Abstract
In the process of coal mining with complex hydrological conditions, underground coal seams are often subjected to corrosion by acidic water, and acidic water–rock chemical interactions can significantly affect the mechanical properties of coal rocks, posing challenges for mine tunnel support and coal seam stability. This study investigates the effects of acidic solution exposure, specifically varying pH levels, on the mechanical and structural properties of coal samples. Static Brazilian splitting tests were conducted to determine the tensile mechanical properties of the treated coal samples. Additionally, the Particle Flow Code (PFC) was utilized to examine the evolution of microcracks, stress fields, and energy conversion characteristics within the coal samples. The results indicate that acidic solutions induce damage and softening of the coal structure, leading to a reduction in tensile strength and elastic modulus as acid corrosion intensifies. The primary mechanism of failure in the coal samples is attributed to the initiation, propagation, nucleation, and rapid consolidation of microcracks within stress concentration zones. A decrease in the area of stress concentration zones, increased stress unevenness, and reduced ultimate tensile strength in corroded coal samples lead to more complex crack propagation paths and lower macroscopic strength. Energy monitoring further reveals that acid-corroded coal has reduced resistance to damage and higher failure rates, highlighting the heightened vulnerability of acid-affected coal in structural applications.
Similar content being viewed by others
Introduction
Coal resources play a dominant role in China’s energy supply. As mining depths increase, the hydrogeological conditions of coal seams become increasingly complex, leading to frequent structural instability issues such as roof collapses and sudden water inrushes in mines. These events pose significant threats to the safety of coal mining operations1,2. Research indicates that mine water is a significant factor contributing to these disasters3,4. Mine water, which accumulates in mining areas, interacts with pyrite and other sulfur-bearing minerals in coal seams, rendering the water acidic or even highly acidic5. This acidic water chemically reacts with coal, causing dissolution, hydrolysis, ion exchange, and redox reactions, which alter the microstructure and physical properties of coal. These changes lead to the degradation of its macroscopic mechanical properties6. Therefore, studying the mechanical properties of coal under acidic water corrosion conditions is crucial for ensuring safe coal mining.
In the engineering field, early studies on the interaction between aqueous chemical solutions and coal primarily aimed to improve the efficiency of coalbed methane production7,8,9. These studies involved using various chemical solvents to enhance the development of fractures and pores in coal, thereby increasing the permeability of coalbed methane reservoirs. In recent years, research in this area has been advanced, and findings suggest that chemical solvents effectively remove various metal cations from coal and reduce the mineral and small-molecule organic content10. For example, HCl primarily affects the aliphatic structures and oxygen-containing functional groups in coal11, while NaOH has a more pronounced influence on aromatic structures and hydroxyl groups12. Organic solvents have also been shown to enhance coal permeability by extracting small organic molecules of coal, effectively expanding its pore structure13. These studies demonstrate that the mineral composition, microscopic defects, and pore structure of coal undergo changes under the influence of aqueous chemical solutions, leading to inevitable alterations in its macroscopic mechanical properties.
The interaction between aqueous chemical solutions and the coal mechanical properties has increasingly attracted researchers’ attention, yielding numerous valuable studies. For instance, Xue et al.14 conducted acoustic emission and uniaxial compression tests on coal samples treated with acidic solutions of different pH values, revealing patterns of fracture development and damage evolution in coal samples under acidic water conditions. Li et al.15 analyzed the mechanical properties and pore structure of acid-treated coal samples through microscopic and uniaxial compression tests. Zhang et al.16 investigated the effects of H2O + CO2 solutions with varying fluid saturations on coal mechanical properties using triaxial tests.
Coal failure under external loads is a complex fracturing process. Laboratory tests, limited by experimental techniques, can only provide stress-strain curves and failure modes of rocks, but they cannot monitor crack propagation and energy conversion processes at a microscopic scale. This limitation poses challenges in accurately understanding the intrinsic mechanisms of macroscopic deformation and failure of rocks17,18. Numerical simulation serves as an excellent supplementary method, which is primarily divided into finite element and discrete element categories. While the finite element method struggles with simulating microcrack propagation in heterogeneous materials like coal, the discrete element-based Particle Flow Code (PFC), in contrast, can characterize microcracks and heterogeneity without the need for complex failure criteria and constitutive models, clearly depicting the entire process of coal crack initiation, expansion, and merging. Thus, PFC is considered an effective technology for simulating the failure processes of rock-like materials and is widely utilized in engineering simulations. Li et al.19 developed a particle flow model for hydraulic fracturing of low-permeability reservoirs considering fluid-solid coupling effects. Zhang et al.20 conducted a holistic study using PFC on the strength, acoustic emission response, damage characteristics, and crack evolution of intact coal samples under triaxial and biaxial compression. Song et al.21. proposed a water-ice particle phase transition expansion method using particle flow programming to explore the initiation and damage evolution of microcracks in freeze-thaw rocks. Liu et al.22 utilized DIC technology and the PFC simulation method to analyze the influence of loading rates on the mechanical properties and failure mechanism of coal samples. Despite extensive simulation research on the microscopic mechanical properties and crack propagation of rocks, there is a lack of simulation research on the internal microcrack evolution, stress fields, and energy conversion characteristics of acid-corroded coal under external load conditions. Therefore, the understanding of the intrinsic rules of macroscopic damage in acid-corroded coal samples remains insufficient.
Among the various mechanical parameters of coal, tensile strength is a key parameter due to its lower value compared to compressive strength. Additionally, in underground coal mining accidents, coal seams typically fracture in tension23. Based on these reasons, this paper conducted Brazilian split tensile tests on coal samples before and after acid treatment to investigate the influence of acidic solutions on the static tensile mechanical properties of coal. Simultaneously, numerical simulations were employed to further elucidate the evolution of microcracks, stress fields, and energy conversion characteristics of coal samples under the influence of acidic solutions. The findings of this research can provide theoretical references for the fundamental understanding of water–rock interactions and the development of protective design in underground coal mining engineering.
Experimental
Coal sample Preparation and acid treatment
Laboratory experiments were conducted as an initial step to establish a foundation for calibrating the microscopic parameters of the numerical model and to assess the reliability of simulation results. The coal samples used in these experiments were sourced from the 3101 working face coal of the Yangcheng mine in Shanxi Province, China. In accordance with ISRM standards, the coal samples were drilled and polished to create standard Brazilian disc samples with a diameter of 50 mm and a thickness of 25 mm. These samples were divided into three groups, each containing three samples. Due to the influence of geological movements and human activities, mine water has a complex composition, including acidic ions such as SO42−, Cl−, and HCO3 −24. The pH of mine water ranges from 2 to 825. To simulate an acidic water environment accurately, this study utilized distilled water, 1 mol/L HCl, and NaHSO4 standard solutions to prepare 500 ml of mixed acid solutions with pH values of 4 and 2, respectively. Two sets of coal samples from the three groups were immersed separately in these two types of solutions for 24 h, with the beaker mouths sealed with plastic wrap during immersion, as shown in Fig. 1. After being removed from the solutions, the coal samples were subjected to a constant temperature drying process in an oven set at 50 °C for 12 h and subsequently subjected to Brazilian splitting tests. The R group represents coal samples in their original state, while the S4 and S2 groups represent coal samples treated with acidic solutions at pH 4 and 2, respectively.
Experimental scheme
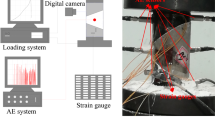
The multifunctional testing machine used was the WDW-200D model, with a maximum load capacity of 200 kN. The tests employed displacement-controlled loading at a constant axial deformation rate of 0.1 mm/min, ensuring quasi-static loading conditions for the coal samples. During the loading process, the system automatically collected load and displacement data. Strain gauges were placed at the center of both the front and back faces of each coal sample to monitor strain changes until sample failure, as illustrated in Fig. 2.
Analysis of Brazilian test results
The mechanical properties of three coal samples obtained from the Brazilian split tests are shown in Table 1. The mean tensile strength of the raw coal samples was 2.54 MPa, and the mean elastic modulus was 0.51 GPa. The mean tensile strength of the specimens soaked in mixed acid solutions with pH values of 4 and 2 was 2.03 MPa and 1.63 MPa, respectively, with corresponding elastic modulus of 0.39 GPa and 0.33 GPa. Compared to the raw coal, the tensile strength of the samples treated with pH 4 and pH 2 solutions decreased in 20% and 33%, respectively. This indicates that the acidic solutions weakened the tensile strength of the coal, with stronger acidity leading to greater reductions in tensile strength. Similarly, the elastic modulus of the coal samples also decreased to varying degrees after treatment with acidic solutions, with average reductions of 24% and 35% for the pH 4 and pH 2 samples, respectively. This suggests that acidic solutions also result in the softening of the coal’s structure, with the softening effect becoming more pronounced as the acidity of the solution increases, leading to greater reductions in elastic modulus. This discovery aligns with earlier studies that acidic solutions soften rocks24,26.
A comparative analysis was conducted using three typical coal samples (R-2, S4-2, S2-2) with tensile strengths close to the average value of the three coal groups. The corresponding axial stress-strain curves are shown in Fig. 3a. During the initial loading stage, due to the presence of initial microcracks and pores inside the coal samples, a noticeable “compaction” phase appears in the stress curves of all three types of samples. As loading continues, the stress curves of the three samples show a generally linear increasing trend until reaching the peak, after which the stress decreases rapidly, exhibiting brittle failure characteristics without a distinct yield point. However, compared to the raw coal, the initial compaction phase is significantly longer in the samples treated with acidic solutions. This is due to the chemical reaction between H⁺ions in the solution and the soluble minerals in the coal, which facilitates the proliferation of internal pores and the expansion of original cracks. As a result, the compaction phase is lengthened and tensile strength is further reduced. As the pH of the solution decreases, more minerals in the coal participate in chemical reactions, intensifying the degradation of the coal and further reducing its tensile strength.
The lateral tensile strains at the center of both the front and back faces of the three coal samples over time are shown in Fig. 3b. The graphical representation indicates that the strain trends at the center of both faces are generally consistent. During loading, the center of the coal samples remains in a state of tensile stress, with the tensile strain increasing over time. After 75 s, the growth rate of the tensile strain accelerates, and when the coal samples fail, the strain value changes significantly in an instant as central cracks pass through the strain gauges. Among the samples, the failure strain at the center of the pH 4 sample is the largest, with an average value of 9.4 × 10− 4. The failure strain of the raw coal is the smallest, at 5.4 × 10− 4. This is because the softening action of the acidic solution significantly increases the plastic deformation capability of the pH 4 coal sample. With an increase in the acidity of the solution, the detrimental impact of the acid on the tensile strength of the coal becomes increasingly evident, but the softening effect is relatively gradual. Under the interaction of these effects, the failure strain of the pH 2 sample is less than that of the pH 4 sample.
Failure mode
Figure 4 illustrates the failure patterns in the coal samples post-Brazilian split test. It can be observed that the failure mode for all three coal samples is tensile splitting, with the main fracture generally propagating along the direction of the loading axis. Due to the inherent heterogeneity and anisotropy in coal samples, the crack paths are not straight. There are no significant differences in the degree of fragmentation among the three coal samples. However, a notable difference is observed in the samples treated with acidic solutions: the paths of the main fractures in these samples are more curved, and the stronger the acidity of the solution, the greater the curvature of the crack propagation paths. This indicates that while the acidic solutions cause degradation and corrosion, they also further increase the heterogeneity and anisotropy of the coal.
PFC numerical simulation
Numerical model establishment
Laboratory experiments alone face challenges in assessing the damage evolution of coal samples from a microscopic perspective. Therefore, the use of a numerical simulation method is necessary to complement laboratory experiments for analysis and research, enabling a deeper understanding of the inherent laws of coal sample failure and the essential characteristics of fracture instability. According to the dimensions of coal samples utilized in the laboratory experiments, a two-dimensional particle model with a radius of 25 mm was created using PFC2D.
This 2D model contains 7,305 particles with radii ranging from 0.2 to 0.3 mm, where particles are randomly positioned within a circular area bordered by frictionless walls, ensuring uniform size distribution.The contacts between particles are modeled using the Parallel Bond Model (PBM). Consistent with the laboratory experiments, the simulation also adopted a displacement-controlled loading method, where loading is achieved by applying a constant loading rate to the walls on either side of the model. The axial loading rate set for this numerical simulation is 0.015 m/s. Figure 5 shows the PFC model of the coal sample.
PFC simulation results primarily depend on the microscopic mechanical parameters of particles and the selected contact model. A ‘trial-and-error’ approach is recommended by the developers of PFC software to calibrate the model parameters. This involves comparing the simulation results with laboratory test results and then continually adjusting them based on differences until the microscopic mechanical parameters that yield consistent mechanical properties between the two are obtained. In the PFC simulation process described in this paper, simulating the effects of acid corrosion was achieved by weakening the microscopic mechanical parameters of particles and bonds. From another perspective, it is precisely because of the deteriorating effects of acid corrosion on the microscopic mechanical parameters of coal that ultimately lead to changes in its macroscopic mechanical properties.
Three typical coal samples (R-2, S4-2, S2-2) were selected as the experimental control group. The stress-strain curves and failure mode comparisons between laboratory experiments and PFC simulations for the three coal samples are shown in Fig. 6. Experimental results are denoted by solid lines, whereas simulation outcomes are indicated by dashed lines. It can be observed that the two curves match quite well. However, it is noteworthy that the numerical simulation curves do not exhibit a distinct compaction phase, as the particles in PFC are rigid and do not accurately display the porosity compaction phenomenon that occurs during the loading process of natural coal samples27. In terms of failure mode, the numerical model also demonstrates clear tensile splitting characteristics, and the paths of crack expansion are very similar to those observed in the experiments. The comparison between simulation and experimental results validates the correctness of the selected PBM parameters and indicates that the numerical model can accurately simulate acid-treated coal samples in actual experiments. The microscopic parameters of the three coal sample models are listed in Table 2.
Analysis of microcrack evolution and expansion
Figure 7b presents the changes in stress and microcrack count with strain in the raw coal model, while Fig. 7a shows the distribution of microcracks at three specific stress points within Fig. 7b, where red represents tensile cracks and yellow represents shear cracks. Based on the characteristics of microcrack evolution, the model’s stress-strain curve can be divided into three stages:
-
(1)
Initial Compaction and Deformation Stage: Under the compression of the walls, the deformation of the model leads to relative movement between particles. However, due to the low level of compression, the stress levels at contacts are relatively low, resulting in only a minimal number of bond breakages forming tensile cracks, which constitute no more than 2% of the total number of cracks formed. During this loading stage, the number of cracks remains roughly constant, with no evident upward trend, and the few cracks that do form are all located at the top of the model.
-
(2)
Stable Crack Expansion Stage: As the loading stress increases, the degree of relative movement between particles increases, further elevating the stress at contacts and gradually accelerating the rate of bond breakage. The number of cracks increases noticeably with strain, showing a stepwise growth trend. Shear cracks begin to appear at this stage, though in small numbers (single digits), with tensile cracks remaining the predominant type. During this stage, the model is in a state of structural damage. As the axial strain increases, structural damage, stress redistribution, and stress concentration within the model intensify until the model completely loses its load-bearing capacity. The presence of cracks leads to a stress concentration around adjacent elements, and new cracks begin to propagate and nucleate at the locations of existing cracks. At the end of this stage (point b), two microcrack aggregation areas can be observed at the top of the model.
-
(3)
Crack Merging and Unstable Expansion Stage: When the loading stress reaches peak intensity, the rate of bond breakage rapidly increases and reaches its maximum, causing internal microcracks to quickly expand and merge, eventually forming a macroscopic main crack along the loading axis, resulting in complete failure of the raw coal model. The path of macroscopic crack expansion is nearly linear, with a higher concentration of cracks in the area near the loading walls at the end of the model, consistent with experimental results. At this stage, the model is in a state of failure, with the number of microcracks increasing rapidly in a short period, and the number and growth rate of tensile cracks far exceeding those of shear cracks.
Figures 8 and 9 illustrate the damage evolution process under Brazilian split conditions for the pH = 4 and pH = 2 models, respectively. It is evident that the microcrack evolution pattern of the acid-corroded coal sample models is broadly similar to that of raw coal. Throughout the loading process, the microcrack evolution can be divided into three stages: initial nucleation, stepwise increase, and abrupt increase. The primary type of microcrack in all three coal sample models is tensile cracks, with virtually no shear cracks, and tensile cracks dominate throughout. The total number of cracks in the raw coal model is 567, with 98% being tensile cracks; the pH = 4 model has 580 cracks, also 98% tensile; and the pH = 2 model has 438 cracks, of which 97% are tensile. The statistical data on microcracks indicate that the initiation, expansion, nucleation, and rapid coalescence of tensile microcracks are the ultimate causes of coal sample failure, which aligns with the theoretical findings of Brazilian splitting tests.
Stress field evolution analysis
Figure 10 shows the horizontal stress distribution at three characteristic stress points for each of the three coal sample models. Tensile stress is denoted as positive, while compressive stress is negative. For the raw coal model, when the stress is loaded to point a, a region of tensile stress concentration begins to appear at the center of the model. Due to the model’s inherent discreteness, the stress concentration area is irregular. Additionally, due to the end effects of the Brazilian split test, a small region of compressive stress concentration is also observed at the end of the model. The large stress differential between these two different stress areas causes the bonds near their interface to be the first to undergo minor tensile failures. At this point, the overall stress intensity of the model has increased from before. When the loading stress reaches its peak value (point b), a large area of tensile stress concentration appears at the center of the model, mainly distributed symmetrically along the axial line. The area of compressive stress concentration at the end also increases, enlarging the stress difference and thereby increasing the number of cracks at the stress interfaces. At this stage, the overall stress intensity of the model reaches its maximum, with the highest tensile stress value being 3.95 MPa. The high tensile stress concentration in the central area ultimately causes a large number of bonds to break, producing many tensile microcracks that quickly merge to form a vertical macroscopic main crack. After the model fails, the tensile stress concentration area in the center of the coal sample model disappears, the area of compressive stress at the ends decreases, and the overall stress intensity of the model is reduced, showing an irregular distribution.
The evolution of tensile stress in the pH = 4 and pH = 2 coal sample models during different loading stages is generally similar to that of the raw coal model. However, notable differences emerge at the stress peak point (point b). Compared to the raw coal model, the overall area of tensile stress concentration in the center of the pH 4 and pH 2 models is noticeably reduced. Additionally, the pH = 4 model includes small areas of low tensile stress within its high tensile stress concentration area, and the pH = 2 model has a smaller high tensile stress concentration area which includes more small areas of low tensile stress. Overall, as the degree of acid corrosion increases, the area of stress concentration in the coal sample models decreases and the unevenness becomes more pronounced. The unevenness in stress suggests an unevenness in model structure, aligning with experimental observations where acid corrosion increased the heterogeneity and anisotropy of the coal samples. Additionally, the maximum tensile stress values in the corroded coal sample models are also reduced, with the pH = 4 model at 2.73 MPa and the pH = 2 model at 2.38 MPa, representing decreases of 31% and 40% compared to the raw coal model, respectively. The complex, irregular, and small areas of stress concentration, along with lower ultimate tensile stress values, not only make the crack propagation paths in the coal sample more curved but also lead to a decrease in macroscopic strength.
Energy conversion analysis
PFC enables real-time monitoring of energy conversion during simulations. In this study, the system’s input energy originates from the boundary energy applied by the wall loading. The evolution of internal cracks in the coal sample over time is driven by several types of energy: strain energy, bond strain energy, kinetic energy generated by particle motion, and friction energy resulting from slip between particles.
Figure 11 illustrates the energy conversion process of the three coal sample models from initial loading to final failure. It is evident that the energy evolution characteristics of the three samples are generally similar. During the initial loading stage, the growth of boundary energy and increases in strain energy are slow, while frictional energy and kinetic energy are nearly zero, indicating that most of the input energy in the experiment is converted into strain energy of the model. Since there is no compaction stage in the numerical simulation, almost all input energy is utilized for the elastic deformation of the particle skeleton. As the wall continues to load, both strain energy and bond strain energy exhibit approximately parabolic growth. At this point, some forces between particles exceed the bonding strength of the bonds, leading to the initiation of cracks within the model. The strain energy stored in the fractured bonds is released, resulting in a slight increase in frictional energy, while kinetic energy remains almost constant. Frictional energy mainly arises from the frictional interaction between particles. Upon reaching peak stress, a significant amount of bonds began to fail, leading to rapid release of both types of strain energy. Kinetic and frictional energy increase rapidly, but kinetic energy increases at a faster rate than frictional energy, indicating that most of the strain energy dissipates in the form of kinetic energy. During the failure stage, the total accumulated strain energy of the raw coal is 27.66 J, with bond strain energy at 16.27 J and strain energy at 11.39 J. For the pH = 4 model, the total accumulated strain energy is 25.32 J, with bond strain energy and strain energy of 14.93 J and 10.39 J, respectively. For the pH = 2 model, the total accumulated strain energy is 19.12 J, with bond strain energy and strain energy of 10.61 J and 8.51 J, respectively. Compared to the raw coal model, the total accumulated strain energy of the pH = 4 and pH = 2 models decreased by 8% and 31%, respectively. For the pH = 4 model, the total accumulated strain energy is 25.32 J, with bond strain energy and strain energy of 14.93 J and 10.39 J, respectively. For the pH = 2 model, the total accumulated strain energy is 19.12 J, with bond strain energy and strain energy of 10.61 J and 8.51 J, respectively. Compared to the raw coal model, the total accumulated strain energy of the pH = 4 and pH = 2 models decreased by 8% and 31%, respectively. Overall, the majority of the accumulated strain energy in the three coal sample models is stored in the bonds. Furthermore, observing the energy curve trends after failure, it can be noted that the slopes of the strain energy decrease segments and the rates of kinetic energy growth for the pH = 4 and pH = 2 coal sample models do not exhibit significant changes compared to the raw coal. This indicates that although acid corrosion weakens the ability of coal accumulation to store strain energy to some extent, it does not significantly slow down the release rate of strain energy. This fully demonstrates the poor resistance to damage and rapid failure characteristics of corroded coal samples, with the prominence of these characteristics increasing with stronger acid corrosion. This indicates that although acid corrosion weakens the coal’s capacity to store strain energy to some extent, it does not significantly slow down the rate of strain energy release. This fully demonstrates the poor resistance to damage and rapid failure characteristics of corroded coal samples, which become more pronounced with stronger acid corrosion.
Conclusions
-
(1)
The laboratory experiments demonstrate that acidic solutions induce damage and soften the structure of coal samples. As the acidity of the solution increases, the degree of structure softening and damage also increases. Additionally, the acidic solutions augment the heterogeneity and anisotropy of the coal. The central failure strain of the coal samples is influenced by the combined effects of acid-induced damage and softening.
-
(2)
The numerical model effectively reproduces the behaviour of acid-treated coal samples observed in real experiments. By analyzing the microcrack evolution characteristics, the stress-strain curve of the coal samples can be divided into three stages, each corresponding to distinct features of microcrack expansion. Under Brazilian splitting conditions, tensile cracks are identified as the root cause of the ultimate failure observed in the coal samples.
-
(3)
In the Brazilian split simulation, the initial tensile failure at the model ends is primarily caused by the stress difference, while the concentrated tensile stress in the center of the model inherently drives the formation of the macroscopic main crack. With an increase in the degree of acid corrosion, the area of stress concentration in the coal sample decreases, and the ultimate tensile stress value decreases accompanied by increased stress inhomogeneity.
-
(4)
During the loading process, nearly all input energy is converted into strain energy of the coal samples. Upon reaching peak stress, a considerable number of bonds break, resulting in a rapid release of stored strain energy, which leads to a sharp escalation in kinetic and friction energy. Although the total accumulated strain energy in the pH = 4 and pH = 2 samples is reduced compared to the raw coal, the rate of energy release does not notably decelerate.
Data availability
The authors confirm that the datasets used during the current study are available from the corresponding author upon reasonable request.
References
Ma, D., Duan, H. Y., Zhang, J. X. & Bai, H. B. A state-of-the-art review on rock seepage mechanism of water inrush disaster in coal mines. Int. J. Coal Sci. Technol. 9, 50 (2022).
Xu, B. et al. Characteristics analysis of correlation factors of coal mine water hazard accidents and prevention and control measures. Saf. Coal Mines 05, 13–19 (2023). in Chinese.
Yu, L. Y. et al. Experimental study on the dynamic fracture mechanical properties of limestone after chemical corrosion. Theoret. Appl. Fract. Mech. 108, 102620 (2020).
Zheng, L. W. et al. Molecular structure characterization of coal under the water–rock interaction in acid mine drainage (AMD). J. Mol. Struct. 1251, 132043 (2022).
Galhardi, J. A. & Bonotto, D. M. Hydrogeochemical features of surface water and groundwater contaminated with acid mine drainage (AMD) in coal mining areas: A case study in Southern Brazil. Environ. Sci. Pollut. Res. 23, 18911–18927 (2016).
Miao, S. J., Cai, M. F., Guo, Q. F., Wang, P. T. & Liang, M. C. Damage effects and mechanisms in granite treated with acidic chemical solutions. Int. J. Rock Mech. Min. Sci. 88, 77–86 (2016).
Ni, X. M., Li, Q. Z., Wang, Y. B. & Gao, S. S. Experimental study on chemical permeability improvement of different rank coal reservoirs using multi-component acid. J. China Coal Soc. S2, 436–440 (2014). in Chinese.
Yandré, L., Rabemanana, V. & Vuataz, F. D. Influence of water–rock interactions on fracture permeability of the deep reservoir at Soultz-sous-Forêts. Fr. Geotherm. 35, 507–531 (2006).
Yang, L. L. et al. Dissolution of Arkose in dilute acetic acid solution under conditions relevant to burial diagenesis. Appl. Geochem. 54, 65–73 (2015).
Meshram, P., Purohit, B. K., Sinha, M. K., Sahu, S. K. & Pandey, B. D. Demineralization of low grade coal: A review. Renew. Sustain. Energy Rev. 41, 745–761 (2015).
Wang, F. F., Zhang, X. D., Ping, X. D., Zhang, S. & Liu, X. Effect of acidizing pretreatment on the composition and structure of soluble organic matter in coking coal. Spectrosc. Spectr. Anal. 03, 896–903 (2022). in Chinese.
Xu, Q. F., Liu, R. L. & Yang, H. T. Effect of acid and alkali solutions on micro-components of coal. J. Mol. Liq. 329, 115518 (2021).
Wang, Z., Lin, B. Q., Yang, W., Li, H. & Lin, M. H. Fracture and pore development law of coal under organic solvent erosion. Fuel 307, 121815 (2022).
Xue, H. F., Li, X. M., Zhang, Y. & Yao, T. Study on damage evolution characteristics of coal samples under acidic water environment. Min. Res. Dev. 02, 90–93 (2022). in Chinese.
Li, X. L., Liu, Z. T., Feng, X. J., Zhang, H. J. & Feng, J. J. Effects of acid sulfate and chloride ion on the pore structure and mechanical properties of sandstone under dynamic loading. Rock Mech. Rock Eng. 54, 6105–6121 (2021).
Zhang, X. G., Ranjith, P. G., Lu, Y. Y. & Ranathunga, A. S. Experimental investigation of the influence of CO2 and water adsorption on mechanics of coal under confining pressure. Int. J. Coal Geol. 209, 117–129 (2019).
Zhang, Y. Y., Shao, Z. S., Wei, W. & Qiao, R. J. PFC simulation of crack evolution and energy conversion during basalt failure process. J. Geophys. Eng. 16, 639–651 (2019).
Wu, T. H., Gao, Y. T., Zhou, Y. & Li, J. W. Experimental and numerical study on the interaction between holes and fissures in rock-like materials under uniaxial compression. Theoret. Appl. Fract. Mech. 106, 102488 (2020).
Li, R. et al. Numerical simulation of hydraulic fracturing for low permeability reservoirs based on particle flow code-discrete element method. Petrol. Sci. Bull. 04, 576–583 (2022). in Chinese.
Zhang, L., Ren, T., Li, X. C. & Tan, L. H. Acoustic emission, damage and cracking evolution of intact coal under compressive loads: Experimental and discrete element modelling. Eng. Fract. Mech. 252, 107690 (2021).
Song, Y. J. et al. Meso-fracture evolution characteristics of freeze-thawed sandstone based on discrete element method simulation. Rock. Soil. Mech. 12, 3602–3616 (2023). in Chinese.
Liu, X. W. et al. Experimental and numerical study on failure characteristics and mechanism of coal under different quasi-static loading rates. Theoret. Appl. Fract. Mech. 121, 103478 (2022).
Zhang, T., Yu, L. Y., Su, H. J., Zhang, Q. & Chai, S. B. Experimental and numerical investigations on the tensile mechanical behavior of marbles containing dynamic damage. Int. J. Min. Sci. Technol. 32, 89–102 (2022).
Xu, D., Gao, M. S., Zhao, Y. C., He, Y. L. & Yu, X. Study on the mechanical properties of coal weakened by acidic and alkaline solutions. Adv. Civil Eng. 2020, 1–15 (2020).
Skousen, J. G., Ziemkiewicz, P. F. & McDonald, L. M. Acid mine drainage formation, control and treatment: approaches and strategies. Extr. Ind. Soc. 6 (1), 241–249 (2019).
Lai, J. et al. Effect of acid–rock reaction on the microstructure and mechanical property of tight limestone. Rock Mech. Rock Eng. 55, 35–49 (2022).
Shen, J. Y., Zhan, S. X., Karakus, M. & Zuo, J. P. Effects of flaw width on cracking behavior of single-flawed rock specimens. Bull. Eng. Geol. Environ. 80, 1701–1711 (2021).
Acknowledgements
This research was financially supported by the State Key Laboratory of Coal and CBM Co-mining opening fund (No. 2022KF10), the Fundamental Research Program of Shanxi Province (No. 202303021222121).
Author information
Authors and Affiliations
Contributions
methodology, W.H., X.Z.; writing—original draft and editing, W.H and X.Z; data curation, X.Z., Q.Y.; validation, Z.L., Q.Y.; All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Han, W., Zheng, X., Li, Z. et al. Crack evolution and energy conversion characteristics of coal under acid corrosion conditions. Sci Rep 15, 10911 (2025). https://doi.org/10.1038/s41598-025-96252-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-96252-8