Abstract
Diet patterns and air pollution have been recognized as factors that affect the onset of chronic obstructive pulmonary disease (COPD). However, the combined effects of these factors on COPD incidence remain unclear. On the basis of a large prospective cohort from UK Biobank, we explored the independent and joint effects of diet patterns and air pollution on the risk of COPD. A total of 314,226 participants from UK Biobank, who were recruited between 2006 and 2010, were included in this study. The participants were randomly divided into the training (70%) and validation (30%) sets, with the air pollution score calculated using the training set. A diet pattern score was constructed in accordance with the intake of fruits, vegetables, fish, processed meat, unprocessed red meat, whole grains, and refined grains. Meanwhile, an air pollution score was calculated by determining the exposure levels to four components: fine particulate matter (PM2.5), PM2.5–10, NO2, and NOx. Cox proportional hazard models with hazard ratios (HRs) and 95% confidence intervals (CIs) were applied to examine the independent effects of diet patterns and air pollution on COPD incidence. Interaction analyses at the multiplicative and additive scales were also applied to assess the joint effects of diet patterns and air pollution on COPD risk. The above analyses were performed on both the training and validation datasets to verify the robustness of the results. In the training set, 6,696 participants were diagnosed with COPD during a median follow-up duration of 13.9 years. In the fully adjusted model, participants with intermediate and low diet pattern scores had higher COPD risk compared with those with high diet pattern score, with HRs being 1.11 (95% CI: 1.05 to 1.17) and 1.31 (95% CI: 1.21 to 1.42), respectively. Moreover, heavier exposure to air pollution was associated with higher COPD risk, with HRs being 1.06 (95% CI: 1.03, 1.14) and 1.13 (95% CI: 1.05, 1.22) for quintiles Q4 and Q5, respectively, compared with that for quintile Q1. Similar results were observed in the validation dataset. Analyses revealed limited evidence of significant joint effects between diet pattern and air pollution scores on either the multiplicative or additive scales, both in the training and validation sets. Targeted intervention on modifiable diet patterns may offset the adverse effect of air pollution exposure on COPD risk.
Similar content being viewed by others
Introduction
Chronic obstructive pulmonary disease (COPD), characterized by the chronic inflammation of the airways and lung parenchyma with progressive and irreversible airflow restriction, is a common chronic respiratory disease that encompasses a group of disorders, including small airway obstruction, emphysema, and bronchitis1. In accordance with the estimates of the study Global Burden of Disease 2019, COPD is one of the leading causes of disease burden worldwide, rising in rank from 11 th in 1990 to 6 th in 2019 in terms of disability-adjusted life year (DALY) among 369 diseases2. COPD led to 3.28 million deaths and 74.43 million DALYs in 20193. The economic burden of COPD due to hospitalizations and recurrent exacerbations is enormous, accounting for $18 billion in annual direct costs in USA and €38.7 billion in Europe. COPD also leads to significant productivity losses and reduced quality of life. These challenges highlight the urgent need for effective prevention strategies and improved management to alleviate its economic and clinical burden4.
In recent years, the understanding of COPD aetiology has significantly advanced, emphasizing its multifactorial nature and the complexity of its risk factors. While smoking and air pollution remain primary causes, research has identified a broader range of exposures, including genetic predispositions, early-life environmental factors, and socioeconomic determinants. However, the COVID- 19 pandemic has exposed the vulnerabilities of COPD patients, highlighting the need for future research to enhance early interventions and precision medicine within the context of current treatment and prevention strategies5.
At present, confirmed risk factors for COPD mainly include genetics, chronic asthma, smoking, diet patterns, occupational exposure, and exposure to air pollution6. Among them, diet patterns have been recognized as a modifiable risk factor for the development and progression of chronic diseases7, and cumulative evidence has suggested its role in obstructive pulmonary diseases, including COPD8,9,10. Over the past few decades, changes in diet patterns, such as low consumption of fruits, vegetables, whole grains, and fish, and increased consumption of processed and refined foods, have been found to be associated with an increasing risk of COPD, mostly in developing countries11. Healthy dietary patterns have been shown as an effective measure for preventing COPD, primarily due to the antioxidant and anti-inflammatory effects of certain nutrients in the diet, such as vitamins and polyunsaturated fatty acids11,12. Moreover, a series of studies has revealed the negative effects of long-term exposure to air pollution on the risk of COPD, particularly fine particulate matter (PM2.5) and NO213,14,15. The effects of air pollution on respiratory diseases can be mediated through a variety of biological pathways, including inflammatory and oxidative stress pathways16. However, only 12% of the world’s population lives in cities that meet the air quality standards of the World Health Organization (WHO)17. In view of the magnitude of the air pollution problem and the heavy global burden of COPD, any link between air pollution and COPD must be firmly established. Notably, previous studies that evaluated the relationship between air pollution and the incidence of COPD have focused on individual air pollutants, such as PM2.5, NO2, or NOx. In reality, ambient air pollution consists of a mixture of particulate matter and gaseous pollutants, whose combined effects on human health may differ from that of individual air pollutants. An air pollution score was proposed by Wang et al. to assess combined exposure to multiple air pollutants, including PM2.5, coarse particulate matter (PM10), PM2.5–10, NOx, and NO2, and it has shown that combined exposure is significantly associated with several chronic diseases, including heart failure and diabetes18,19. Compared with research on single air pollutants, the air pollution score reflects a more comprehensive measure of the effect of joint exposure to various air pollutants on human health, and thus, investigating the association between combined exposure to multiple air pollutants and the incidence of COPD becomes possible.
Recent research on the occurrence and development of COPD has largely focused on the separate effects of air pollutants or dietary patterns. However, based on the similarities in biological mechanisms, a joint effect between air pollution and diet patterns may exist on COPD incidence. A recent cohort study showed that Mediterranean dietary patterns can significantly reduce the risk of cardiovascular and cerebrovascular diseases caused by long-term exposure to PM2.5 and NO2, in which reduced inflammation may be the underlying mechanism20. However, the joint effect between air pollution exposure and dietary patterns on COPD remains unknown. Therefore, we conducted this study on the basis of a large UK cohort to explore the joint effect between diet and air pollution on COPD incidence. We aim to provide more reliable clues for the prevention and reduction of COPD burden.
Methods
Study population
Our study applied data from the UK Biobank cohort study (application number: 97101). The UK Biobank protocol is available online (http://www.ukbiobank.ac.uk/wp-content/uploads/2011/11/UK-Biobank-Protocol.pdf). UK Biobank is a continuous longitudinal cohort study that included over 500,000 participants between 2006 and 2010, with a baseline age of 39–73 years old. The study was mostly conducted in urban areas in England, Scotland, and Wales through the UK National Register of Health Services13. All the participants completed a series of baseline assessments, including social demographic factors, socioeconomic factors, lifestyle and behavioral factors, medication and surgical history, and biological samples, such as blood and urine samples21. The UK Biobank study has been approved by the North West Multi-centre Research Ethics Committee (11/MW/0382) and has obtained informed consent from all the participants22.
The inclusion and exclusion procedures of the study population in the present work are shown in Fig. 1. Among the 502,379 participants in UK Biobank, 17,149 with COPD diagnosis at the baseline were excluded. We further excluded 170,984 participants due to the lack of information on diet pattern, air pollution, and covariates, leaving 314,226 participants for the final analysis. All included participants were randomly split into training (70%) and validation (30%) sets, following a 7:3 ratio. The training set was used to construct the air pollution score and perform the primary analyses, while the validation set was used to validate the results.
Diet pattern score
Within the UK Biobank framework, the diet habits of participants were collected through the validated Oxford WbeQ online questionnaires. The Oxford WbeQ included 206 food types and 32 different beverages consumed within the previous 24 h23. Participants completed their first 24-h dietary assessment in at recruitment, followed by up to four further assessments until June 2013. For the repeated measurements, we averaged the values to reflect the long-term diet pattern and reduce the measurement error bias in assessment of diet-disease associations.
The questions used in our study were those that focus on the intake of fruits, vegetables, fish, processed meat, unprocessed red meat, whole grains, and refined grains, which comprise the diet score developed recently by Lourida et al. to reflect the comprehensive diet pattern24. For each participant, the overall diet score was calculated by summing the points for each of the seven food components (Table S1). If the cutoff value of each item is met, then it will be scored as 1 point; otherwise, it will be scored as 0 point. A higher diet score indicates a healthier diet pattern, with a range of 0–7 points. To assess whether diet was associated with COPD, three diet score categories were defined: low (0–1 point), intermediate (2–5 points), and high (6–7 points) categories.
Air pollution score
The annual average concentrations of PM2.5, PM2.5–10, NO2, and NOxwere estimated with a land use regression (LUR) model developed by the European Study of Cohorts for Air Pollution Effects18,25. A series of predictive variables derived from geographic information systems, such as traffic intensity, population, topography, and land use, was used to calculate spatial changes in air pollution concentrations at individual residential addresses at the baseline through the LUR model. In the training set, a weighted air pollution score was created by adding the concentrations of the four air pollutants, weighted by the βcoefficients of multivariable adjusted Cox proportional hazard regression models for COPD18 (Table S2). The equation was as follows: air pollution score = (β[PM2.5] × PM2.5 + β[PM2.5–10] × PM2.5–10 + β[NOX] × NOX + β[NO2] × NO2) × (4/sum of the β coefficients). The air pollution score ranged from 23.23 to 191.99. A higher score indicates higher exposure to ambient air pollution. The participants were divided into five groups in accordance with the quintiles of the air pollution score.
Outcome ascertainment
COPD diagnoses were coded as J40–J44 in accordance with the International Classification of Diseases version 10. The participants were followed up from enrollment until the first occurrence of COPD, death, or the end date of follow-up, whichever came first. COPD cases were identified using self-reported information, primary care data, and admission data through a link to the UK National Health Service Register26.
Covariates
We included demographic characteristics (age, sex, ethnicity, and body mass index [BMI]), socioeconomic factors (Townsend deprivation index [TDI], average total household income before tax, qualifications, and employment status), and lifestyle factors (smoking status, secondhand smoke exposure, sleep duration, and regular physical activity) as potential confounders. Age was calculated from date of birth and date of baseline assessment. Ethnic background was classified as White, Black, Asian, and others. The TDI is an indicator that reflects socioeconomic status, which is calculated on the basis of four census variables: unemployment, non-car ownership, non-house ownership, and household overcrowding27. Self-reported smoking status was categorized into never, previous, and current smokers. Exposure to secondhand smoke was classified as “yes” and “no”. Physical activity was assessed using the number of days per week that included at least 10 min of moderate or vigorous physical activity. During the baseline assessment center visit, trained nurses measured height and weight, and BMI (kg/m2) was calculated by dividing weight (kg) by the square of height (m).
Statistical analysis
Continuous variables with normal distribution were presented as mean [standard deviation (SD)], and the independent-samples t-test was used to test the difference between groups. Continuous variables with non-normal distribution were presented as median and interquartile range (IQR), and the Kruskal–Wallis test was used to examine the between-group difference. Categorical variables were presented as percentages, and the chi-square test was adopted to test the difference between groups.
Cox proportional hazard regression models with hazard ratios (HRs) and 95% confidence intervals (CIs) were applied to analyze the associations of diet patterns and air pollution with COPD incidence. This study developed a five-model analysis protocol by adding various covariates (e.g., demographic characteristics, socioeconomic factors, and lifestyle factors) incrementally. First, the independent associations of diet patterns or air pollution with COPD incidence were investigated by building Model 0 (crude model). Model 1 was adjusted for demographic characteristics, including age, sex, BMI, and ethnicity. In addition to the covariates in Model 1, Model 2 was adjusted for socioeconomic factors, including TDI, average total household income before tax, qualifications, and employment status. Model 3 was further adjusted for lifestyle factors, including smoking status, secondhand smoke exposure, sleep status, and physical activity. Finally, Model 4 was further mutually adjusted by diet pattern score (effect estimation of air pollution) or air pollution (effect estimation of diet patterns). Subgroup analyses stratified by the five quintiles of the air pollution score or the three levels of the diet pattern score (low, intermediate, and high) were also conducted based on Model 3. Restricted cubic spline for the multivariate Cox regression models was applied to examine the linear relationship between diet quality sores or air pollution scores and the risk of COPD.
On the basis of the Cox proportional hazard regression model, our study further investigated the potential joint effects between diet patterns and air pollution exposure at the additive and multiplicative scales. Additive joint effect evaluates whether the combined effect of two exposures exceeds the sum of their individual effects on absolute risk, while multiplicative joint effect assesses whether the combined effect exceeds the product of their individual effects on relative risk28. These two scales have distinct interpretative goals and statistical measures, providing complementary insights into the nature of joint effects. For additive joint effect, a new term with 15 categories (3 × 5) was created based on the five quintiles of air pollutant score and the three levels of diet pattern score (low, intermediate, and high) by using participants with the first quintile of the air pollution score and the high diet pattern score level as the reference group. Moreover, the relative excess risks (RERIs), along with their corresponding 95% CIs and Pvalues, were calculated to comprehensively assess the additive joint effects. RERI measures the combined excess risk in both exposed groups due to the joint effects. An RERI of 0 indicates no additive joint effects (i.e., the combined excess risk is the sum of the individual excess risks, and the excess risk is calculated as 1). An RERI greater than 0 with its 95% CI > 0 indicates positive interaction (i.e., the combined excess risk is greater than the sum of the individual excess risks), while an RERI less than 0 with its 95% CI not exceeding 0 indicates negative interaction (i.e., the combined excess risk is less than the sum of the individual excess risks)29. At the multiplicative scale, we created a product term between diet pattern score categories and air pollution score subgroups. Likelihood tests were applied to test the significance of the interaction term by comparing the model with and without the interaction term. Additionally, we took air pollution score and dietary score as continuous variables, added their product terms to the Cox regression model, and used the likelihood ratio test to evaluate the significance of their joint effect30.
Our study performed a series of sensitivity analyses to examine the robustness of our results. First, we excluded events that occurred within the first 5 years of follow-up to reduce potential reverse causation31. Second, we did not consider death as censored in our primary analysis but rather as a competing risk, and then performed Fine–Gray hazards regression model analysis to validate the results32.
All the analyses were conducted using R 4.3.3. A two-sided P value of < 0.05 was considered statistically significant.
Result
Baseline characteristics of participants
The study population comprised 219,958 and 94,268 individuals in the training and validation sets, respectively. No significant differences in baseline characteristics were observed between the training and validation sets (Table S3). As shown in Table 1, the participants in the training set had a mean age of 56.1 ± 8.1 years at baseline. Females (52.8%) outnumbered the males, and over 90% of the participants were of White ethnicity. During the median follow-up duration of 13.9 years, 6,696 participants were diagnosed with COPD, including 3,761 males and 2,935 females. About 50% of the participants were classified under intermediate diet score. The average air pollution concentration at baseline was higher among COPD cases than among non-COPD cases. In the validation set, significant difference of demographic features, diet score, and air pollution exposure were also observed between COPD and non-COPD case (Table S4).
Association between diet patterns and COPD incidence
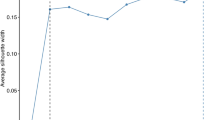
Table 2 presents the associations between diet pattern score and COPD risk. In the training set, the crude model revealed that participants with intermediate and low diet scores had a higher risk of COPD than those with high diet score, with HRs being 1.16 (95% CI: 1.10 to 1.23) and 1.40 (95% CI: 1.30 to 1.52), respectively. After adjustments for demographic characteristics, socioeconomic factors, and lifestyle factors, the relationships between diet pattern score and the onset of COPD remained significant with HR being 1.11 (95% CI: 1.05 to 1.17) for participants with intermediate diet score group and 1.31 (95% CI: 1.21 to 1.42) for those with low diet score group in Model 3. The negative association between diet score and COPD risk remained strong after adjusting for demographic characteristics, socioeconomic status, lifestyle, and air pollution (Model 4) with HRs being 1.11 (95% CI: 1.05 to 1.17) and 1.31 (95% CI: 1.21 to 1.42) for individuals with intermediate and low diet pattern scores compared with those with high diet score, respectively. The results derived from the validation set further validated these associations (Table 2). The association of diet score and COPD risk remained significant in participants exposed to different air pollution levels in both training and validation sets (Table 3). The cumulative incidence rate curves of COPD grouped in accordance with diet score categories are shown in Fig. 2A, indicating that a lower diet pattern score is associated with a higher cumulative incidence rate of COPD. As shown in Fig. 3A, a linear relationship was observed between diet pattern score and COPD risk with a gradual decrease in the incidence of COPD as diet pattern score increases (P for nonlinearity = 0.333). Similar trends were also observed in the validation set (Figs. 2B and 3B).
Dose-response relationship between COPD and diet patterns score (A: training set; B: validation set) or air pollution score (C: training set; D: validation set). Restricted cubic spline for the multivariate Cox regression models was applied by adjusting for age, sex, ethnicity, Townsend deprivation index, average total household income, qualifications, employment status, secondhand smoke exposure, BMI. HR, hazard ratio; CI, confidence interval.
Association between air pollution and COPD incidence
As indicated in Table 2, a positive association of air pollution exposure with COPD incidence was observed in both the training and validation sets, which remained significant after adjustment for various covariates. In Model 3 of the training set, the HRs of quintiles Q4 and Q5 of air pollution score was 1.06 (95% CI: 1.03, 1.14) and 1.13 (95% CI: 1.05, 1.22), respectively, compared with the quintile Q1. Similar results were achieved for Model 4 after additionally adjusting by diet pattern scores on the basis of Model 3. The positive association between air pollution score and COPD risk were also observed in the validation set (Table 2). When stratified in accordance with diet pattern score categories, the positive associations remained significant in the low and intermediate diet score groups, but not in the high diet pattern score groups (Table 4). The cumulative incidence rate curves of COPD grouped by air pollution score are shown in Fig. 2C, indicating that a higher pollution score is associated with a higher risk of COPD. As depicted in Fig. 3C, a linear relationship was observed between air pollution score and COPD risk, with a higher dosage of air pollution exposure resulting in a higher risk of COPD (P for nonlinearity = 0.576). All of the above results are based on the training set. Similar trends were also observed in the validation set (Figs. 2D and 3D).
Joint effect between diet patterns and air pollution on COPD incidence
Table 5 provides the joint effects of diet patterns and exposure to air pollution on COPD incidence at the additive scale. By using participants with the highest level of diet pattern score and the lowest level of air pollution score as reference, participants with lower diet pattern score or higher air pollution score generally exhibited an increased risk of COPD incidence. Compared with the reference group, the highest risk of developing COPD was observed among participants with the lowest level of diet pattern score and the highest air pollution score, with HR being 1.53 (95% CI: 1.29, 1.80). However, no evidence of the additive joint effect between diet patterns and air pollution on the onset of COPD was found, because the RERIs were not statistically significant. These findings were also consistent across the validation set (Table 5). When diet pattern score and air pollution score were treated as continuous variables, their additive joint effect was also not significant (Table 5).
The results of the multiplicative joint effects between diet patterns and air pollution on the risk of COPD are presented in Table 6. Whether air pollution score and diet pattern score were treated as categorical or continuous variables, their multiplicative joint effect on COPD risk were not significant.
Sensitivity analyses
After excluding the participants who developed COPD within 5 years of enrollment, the negative correlations between diet pattern score and COPD incidence and the positive associations between air pollution score and COPD risk remained stable (Table S5). As shown in Tables S6 and S7, when sensitivity analyses were performed by excluding all the participants who developed COPD within 5 years of enrollment, the results of the additive and multiplicative interactions of diet patterns and air pollution on the risk of COPD were not substantially altered. Moreover, the independent effect of diet patterns or air pollution on COPD incidence remained robust under the framework of the competing risk model (Table S8).
Discussion
In this study, we explored the independent and comprehensive effects of diet patterns and air pollution on the pathogenesis of COPD. Regardless of the level of air pollution score, a high diet pattern score was significantly associated with a decreased risk of COPD. Moreover, a positive correlation was observed between air pollution exposure and COPD incidence, but this association varies across dietary patterns. However, limited evidence of the interaction between diet patterns and air pollution exposure on the development of COPD was observed at the additive and multiplicative scales. Although our primary objective was to study interactions, the non-significant likelihood ratio test suggests that the joint effect of diet and air pollution may not deviate significantly from their independent effects.
Previous epidemiological studies have associated the onset of COPD with long-term exposure to particulate matter, particularly PM2.5 and PM10. A longitudinal cohort study found that compared with the participants exposed to the first quartile of PM2.5, participants exposed to the second, third, and fourth quartiles of PM2.5exhibited an increased risk of COPD by 23%, 30%, and 39%, respectively33. According to a cohort study from the United States, a 2 micro/m3 increase in long-term PM2.5exposure was associated with an 11% increase in emphysema prevalence over a 10-year period, as assessed by imaging34. Moreover, nitrogen oxides, particularly NO2, play an important role in the occurrence and development of COPD. In accordance with a study conducted in Europe, the risk of COPD increases by 11% for every 10 micro/m3 increase in NO235. However, current studies have focused on assessing the effect of a single air pollutant, and only a few studies have comprehensively evaluated the combined effects of multiple air pollutants on COPD risk. The combined effects of air pollution on human health are of increasing concern because various pollutants may originate from the same emission sources36. Moreover, similarity exists in the mechanism of different air pollutants on the lungs. Apparently, air pollution is more likely to remain in the lungs and translocate to the lung interstitium; this condition may generate free radicals on the surface, leading to oxidative stress and the activation of pro-inflammatory gene transcription factors in macrophages and epithelial cells and triggering greater inflammation and fibrosis37,38. Therefore, our study referenced the air pollution score developed by Wang et al.18, which included PM2.5, PM2.5–10, NO2, and NOX. This score made comprehensively assessing the association between long-term exposure to multiple air pollutants and COPD incidence feasible. Wang et al.18 found that the combined effects of multiple air pollution components on the risk of heart failure were significantly stronger than that of an individual air pollutant. In addition, a study from South Korea formulated an air pollution index by incorporating PM10, NO2, SO2, and ozone and found that the effect of this combined index on the risk of all-cause mortality was considerably stronger than that of a single air pollutant39. In our study, a high air pollution score was associated with an increased risk of COPD. Given the widespread exposure of people to many different air pollutants in their daily lives, the application of an air pollutant score that involves multiple air pollutants may better explain the disease burden caused by air pollution on COPD and enable policymakers to provide comprehensive guidance on disease prevention and management strategies.
Our study also constructed a diet pattern score to assess the relationship between diet patterns and COPD incidence. It determined that a high diet patterns score was significantly associated with a decreased risk of COPD. Two prospective cohort studies in the US found that prudent diet patterns with a high intake of fruits, vegetables, and whole grains were significantly associated with a reduced risk of COPD; by contrast, diet patterns with high intake of refined grains, processed meat, red meat, desserts, candies, and French fries were associated with an increased risk of COPD40,41. Antioxidants and anti-inflammatory components in food may be the basis for healthy diet patterns to reduced COPD risk. Antioxidant vitamins in fruits and vegetables, such as vitamins A, C, D, E, and β-carotene, contribute to the proliferation and maintenance of respiratory epithelial cells, alleviate oxidative damage by eliminating various free radicals and oxidants, and regulate gene expression and mRNA translation, improving lung function and reducing the risk of COPD42,43. Moreover, the protective effects of whole grains, fruits, and vegetables on the lungs are partially attributed to the antioxidant and anti-inflammatory effects of fibers44. An increased fiber intake is associated with low levels of C-reactive protein and pro-inflammatory cytokines (e.g., tumor necrosis factor alpha and interleukin 6), and a high level of adiponectin, which is an insulin-sensitized adipocyte factor with anti-inflammatory properties in the serum45. Previous studies have observed that a high intake of omega- 3 polyunsaturated fatty acids (n- 3 PUFA) is associated with a low risk of COPD46,47. Eicosapentaenoic acid and docosahexaenoic acid are polyunsaturated fatty acids of the n- 3 family, whose lipid metabolites are potent vasodilators and bronchodilators with anti-inflammatory properties48. By contrast, saturated fatty acids found in red meat and processed meat are linked to airway inflammation and decreased lung function due to their pro-inflammatory properties49. In addition, processed meat contains a high level of nitrite. The reactive nitrogen species produced by nitrite during metabolism may promote and amplify the inflammatory process of the airway and lung parenchyma, and long-term persistence of nitrosative stress may lead to the progressive deterioration of lung functions50. Processed meat also contains a high amount of sodium, which may increase bronchial hyperresponsiveness and trigger inflammation51. In summary, our study suggests that healthy dietary patterns characterized by a high intake of fruits, vegetables, and fish, and a low intake of red meat, and processed meat, are significantly associated with a declined risk of COPD. Given that diet patterns are a modifiable risk factor, people should be encouraged to adopt healthy diet patterns, providing a simple and feasible public health strategy to reduce the disease burden of COPD.
The results of this study indicated that the negative association between dietary patterns and COPD remained stable regardless of air pollution levels. Oxidative stress has been identified as one of the most important mechanisms of air pollution that affects health35. Air pollutants are powerful oxidants that may induce excessive production of reactive oxygen species (ROS) in cells, trigger oxidative stress, and lead to adverse health effects on the lungs, including changes in cell permeability, increased airway hyperactivity, decreased lung functions, and elevated levels of lung inflammation and damage37. However, antioxidants in food can protect against the adverse health effects associated with exposure to air pollution. For example, n- 3 PUFA, which is abundant in fish oil and soy oil, can reduce ROS production and mitigate oxidative stress responses caused by stimuli, including air pollution, due to its anti-inflammatory properties52. A randomized controlled trial suggested that supplementation with n- 3 PUFA may modulate oxidative response to PM2.5exposure53. In addition, sulforaphane, a molecule in cruciferous vegetables, exhibits anti-inflammatory and antioxidant properties by affecting the expression of oxidative stress genes, and thus, it may protect against the harmful effects of air pollutants54,55. Moreover, the antioxidant vitamins C and E may protect the lungs from inflammation caused by short-term exposure to air pollution56. Overall, diet patterns and air pollution play a role in the pathogenesis of COPD through similar mechanisms, but the protective effect of diet patterns on COPD is not affected by air pollution, but rather, it offsets the adverse effects of air pollution on COPD to some extent. Furthermore, a dose-response relationship between air pollution exposure and COPD risk was observed to a certain degree; that is, the more severe the air pollution exposure, the higher the COPD risk. However, this association was insignificant in people with low diet pattern score, suggesting that high quality diet patterns may reverse the effect of air pollution on COPD risk to a certain extent. If a healthy diet pattern is adopted, then the effect of air pollution exposure on the onset of COPD may be reduced. Moreover, the current study did not observe any interaction between diet patterns and air pollution on the onset of COPD, which is consistent with the findings or Whyand et al.57. This previous study suggested that some nutrients, such as carotenoids and vitamins D and E, contribute to protection against pollution damage that can trigger COPD, but no evidence was found on the protective role of a Mediterranean diet against air pollution57. Although certain nutrients in food have been shown to protect against the harmful effects of air pollutants on COPD, the food and nutrients included in diet patterns are numerous and complex, and thus, whether a healthy dietary pattern can protect against the adverse effects of air pollution on COPD remains unknown. Therefore, further research is necessary to better characterize the potential interaction between diet patterns and air pollution.
Air pollution is a major public health concern associated with a high incidence of respiratory diseases, including COPD, worldwide. Although reducing air pollution levels can effectively decrease the burden of COPD, fully achieving sustainable environmental emission reduction in the foreseeable future is unlikely due to population expansion and industrial development. In addition to reducing air pollution, the most effective and feasible route of intervention may be to recommend healthy dietary patterns to improve the inflammatory response and oxidative stress processes associated with COPD. In an environment with polluted air, adopting a healthy dietary pattern, particularly increasing the intake of antioxidant and anti-inflammatory nutrients, can alleviate oxidative stress and inflammatory processes caused by air pollution, providing enormous potential health and economic benefits for disease management through a non-pharmacological method. On one hand, people should be encouraged to change unhealthy dietary patterns and increase intake of antioxidant nutrients, particularly among susceptible populations, such as children, the elderly, and those exposed to smoking and occupational air pollution. On the other hand, further research is necessary to validate whether antioxidant dietary patterns are beneficial for reducing oxidative stress caused by air pollution, and thus, provide a useful complement to current management strategies for chronic inflammatory diseases, including COPD.
Strengths and limitations
The strengths of our study included a large sample size of participants from UK Biobank, abundant confounding factor information, prospective design, and long-term follow-up. However, several limitations should be considered. First, data from the UK Biobank cohort differ from the general population in terms of socio-demographic and health-related factors, and this condition may lead to biases in the estimates58. Second, our study excluded a large number of participants due to insufficient data. The missing data are not randomly lost and difficult to solve with imputation. Therefore, selection bias was inevitable, which may affect the generalizability of the results. Third, dietary information in UK Biobank was self-reported and may be subject to recall bias and misreporting. Fourth, dietary data were obtained from a simple questionnaire that includes main foods and food groups, which may not provide adequate information regarding energy intake and may not accurately reflect overall healthy eating behavior and long-term dietary habits. Fifth, the assessment of air pollution was based on the outdoor exposure of habitation, but not indoor exposure. Sixth, our study used the ESCAPE project’s LUR model for air pollution exposure assessment instead of empirical measurements. Although the LUR model has been widely validated and shown strong predictive performance, uncertainties remain due to input data quality, model assumptions, and potential extrapolation limitations. Seventh, due to insufficient data on the spatial mobility of participants during follow-up, we were unable to analyze the changes in air pollution exposure caused by address changes. Finally, the interaction coefficients in this study were calculated on a limited population sample, which generally had low statistical power. Thus, this study lacked sufficient power to determine whether the effects of air pollution and diet pattern were additive or multiplicative, emphasizing the need for cautious interpretation.
Conclusion
Long-term exposure to high levels of air pollution is independently associated with an increased risk of COPD incidence, but this association varies across dietary patterns. Moreover, healthy diet patterns contribute to a deceased risk of COPD regardless of the air pollution exposure level. Healthy diet patterns may reverse the harmful effects of air pollution on COPD risk. Given that diet pattern is a modifiable risk factor, targeted intervention on diet patterns may yield potential benefit on preventing COPD among the population with severe air pollution exposure. Although we adjusted for various confounding factors, including demographic, socioeconomic, and lifestyle factors, to better clarify the association between dietary patterns, air pollution, and COPD risk, the evidence for their joint effect remains limited. More rigorous epidemiological studies are needed to further explore these complex relationships.
Data availability
The data that support the findings of this study are available from UK Biobank but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the corresponding authors ([email protected]) upon reasonable request and with permission of UK Biobank.
References
HalpinDMG et al. Global initiative for the diagnosis, management, and prevention of chronic obstructive lung disease. The 2020 GOLD science committee report on COVID-19 and chronic obstructive pulmonary disease. Am. J. Respir Crit. Care Med. 203 (1), 24–36. https://doi.org/10.1164/rccm.202009-3533SO (2021).
GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet Lond. Engl. 396 (10258), 1204–1222. https://doi.org/10.1016/S0140-6736(20)30925-9 (2020).
Zou, J. et al. Distributions and trends of the global burden of COPD attributable to risk factors by SDI, age, and sex from 1990 to 2019: a systematic analysis of GBD 2019 data. Respir Res. 23 (1), 90. https://doi.org/10.1186/s12931-022-02011-y (2022).
López-Campos, J. L., Tan, W. & Soriano, J. B. Global burden of COPD. Respirology 21 (1), 14–23. https://doi.org/10.1111/resp.12660 (2016).
Christenson, S. A., Smith, B. M., Bafadhel, M. & Putcha, N. Chronic obstructive pulmonary disease. Lancet 399 (10342), 2227–2242. https://doi.org/10.1016/S0140-6736(22)00470-6 (2022).
Interventions for promoting physical activity in people with chronic obstructive pulmonary disease (COPD) - PubMed. Accessed September 23, (2024). https://pubmed.ncbi.nlm.nih.gov/32297320/
Schulze, M. B., Martínez-González, M. A., Fung, T. T., Lichtenstein, A. H. & Forouhi, N. G. Food based dietary patterns and chronic disease prevention. BMJ 361, k2396. https://doi.org/10.1136/bmj.k2396 (2018).
Seyedrezazadeh, E. et al. Dietary factors and risk of chronic obstructive pulmonary disease: a systemic review and Meta-Analysis. Tanaffos 18 (4), 294–309 (2019).
Zheng, P. F. et al. Dietary patterns and chronic obstructive pulmonary disease: A Meta-analysis. COPD 13 (4), 515–522. https://doi.org/10.3109/15412555.2015.1098606 (2016).
Vasankari, T. et al. Predictors of new airway Obstruction - An 11 year’s Population-Based Follow-Up study. COPD 16 (1), 45–50. https://doi.org/10.1080/15412555.2019.1576163 (2019).
Scoditti, E., Massaro, M., Garbarino, S. & Toraldo, D. M. Role of diet in chronic obstructive pulmonary disease prevention and treatment. Nutrients 11 (6), 1357. https://doi.org/10.3390/nu11061357 (2019).
van Iersel, L. E. J., Beijers, R. J. H. C. G., Gosker, H. R. & Schols, A. M. W. J. Nutrition as a modifiable factor in the onset and progression of pulmonary function impairment in COPD: a systematic review. Nutr. Rev. 80 (6), 1434–1444. https://doi.org/10.1093/nutrit/nuab077 (2022).
Doiron, D. et al. Air pollution, lung function and COPD: results from the population-based UK biobank study. Eur. Respir J. 54 (1), 1802140. https://doi.org/10.1183/13993003.02140-2018 (2019).
Hendryx, M., Luo, J., Chojenta, C. & Byles, J. E. Air pollution exposures from multiple point sources and risk of incident chronic obstructive pulmonary disease (COPD) and asthma. Environ. Res. 179 (Pt A), 108783. https://doi.org/10.1016/j.envres.2019.108783 (2019).
Yang, X., Zhang, T., Zhang, Y., Chen, H. & Sang, S. Global burden of COPD attributable to ambient PM2.5 in 204 countries and territories, 1990 to 2019: A systematic analysis for the global burden of disease study 2019. Sci. Total Environ. 796, 148819. https://doi.org/10.1016/j.scitotenv.2021.148819 (2021).
Kelly, F. J. & Fussell, J. C. Air pollution and airway disease. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 41 (8), 1059–1071. https://doi.org/10.1111/j.1365-2222.2011.03776.x (2011).
Berend, N. Contribution of air pollution to COPD and small airway dysfunction. Respirol. Carlton Vic. 21 (2), 237–244. https://doi.org/10.1111/resp.12644 (2016).
Wang, M. et al. Joint exposure to various ambient air pollutants and incident heart failure: a prospective analysis in UK biobank. Eur. Heart J. 42 (16), 1582–1591. https://doi.org/10.1093/eurheartj/ehaa1031 (2021).
Li, X. et al. Obesity and the relation between joint exposure to ambient air pollutants and incident type 2 diabetes: A cohort study in UK biobank. PLoS Med. 18 (8), e1003767. https://doi.org/10.1371/journal.pmed.1003767 (2021).
Lim, C. C. et al. Mediterranean diet and the association between air pollution and cardiovascular disease mortality risk. Circulation 139 (15), 1766–1775. https://doi.org/10.1161/CIRCULATIONAHA.118.035742 (2019).
Gao, M. et al. Associations between dietary patterns and the incidence of total and fatal cardiovascular disease and all-cause mortality in 116,806 individuals from the UK biobank: a prospective cohort study. BMC Med. 19 (1), 83. https://doi.org/10.1186/s12916-021-01958-x (2021).
Sudlow, C. et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 12 (3), e1001779. https://doi.org/10.1371/journal.pmed.1001779 (2015).
Bradbury, K. E., Murphy, N. & Key, T. J. Diet and colorectal cancer in UK biobank: a prospective study. Int. J. Epidemiol. 49 (1), 246–258. https://doi.org/10.1093/ije/dyz064 (2020).
Lourida, I. et al. Association of lifestyle and genetic risk with incidence of dementia. JAMA 322 (5), 430–437. https://doi.org/10.1001/jama.2019.9879 (2019).
Eeftens, M. et al. Development of land use regression models for PM(2.5), PM(2.5) absorbance, PM(10) and PM(coarse) in 20 European study areas; results of the ESCAPE project. Environ. Sci. Technol. 46 (20), 11195–11205. https://doi.org/10.1021/es301948k (2012).
Tikkanen, E., Gustafsson, S. & Ingelsson, E. Associations of fitness, physical activity, strength, and genetic risk with cardiovascular disease: longitudinal analyses in the UK biobank study. Circulation 137 (24), 2583–2591. https://doi.org/10.1161/CIRCULATIONAHA.117.032432 (2018).
Parra-Soto, S. et al. Association of meat, vegetarian, pescatarian and fish-poultry diets with risk of 19 cancer sites and all cancer: findings from the UK biobank prospective cohort study and meta-analysis. BMC Med. 20 (1), 79. https://doi.org/10.1186/s12916-022-02257-9 (2022).
de Mutsert, R., Jager, K. J., Zoccali, C. & Dekker, F. W. The effect of joint exposures: examining the presence of interaction. Kidney Int. 75 (7), 677–681. https://doi.org/10.1038/ki.2008.645 (2009).
Chu, H., Nie, L. & Cole, S. R. Estimating the relative excess risk due to interaction: a bayesian approach. Epidemiol. Camb. Mass. 22 (2), 242–248. https://doi.org/10.1097/EDE.0b013e318208750e (2011).
Sun, S. et al. Benefits of physical activity not affected by air pollution: a prospective cohort study. Int. J. Epidemiol. 49 (1), 142–152. https://doi.org/10.1093/ije/dyz184 (2020).
Zhang, Y. B. et al. Associations of healthy lifestyle and socioeconomic status with mortality and incident cardiovascular disease: two prospective cohort studies. BMJ 373, n604. https://doi.org/10.1136/bmj.n604 (2021).
Honigberg, M. C. et al. Association of premature natural and surgical menopause with incident cardiovascular disease. JAMA 322 (24), 2411–2421. https://doi.org/10.1001/jama.2019.19191 (2019).
Guo, C. et al. Effect of long-term exposure to fine particulate matter on lung function decline and risk of chronic obstructive pulmonary disease in Taiwan: a longitudinal, cohort study. Lancet Planet. Health. 2 (3), e114–e125. https://doi.org/10.1016/S2542-5196(18)30028-7 (2018).
Wang, M. et al. Association between Long-term exposure to ambient air pollution and change in quantitatively assessed emphysema and lung function. JAMA 322 (6), 546–556. https://doi.org/10.1001/jama.2019.10255 (2019).
Liu, S. et al. Long-term exposure to low-level air pollution and incidence of chronic obstructive pulmonary disease: the ELAPSE project. Environ. Int. 146, 106267. https://doi.org/10.1016/j.envint.2020.106267 (2021).
Dominici, F., Peng, R. D., Barr, C. D. & Bell, M. L. Protecting human health from air pollution: shifting from a single-pollutant to a multipollutant approach. Epidemiol. Camb. Mass. 21 (2), 187–194. https://doi.org/10.1097/EDE.0b013e3181cc86e8 (2010).
Kelly, F. J. Oxidative stress: its role in air pollution and adverse health effects. Occup. Environ. Med. 60 (8), 612–616. https://doi.org/10.1136/oem.60.8.612 (2003).
Persinger, R. L., Poynter, M. E., Ckless, K. & Janssen-Heininger, Y. M. W. Molecular mechanisms of nitrogen dioxide induced epithelial injury in the lung. Mol. Cell. Biochem. 234–235 (1–2), 71–80 (2002).
Hong, Y. C., Leem, J. H., Ha, E. H. & Christiani, D. C. PM(10) exposure, gaseous pollutants, and daily mortality in Inchon, South Korea. Environ. Health Perspect. 107 (11), 873–878. https://doi.org/10.1289/ehp.99107873 (1999).
Varraso, R., Fung, T. T., Hu, F. B., Willett, W. & Camargo, C. A. Prospective study of dietary patterns and chronic obstructive pulmonary disease among US men. Thorax 62 (9), 786–791. https://doi.org/10.1136/thx.2006.074534 (2007).
Varraso, R. et al. Prospective study of dietary patterns and chronic obstructive pulmonary disease among US women. Am. J. Clin. Nutr. 86 (2), 488–495. https://doi.org/10.1093/ajcn/86.2.488 (2007).
Meteran, H. et al. Self-reported intake of fruit and vegetables and risk of chronic obstructive pulmonary disease: A nation-wide twin study. Respir Med. 144, 16–21. https://doi.org/10.1016/j.rmed.2018.09.013 (2018).
Garcia-Larsen, V. et al. Dietary antioxidants and 10-year lung function decline in adults from the ECRHS survey. Eur. Respir J. 50 (6), 1602286. https://doi.org/10.1183/13993003.02286-2016 (2017).
Fonseca Wald, E. L. A., van den Borst, B., Gosker, H. R. & Schols, A. M. W. J. Dietary fibre and fatty acids in chronic obstructive pulmonary disease risk and progression: a systematic review. Respirol. Carlton Vic. 19 (2), 176–184. https://doi.org/10.1111/resp.12229 (2014).
Esposito, K. & Giugliano, D. Whole-grain intake cools down inflammation. Am. J. Clin. Nutr. 83 (6), 1440–1441. https://doi.org/10.1093/ajcn/83.6.1440 (2006). author reply 1441–1442.
Lemoine, S. C. M. et al. Omega-3 fatty acid intake and prevalent respiratory symptoms among U.S. Adults with COPD. BMC Pulm Med. 19 (1), 97. https://doi.org/10.1186/s12890-019-0852-4 (2019).
Lemoine, C. et al. Relationship between Omega-3 and Omega-6 fatty acid intake and chronic obstructive pulmonary disease morbidity. Ann. Am. Thorac. Soc. 17 (3), 378–381. https://doi.org/10.1513/AnnalsATS.201910-740RL (2020).
Fritsche, K. L. The science of fatty acids and inflammation. Adv. Nutr. Bethesda Md. 6 (3), 293S–301S. https://doi.org/10.3945/an.114.006940 (2015).
Wood, L. G., Attia, J., McElduff, P., McEvoy, M. & Gibson, P. G. Assessment of dietary fat intake and innate immune activation as risk factors for impaired lung function. Eur. J. Clin. Nutr. 64 (8), 818–825. https://doi.org/10.1038/ejcn.2010.68 (2010).
Ricciardolo, F. L. M., Di Stefano, A., Sabatini, F. & Folkerts, G. Reactive nitrogen species in the respiratory tract. Eur. J. Pharmacol. 533 (1–3), 240–252. https://doi.org/10.1016/j.ejphar.2005.12.057 (2006).
Yi, B. et al. Effects of dietary salt levels on monocytic cells and immune responses in healthy human subjects: a longitudinal study. Transl Res. J. Lab. Clin. Med. 166 (1), 103–110. https://doi.org/10.1016/j.trsl.2014.11.007 (2015).
De Caterina, R., Liao, J. K. & Libby, P. Fatty acid modulation of endothelial activation. Am. J. Clin. Nutr. 71 (1 Suppl), 213S–23S. https://doi.org/10.1093/ajcn/71.1.213S (2000).
Romieu, I. et al. The effect of supplementation with omega-3 polyunsaturated fatty acids on markers of oxidative stress in elderly exposed to PM(2.5). Environ. Health Perspect. 116 (9), 1237–1242. https://doi.org/10.1289/ehp.10578 (2008).
Riedl, M. A., Saxon, A. & Diaz-Sanchez, D. Oral Sulforaphane increases phase II antioxidant enzymes in the human upper airway. Clin. Immunol. Orlando Fla. 130 (3), 244–251. https://doi.org/10.1016/j.clim.2008.10.007 (2009).
de Figueiredo, S. M., Binda, N. S., Nogueira-Machado, J. A., Vieira-Filho, S. A. & Caligiorne, R. B. The antioxidant properties of organosulfur compounds (sulforaphane). Recent. Pat. Endocr. Metab. Immune Drug Discov. 9 (1), 24–39. https://doi.org/10.2174/1872214809666150505164138 (2015).
Tong, H. Dietary and Pharmacological intervention to mitigate the cardiopulmonary effects of air pollution toxicity. Biochim. Biophys. Acta. 1860 (12), 2891–2898. https://doi.org/10.1016/j.bbagen.2016.05.014 (2016).
Whyand, T., Hurst, J. R., Beckles, M. & Caplin, M. E. Pollution and respiratory disease: can diet or supplements help? A review. Respir Res. 19 (1), 79. https://doi.org/10.1186/s12931-018-0785-0 (2018).
Fry, A. et al. Comparison of sociodemographic and Health-Related characteristics of UK biobank participants with those of the general population. Am. J. Epidemiol. 186 (9), 1026–1034. https://doi.org/10.1093/aje/kwx246 (2017).
Acknowledgements
We are grateful to all the participants of UK Biobank and all the people involved in building the UK Biobank study.
Funding
This study was supported by Competitive Allocation Project of Science and Technology Development Special Funds of Zhanjiang (2022A01161), Affiliated Hospital of Guangdong Medical University Clinical Research Program (LCYJ2019B011), and High-level Talent Research Start-up Project (GCC2023004), and the Big Data Platform of Affiliated Hospital of Guangdong Medical University.
Author information
Authors and Affiliations
Contributions
Literature search: XFH, YBW, CXZ; Study design: ELY, ZHX and JFX; Data collection: ZHX, YBW and JW; Data analysis: ELY, ZHX and XFH; Model construction: CXZ, JFX and JW; Manuscript writing: ELY, JW, SRY, JYW. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The UK Biobank was approved by the North West Multi-center Research Ethics committee (11/NW/0382). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent for publication
Not applicable
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ye, E., Xu, Z., Hou, X. et al. Joint effects of air pollution and diet patterns on the risk of chronic obstructive pulmonary disease. Sci Rep 15, 13939 (2025). https://doi.org/10.1038/s41598-025-96603-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-96603-5