating the metabolic status of NO. ADMA, as an endogenous NO synthase inhibitor, is capable of competitively binding to NOS with l-arginine, thereby affecting the production of NO64,65. This may be one of the adverse mechanisms by which HLA DR + + monocyte %leukocyte leads to osteoporosis.
These findings reveal fundamental heterogeneity in monocyte-mediated osteoimmunological regulation, where distinct subsets differentially engage immunometabolic checkpoints to modulate bone homeostasis. The paradoxical bidirectional suppression in HLA DR++ monocytes implies potential counterregulatory mechanisms governing hematopoietic-osteogenic crosstalk, with directional concordance—despite lacking statistical significance—pointing to biologically coherent immunometabolic rewiring that merits deeper mechanistic exploration.
This study utilized Mendelian randomization (MR) methodology to investigate the causal effects of immune cells on osteoporosis and the mediating role of serum metabolites. Our study incorporated comprehensive methods, integrating various rigorous analyses. The conclusions are based on exploring the causal relationship between the two at the genetic level. Consequently, the results of this study are robust and not confounded by horizontal pleiotropy or other biases. Despite the numerous strengths of MR analysis, including large sample size and the capacity to apply various sensitivity analyses to ensure reliability, there remain inherent limitations. Firstly, we employed a p-value threshold of p < 1 × 10–5 to screen IVs. Despite the ability to comprehensively assess the relationship between immune cell phenotypes and osteoporosis, the correlation strength of IVs was not sufficient. Secondly, the study is based on a European database, and its applicability to other ethnic groups is controversial. Subsequently, we verified heterogeneity and horizontal pleiotropy by Q-test, Egger intercept, and MR PRESSO. Despite the statistical elimination of heterogeneity and horizontal pleiotropy, it still cannot be fully guaranteed that there is no heterogeneity and horizontal pleiotropy in clinical settings. At the same time, although we use statistical methods to reject the existence of horizontal pleiotropy, it is not ruled out that existing methods cannot completely eliminate their impact. Lastly, our verification results preliminarily verify the relationship between immune cell phenotype, plasma metabolites and osteoporosis. The resulting causal pathways still need to be further explained. Therefore, our conclusions require validation through cellular or animal experiments to elucidate the specific mechanisms of action.
Conclusion
In summary, we evaluated the association between 731 immune cells and osteoporosis using publicly available genetic information, aiming to investigate the association between immune phenotypes and osteoporosis, as well as the mediating role of serum metabolites. The research results show that there is a potential causal relationship between immune cells, serum metabolites and osteoporosis, which is of great significance to further understand the pathogenesis of osteoporosis and identify potential biomarkers. Further basic experiments and clinical experimental verification are still needed to provide more convincing and authoritative evidence results before future applications and development of targeted immunomodulatory treatments.
Data availability
Data is provided within the manuscript or supplementary information files.
References
Okamoto, K. et al. Osteoimmunology: The conceptual framework unifying the immune and skeletal systems. Physiol. Rev. 97(4), 1295–1349 (2017).
Reid, I. R. & Billington, E. O. Drug therapy for osteoporosis in older adults. Lancet 399(10329), 1080–1092 (2022).
Compston, J. E., McClung, M. R. & Leslie, W. D. Osteoporosis. Lancet 393(10169), 364–376 (2019).
Qaseem, A. et al. Pharmacologic treatment of primary osteoporosis or low bone mass to prevent fractures in adults: A living clinical guideline from the American College of Physicians. Ann. Intern. Med. 176(2), 224–238 (2023).
Mäkinen, T. J. et al. The incidence of osteopenia and osteoporosis in women with hip osteoarthritis scheduled for cementless total joint replacement. Bone 40(4), 1041–1047 (2007).
Binkley, N., Nickel, B. & Anderson, P. A. Periprosthetic fractures: An unrecognized osteoporosis crisis. Osteoporos. Int. 34(6), 1055–1064 (2023).
Park, Y. B., et al. Total knee arthroplasty and periprosthetic distal femoral fracture: Looking beyond the osteoporosis to previous osteoporotic fracture. Osteoporosis International, 2024.
Takayanagi, H. Osteoimmunology: Shared mechanisms and crosstalk between the immune and bone systems. Nat. Rev. Immunol. 7(4), 292–304 (2007).
Arron, J. R. & Choi, Y. Bone versus immune system. Nature 408(6812), 535–536 (2000).
Fischer, V. & Haffner-Luntzer, M. Interaction between bone and immune cells: Implications for postmenopausal osteoporosis. Semin. Cell Dev. Biol. 123, 14–21 (2022).
Srivastava, R. K., Dar, H. Y., and Mishra, P. K. Immunoporosis: Immunology of osteoporosis—role of T cells. Front. Immunol. 2018. 9.
Fu, H. et al. Acid neutralization and immune regulation by calcium–aluminum-layered double hydroxide for osteoporosis reversion. J. Am. Chem. Soc. 144(20), 8987–8999 (2022).
Gao, P. et al. Identification of the transcriptome signatures and immune-inflammatory responses in postmenopausal osteoporosis. Heliyon 10(1), e23675 (2024).
Wang, S., et al. Early changes of bone metabolites and lymphocyte subsets may participate in osteoporosis onset: A preliminary study of a postmenopausal osteoporosis mouse model. Front. Endocrinol. 2024. 15.
Yang, Z. et al. Nanoengineering multifunctional extracellular vesicles availably mitigate bone loss in osteoporosis through binding to RANKL and rebalancing the Treg/Th17 cells. Chem. Eng. J. 467, 143391 (2023).
Cai, X. et al. Glycolithocholic acid increases the frequency of circulating Tregs through constitutive androstane receptor to alleviate postmenopausal osteoporosis. Biochem. Pharmacol. 219, 115951 (2024).
Zou, X., et al. Promising therapeutic targets for ischemic stroke identified from plasma and cerebrospinal fluid proteomes: A multicenter Mendelian randomization study. Int. J. Surg. 2024. 110(2).
Zhang, F., Xiong, Y. & Zhang, B. Causal effects of gut microbiota on renal tumor: A Mendelian randomization study. Int. J. Surg. 110(3), 1870–1872 (2024).
Zhou, D. Q., et al. An exploration of the causal relationship between 731 immunophenotypes and osteoporosis: A bidirectional Mendelian randomized study. Front. Endocrinol. 2024. 15.
Gao, J. et al. MACC: A visual interactive knowledgebase of metabolite-associated cell communications. Nucleic Acids Res. 52(D1), D633–D639 (2023).
Podojil, J. R. & Miller, S. D. Molecular mechanisms of T-cell receptor and costimulatory molecule ligation/blockade in autoimmune disease therapy. Immunol. Rev. 229, 337–355 (2009).
Cao, R. R., et al. The immune factors have complex causal regulation effects on bone mineral density. Front. Immunol. 2022. 13.
Bode-Böger, S. M. et al. Symmetrical dimethylarginine: A new combined parameter for renal function and extent of coronary artery disease. J. Am. Soc. Nephrol. 17(4), 1128–1134 (2006).
Peschard, S. et al. Genetic evidence of causal relation between intestinal glucose absorption and early postprandial glucose response: A Mendelian randomization study. Diabetes 73(6), 983–992 (2024).
Yang, J. et al. Blood lipid levels mediating the effects of sex hormone-binding globulin on coronary heart disease: Mendelian randomization and mediation analysis. Sci. Rep. 14(1), 11993 (2024).
Orrù, V. et al. Complex genetic signatures in immune cells underlie autoimmunity and inform therapy. Nat. Genet. 52(10), 1036–1045 (2020).
Larsson, S. C. et al. Risk factors for intracerebral hemorrhage: Genome-wide association study and mendelian randomization analyses. Stroke 55(6), 1582–1591 (2024).
Chen, Y. et al. Genomic atlas of the plasma metabolome prioritizes metabolites implicated in human diseases. Nat. Genet. 55(1), 44–53 (2023).
Wang, A., Zhang, J. Causal role of immune cells in psoriasis: a Mendelian randomization analysis. Front. Immunol. 2024. 15.
Li, Y. & Yang, Z. The causal effect of exposure to air pollution on risk of adverse pregnancy outcomes: A two-sample Mendelian randomisation study. Sci. Total Environ. 928, 172234 (2024).
Zuber, V. et al. Combining evidence from Mendelian randomization and colocalization: Review and comparison of approaches. Am. J. Hum. Genet. 109(5), 767–782 (2022).
Sobczyk, M. K. et al. Causal relationships between anthropometric traits, bone mineral density, osteoarthritis and spinal stenosis: A Mendelian randomisation investigation. Osteoarthritis Cartil. 32(6), 719–729 (2024).
Yang, S. et al. Roles of blood metabolites in mediating the relationship between vitiligo and autoimmune diseases: Evidence from a Mendelian randomization study. Int. Immunopharmacol. 133, 112132 (2024).
Liao, Q. et al. Mendelian randomization study on the causal effect of serum IgA levels on H7N9 avian influenza A virus susceptibility. J. Med. Virol. 95(11), e29266 (2023).
Lee, J. et al. Current advances in immunomodulatory biomaterials for bone regeneration. Adv. Healthcare Mater. 8(4), 1801106 (2019).
Puchner, A. et al. Bona fide dendritic cells are pivotal precursors for osteoclasts. Ann. Rheum. Dis. 83(4), 518 (2024).
Grčević, D., Sanjay, A. & Lorenzo, J. Interactions of B-lymphocytes and bone cells in health and disease. Bone 168, 116296 (2023).
Li, Y. et al. B cells and T cells are critical for the preservation of bone homeostasis and attainment of peak bone mass in vivo. Blood 109(9), 3839–3848 (2007).
Sun, W., et al. B cells inhibit bone formation in rheumatoid arthritis by suppressing osteoblast differentiation. Nature Commun. 2018. 9(1).
Taxel, P. et al. Estradiol rapidly inhibits osteoclastogenesis and RANKL expression in bone marrow cultures in postmenopausal women: a pilot study. Osteoporos. Int. 19(2), 193–199 (2008).
Su, Y.-J. et al. The role of monocyte percentage in osteoporosis in male rheumatic diseases. Am. J. Mens Health 11(6), 1772–1780 (2017).
Opelz, G. & Döhler, B. Association of mismatches for HLA-DR with incidence of posttransplant hip fracture in kidney transplant recipients. Transplantation 91(1), 65–69 (2011).
Eastell, R. & Szulc, P. Use of bone turnover markers in postmenopausal osteoporosis. Lancet Diabetes Endocrinol. 5(11), 908–923 (2017).
Gao, K. et al. The predictive role of monocyte-to-lymphocyte ratio in osteoporosis patient. Medicine 98(34), e16793 (2019).
Puchner, A., et al. Bona fide dendritic cells are pivotal precursors for osteoclasts. Ann. Rheumat. Dis. 2023.
Shi, X.L., et al. N-myc downstream regulated gene 1 suppresses osteoblast differentiation through inactivating Wnt/β-catenin signaling. Stem Cell Res. Ther. 2022. 13(1).
Liu, T. T., et al. Stephanine protects against osteoporosis by suppressing osteoclastogenesis via inhibition of the RANKL-RANK interaction. J. Cell. Mol. Med. 2024; 28(23).
Tao, L. et al. Single-cell RNA sequencing reveals that an imbalance in monocyte subsets rather than changes in gene expression patterns is a feature of postmenopausal osteoporosis. J. Bone Miner. Res. 39(7), 980–993 (2024).
Leung, R. Y. H. et al. Serum metabolomic profiling and its association with 25-hydroxyvitamin D. Clin. Nutr. 39(4), 1179–1187 (2020).
Macêdo, A. P. A., et al. 12,13-diHOME as a new therapeutic target for metabolic diseases. Life Sci. 2022. 290.
Choi, S. J., et al. 2,4-Di--butylphenol from sweet potato protects against oxidative stress in PC12 cells and in Mice. J. Med. Food 2013; 16(11), 977–983.
Gao, S. Z. et al. The association between inflammatory and oxidative stress biomarkers and plasma metabolites in a longitudinal study of healthy male welders. J. Inflamm. Res. 14, 2825–2839 (2021).
Wang, J. et al. Discovery of potential biomarkers for osteoporosis using LC-MS/MS metabolomic methods. Osteoporos. Int. 30(7), 1491–1499 (2019).
Tokarz, J., et al. Common muscle metabolic signatures highlight arginine and lysine metabolism as potential therapeutic targets to combat unhealthy aging. Int. J. Mol. Sci. 2021. 22(15).
Gomez-Muñoz, A. et al. New insights on the role of ceramide 1-phosphate in inflammation. BBA-Mol. Cell Biol. L. 1831(6), 1060–1066 (2013).
Vianey-Saban, C. et al. Fifty years of research on mitochondrial fatty acid oxidation disorders: The remaining challenges. J. Inherit. Metab. Dis. 46(5), 848–873 (2023).
Lu, Y., et al. Gut microbiota, plasma metabolites, and osteoporosis: unraveling links via Mendelian randomization. Front. Microbiol. 2024. 15.
Ballesteros, J., Rivas, D., Duque, G. The role of the kynurenine pathway in the pathophysiology of frailty, sarcopenia, and osteoporosis. Nutrients, 2023. 15(14).
Takakura, M. et al. Biogenesis and functions of aminocarboxypropyluridine in tRNA. Nat. Commun. 10(1), 5542 (2019).
Ito, Y. et al. CD62L expression level determines the cell fate of myeloid progenitors. Stem Cell Rep. 16(12), 2871–2886 (2021).
Molek, P., et al. The shifted balance of arginine metabolites in patients with acute myocardial infarction and its association with the composition of the infarct-related culprit lesion. Circulation, 2021. 144.
Wang, D., et al. Gut microbial alterations in arginine metabolism determine bone mechanical adaptation. Cell Metabolism, 2024. 36(6).
Lee, J.-K. et al. Nitric oxide-releasing bioinspired scaffold for exquisite regeneration of osteoporotic bone via regulation of homeostasis. Adv. Sci. 10(6), 2205336 (2023).
Shin, S. et al. Simultaneous bioanalysis of l-arginine, l-citrulline, and dimethylarginines by LC–MS/MS. J. Chromatogr. B 879(7), 467–474 (2011).
Fleszar, M. G. et al. Quantitative analysis of l-arginine, dimethylated arginine derivatives, l-citrulline, and dimethylamine in human serum using liquid chromatography-mass spectrometric method. Chromatographia 81(6), 911–921 (2018).
Funding
This study was supported by funding from Jilin Scientific and Technological Development Program (20230204075YY, 20240305034YY), the Graduate Innovation Program of Jilin University (2024CX142).
Author information
Authors and Affiliations
Contributions
Hao Wang: conceptualization, methodology, software, validation, formal analysis, data curation, and writing – original draft; Zehao Yu: investigation, writing – review and editing,revised manuscript; Bo Chen: validation, and resources; Shenghao Xu: data curation, and formal analysis; Yichuan Zhou: writing – review and editing; Xiongfeng Tang and Yanguo Qin: project administration, funding acquisition, supervision, writing – review and editing, and conceptualization.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, H., Yu, Z., Chen, B. et al. Causal relationship between immune cells and osteoporosis based on genetic prediction: a bidirectional two-sample mediated Mendelian randomization analysis. Sci Rep 15, 19524 (2025). https://doi.org/10.1038/s41598-025-95740-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-95740-1