Abstract
Soil contaminated by Pb and Cd has aroused worldwide concern due to the environmental hazards they pose. The effects, mechanisms, and evaluation of Pb and Cd contaminated agricultural soil remediation by nonmetallic minerals are still poorly understood. In this study, solidification/stabilization experiments were used to screen nonmetallic mineral materials and optimize their dosages. Stabilization mechanisms of Pb and Cd by nonmetallic mineral materials were investigated by adsorption kinetics, X-ray diffraction spectroscopy, and Fourier transform infrared spectroscopy. The effectiveness of soil remediation was further confirmed through a pot experiment with pak choi (Brassica rapa L. subsp. chinensis), an important non-heading leafy vegetable. Results demonstrated that the SL composite (composed of 2.5% sepiolite and 1.5% limestone, with a total dosage of 4.0%) exhibits the optimal stabilization effect on soil contaminated with Pb and Cd. In soils with low, medium, and high contamination levels, SL reduced the bioavailability of Pb by 97.97%, 96.78%, and 95.82%, and the bioavailability of Cd by 92.96%, 91.76%, and 91.02%, respectively. SL surfaces are rich in hydroxyl (–OH) and carbonate (CO32−) groups, enabling binding with Pb and Cd ions to form hydroxide and carbonate precipitates. Such interactions suggest that chemical adsorption primarily drives Pb and Cd ion stabilization, reducing their bioavailability in soil. Pak choi grown in SL-remediated soil exhibited Pb and Cd contents compliant with China’s food safety standards. These findings further validate the bioavailability reduction rate as a suitable metric for evaluating the remediation effectiveness of heavy metal pollution in agricultural soils. This study provides a new strategy for evaluating the remediation efficiency of heavy metal-contaminated agricultural soil.
Similar content being viewed by others
Agricultural land is the primary base of food production and plays a vital role in affecting human survival and food security1. It is also an essential component for preserving ecological balance and biodiversity. However, with the acceleration of industrialization, the issue of heavy metal contamination in soil has intensified. In particular, industries such as mining and smelting release significant amounts of heavy metals into the environment during production2,3,4. In addition, the large-scale application of pesticides and chemical fertilizers in agricultural production results in the accumulation of heavy metals in the soil, which degrades soil function, reduces fertility, and ultimately lowers crop yields5,6. In the southwest region of China, which is a major hub for the non-ferrous metal industry, the issue of heavy metal contamination in local soil is particularly severe, especially the Pb and Cd content that significantly exceeds the standard, posing serious risks to the health of local residents and food production7,8. Excessive levels of Pb and Cd in the soil inhibit crop growth, reduce yields, and accumulate in crops, eventually entering the human body through the food chain and harming the digestive, nervous, and circulatory systems, kidneys, and other vital organs9,10,11. Heavy metals exist in various forms in soil, but not all forms pose the same level of risk. Among different forms of heavy metals, bioavailable heavy metals are particularly hazardous as they are readily absorbed by plants. These contaminated plants enter the human body through the food chain, posing a potential threat to human health. Therefore, the primary goal of remediation efforts is to reduce the bioavailability of heavy metals, thereby mitigating their adverse effects on human health and the ecological environment12,13,14,15.
Solidification and stabilization technology has proven effective in reducing the bioavailability and mobility of heavy metals in soil16,17. Nonmetallic minerals such as apatite18, limestone19, sepiolite20, attapulgite21, and zeolite22 are widely used in soil remediation due to their abundance, low cost, non-toxicity, and sustainability. These minerals effectively stabilize Pb and Cd, reducing their bioavailability in contaminated soils. Current research primarily focuses on the independent application and remediation effects of specific nonmetallic minerals in controlling soil Pb and Cd pollution, but there is a lack of in-depth exploration of their stabilization mechanisms23. Clarifying the adsorption mechanisms of heavy metals by stabilization materials not only aids in formulating more reasonable and cost-effective soil remediation strategies but also enhances remediation efficiency, reduces environmental risks, and promotes technological advancements24,25. Additionally, there is still a lack of recent scientific data on the potential synergistic effects of these minerals in agricultural soils with varying degrees of Pb and Cd contamination26.
The objective of stabilization remediation technologies is to reduce the mobility and bioavailability of heavy metals, while the total concentration of heavy metals remains unchanged during the remediation process. Therefore, the reduction rate of heavy metal bioavailability is commonly used as a metric to evaluate the effectiveness of stabilization remediation25. In addition, in the context of agricultural land remediation, it is crucial to further evaluate the effects of remediated soils on crop growth, including heavy metal accumulation in crops and crop yields27,28.
Revealing the effectiveness and mechanisms of nonmetallic minerals in treating Pb and Cd contaminated soil, along with systematically evaluating remediation outcomes, is of great significance for addressing heavy metal contamination in agricultural land. In this study, four nonmetallic minerals with distinct properties—apatite, sepiolite, limestone, and attapulgite—were selected as stabilizers for Pb and Cd-contaminated soil. An optimal stabilizer combination (SL) was identified through screening. The immobilization efficiency of SL in soils with varying levels of Pb and Cd contamination was evaluated using the DTPA extraction method and pot experiments. Additionally, the stabilization mechanisms of SL for Pb and Cd were investigated using characterization techniques. The purpose of this study is to verify the scientific validity and feasibility of using nonmetallic minerals for the remediation of Pb and Cd contaminated soil through rigorous experimental design and appropriate evaluation methods.
Materials and methods
Materials
The non-contaminated soil was obtained from agricultural land around the Gejiu smelting plant in Yunnan, China. The soils were characterized by the following basic properties: a pH of 7.63, organic matter content of 9.18 g/kg, cation exchange capacity of 6.7 cmol/kg, and total Pb and Cd contents of 73.35 mg/kg and 0.15 mg/kg, respectively. The available Pb and Cd contents were determined to be 9.55 mg/kg and 0.09 mg/kg, respectively. Air-dried soil passed through an 80-mesh sieve, and sieved soils were stored in sealed bags. Soil was mixed with a specific amount of CdCl2 and PbCl2 solution to prepare low-contaminated soil (Pb content: 500 mg/kg, Cd content: 1.5 mg/kg), moderately contaminated soil (Pb content: 800 mg/kg, Cd content: 3 mg/kg), and highly contaminated soil (Pb content: 1000 mg/kg, Cd content: 5 mg/kg). Highly contaminated soil was used to screen the optimal combination scheme, while soils with low, moderate, and high contamination levels were utilized to evaluate the stability of the selected scheme at different contamination concentrations. The contaminated soil was maintained at a moisture content of 30% in open containers and exposed to air for 90 days of incubation to simulate natural oxidative conditions and prepare for testing. Each group had three parallel experiments. Pak choi (Jinzhi 30) was provided by Zhongshu Seed Industry Technology (Beijing) Co, Ltd. nonmetallic minerals included attapulgite (AT), sepiolite (SP), apatite (AP), and limestone (LS). pH, along with Pb and Cd content in four mineral materials, is presented in Table 1.
Stabilization test
50 g of highly contaminated soil was mixed evenly with stabilizers (dosage 0, 0.5, 1, 1.5, 2, 2.5 wt %), placed in a plastic box, and maintained at a moisture content of 70%. Soil was incubated under open conditions for 30 days at 25℃ and then taken for subsequent testing. Each group had three parallel experiments. Results of material screening tests showed that optimum dosages of attapulgite (AT), sepiolite (SP), apatite (AP), and limestone (LS) were 2%, 2.5%, 2%, and 1.5%, respectively. Four nonmetallic mineral combination tests are shown in Table 2. The subsequent experimental process followed the same method as dosage experiments.
Sorption kinetics
The adsorption of Pb and Cd ions by the optimal combination of nonmetallic minerals was investigated under the following conditions: temperature of 25 °C, pH of 8.6, and agitation speed of 150 rpm. The experiments were conducted with varying ion concentrations and shaking time intervals of 1, 2, 3, 4, and 5 h. The stabilizer concentration was 4 g/L, and PbCl2 and CdCl2 solutions were used to adjust the ion concentration of Pb2+ and Cd2+. Following adsorption experiments, the suspension was centrifuged at 4000 rpm for 10 min and passed through a 0.45 μm filter. Filtrate was collected for Pb and Cd analysis. And the obtained data were fitted to five kinetic models.
Pot experiment
Soil samples with all contamination levels were mixed with the prepared optimal stabilizer (dosage 4 wt %) and the same dosage of nitrogen, phosphorus and potassium, while the contaminated soil without stabilizer was represented as a control group. The soil was placed in plastic pots and maintained at a moisture content of 70% for incubation. Pak choi was sown at different time points (0, 6, 12, 18, 24, and 30 days), with each batch grown for 30 days. Four seeds were planted per pot, and after stable growth, only one plant was retained per pot. After 30 days of growth, the mature plants were harvested to determine the Pb and Cd contents. Each experimental group was conducted in triplicate.
Analysis and testing methods
The bioavailability of Pb and Cd in the samples was detected according to “Extraction with buffered DTPA solution/inductively coupled plasma optical emission spectrometry” (HJ 804–2016, China)25. 10.0 g of soil samples was mixed with 20 mL DTPA extracting agent. Then, the mixture was oscillated at 200 rpm for 2 h under 20 °C. Following the oscillation, the suspension was centrifuged at 4000 rpm for 10 min. The suspension was centrifuged at 4000 rpm for 10 min and passed through a 0.45 μm filter. The filtrate was collected for Pb and Cd analysis.
The stabilization effects of nonmetallic minerals (including individual and combined applications) on Pb and Cd in soil were evaluated using the bioavailability reduction rate, and it can be calculated by using the following Eq. (1):
where P (%) is the reduction rate of bioavailability, C0 (mg/kg) is the initial content of bioavailability Pb and Cd in soil, and Ct (mg/kg) represents the bioavailability of Pb and Cd in soil after stabilization.
Bioconcentration factor (BCF) is the ratio of the content of heavy metals of a plant to that in the soil, and it can be calculated by using the following Eq. (4):
where BCF (%) is the biological factor, Ccrop (mg/kg) is the content of heavy metals in plant, and Csoil (mg/kg) is the content of heavy metals in soil.
Statistical analyses
All experimental data were expressed as mean ± standard deviation. Statistical significance was assessed using the F-test in analysis of variance (ANOVA). Duncan’s test was used to compare mean values between treatment groups and the control group, while the least significant difference (LSD) test was applied for pairwise comparisons among all treatment groups, with P < 0.05 considered statistically significant. Relationships between variables were evaluated using Pearson correlation analysis. Data analysis was performed using Excel 2010 and SPSS 22.0, while graphs and adsorption kinetic models were generated using Origin 2022 software.
Results
Effect of four types of nonmetallic minerals (apatite, sepiolite, limestone, and attapulgite) and dosage on Pb and Cd stabilization
This study investigated the effects of the type and dosage of nonmetallic minerals on the stabilization of Pb and Cd in contaminated soils, with a particular focus on evaluating the remediation efficiency of different stabilizers in reducing the bioavailability of Pb and Cd. The results demonstrated that all four nonmetallic minerals (AT: attapulgite, AP: apatite, SP: sepiolite, LS: limestone) exhibited significant stabilization performance for both Pb and Cd, with their stabilization efficiency ranked as LS > SP > AP > AT. As shown in Fig. 1, the bioavailability of Pb (Fig. 1a) and Cd (Fig. 1b) in the soil decreased significantly with increasing dosages of the stabilizers. Initially, the remediation efficiency improved markedly with higher dosages; however, beyond certain thresholds (AT-2%, AP-2%, SP-2.5%, and LS-1.5%), further increases in dosage provided only marginal improvements, leading to a stabilization in the reduction rates of bioavailability. Specifically, the reduction rates of Pb and Cd bioavailability stabilized at dosages of AT-2%, AP-2%, SP-2.5%, and LS-1.5%, indicating these as the optimal thresholds for remediation. Among these, LS-1.5% demonstrated the most pronounced stabilization effect, reducing the bioavailability of Pb from 299.72 to 102.52 mg/kg and that of Cd from 2.56 to 0.96 mg/kg, with statistically significant differences between the treated and control groups (p < 0.05). In comparison, at dosages of SP-2.5%, AP-2%, and AT-2%, the bioavailability of Pb was reduced to 182.11 mg/kg, 211.02 mg/kg, and 175.9 mg/kg, respectively, while that of Cd was reduced to 1.45 mg/kg, 1.96 mg/kg, and 1.7 mg/kg, respectively, with statistically significant differences between the treated and control groups (p < 0.05). These findings highlight that both the type and dosage of nonmetallic minerals significantly influence the stabilization efficiency of Pb and Cd, with LS demonstrating the most effective remediation performance at a relatively low dosage.
The bioavailability of Pb (a) and Cd (b) in soil after remediation with different species and doses of nonmetallic minerals (AT: Attapulgite, SP: Sepiolite, AP: Apatite, LS: Limestone; the control group (CK) was not treated with any mineral materials). The different letters indicate significant differences at p < 0.05 (Duncan’s test).
The bioavailability reduction rates of Pb and Cd after remediation with different species and doses of nonmetallic minerals are shown in Fig. 2. As shown in Fig. 2, all four nonmetallic minerals increased the bioavailability reduction rates of Pb and Cd. Specifically, the bioavailability reduction rates of Pb (Fig. 2a) and Cd (Fig. 2b) in each soil group increased with increasing stabilizer dosage. Similarly, in each group, the bioavailability reduction rates of Pb and Cd gradually stabilized at dosages of AT-2%, AP-2%, SP-2.5%, and LS-1.5%, respectively. In particular, LS-1.5% demonstrated the best performance, with the bioavailability reduction rates of Pb and Cd increasing to 65.79% and 62.44%, respectively, while SP-2.5%, AP-2%, and AT-2% resulted in bioavailability reduction rates for Pb of 39.24%, 29.59%, and 41.31%, respectively, and for Cd of 43.27%, 23.32%, and 30.36%, respectively. Finally, based on the stabilization effect and cost, the optimal dosages of attapulgite (AT), sepiolite (SP), apatite (AP), and limestone (LS) were determined as 2%, 2.5%, 2%, and 1.5%, respectively..
Effect of nonmetallic mineral combination scheme on the stability of Pb and Cd
Based on the results of the dosage experiments, this study further investigated the effects of different combinations of four nonmetallic minerals on the stabilization of Pb and Cd. The changes in the bioavailability and reduction rates of Pb and Cd following remediation with different stabilizer combinations are illustrated in Fig. 3. The results indicated that all six stabilizer combinations exhibited excellent stabilization performance for both Pb and Cd, with their effectiveness ranked as A4 > A1 > A5 > A3 > A2 > A6. Specifically, after remediation with the A4 scheme, the bioavailability of Pb (Fig. 3a) was significantly reduced from 299.72 to 12.43 mg/kg, resulting in a bioavailability reduction rate of 95.82%, with statistically significant differences compared to the control group (p < 0.05). After remediation with the A1 and A5 schemes, the reduction rates of Pb bioavailability were 88.34% and 80.77%, respectively, while the reduction rates for the remaining schemes were below 70%, with statistically significant differences compared to the control group (p < 0.05). Similarly, as shown in Fig. 3b, the stabilization effects of the stabilizer combinations on Cd were assessed. After remediation with the A4 scheme, the bioavailability of Cd (Fig. 3b) was significantly reduced from 2.56 to 0.23 mg/kg, resulting in a bioavailability reduction rate of 91.02%, with statistically significant differences compared to the control group (p < 0.05). After remediation with the A1 and A5 schemes, the reduction rates of Cd bioavailability were 83.18% and 74.96%, respectively, while the reduction rates for the remaining schemes were below 60%, with statistically significant differences compared to the control group (p < 0.05). In conclusion, based on both stabilization effectiveness and economic cost, the A4 scheme, composed of 2.5% sepiolite and 1.5% limestone (SL), was identified as the optimal combination.
The effects of various combinations of four nonmetallic minerals on the stabilization of Pb (a) and Cd (b) in soil (AT: Attapulgite, SP: Sepiolite, AP: Apatite, LS: Limestone; the control group (CK) was not treated with any mineral materials). The different letters indicate significant differences at p < 0.05 (Duncan’s test).
The bioavailability of Pb and Cd showed temporal changes during the 30-day remediation period using the 4% SL stabilizer (Fig. 4). Samples treated with 4% SL stabilizer for 0 days served as the control group. As shown in Fig. 4a, the bioavailability of Pb in soils with varying contamination levels showed significant temporal variations. In the initial phase (0–18 days), the bioavailability of Pb in soils of all contamination levels decreased rapidly. This was followed by a stabilization phase (18–30 days), during which the bioavailability of Pb remained stable. By the end of the 30-day remediation period, the stabilization effect reached its maximum, with the bioavailability of Pb in low-, moderate-, and high-contamination soils significantly reduced by 97.97%, 96.78%, and 95.82%, respectively, with statistically significant differences compared to the control group (p < 0.05). Similarly, as depicted in Fig. 4b, the trend in Cd bioavailability followed a similar trend to that of Pb. During the same 30-day remediation period, the bioavailability of Cd also decreased rapidly in the initial phase (0–18 days) and stabilized in the subsequent phase (18–30 days). By day 30, the bioavailability of Cd in low-, moderate-, and high-contamination soils was significantly reduced by 92.96%, 91.76%, and 91.02%, respectively, with statistically significant differences compared to the control group (p < 0.05), demonstrating a remediation effect similar to that of Pb.
Stabilization mechanisms of Pb and Cd by SL
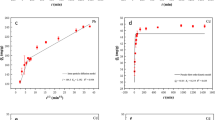
Five adsorption kinetic models—the pseudo-first-order model, pseudo-second-order model, Elovich model, parabolic diffusion model, and power function model—were used to fit the adsorption kinetic data of SL under three different mixed ion concentrations (Pb: 500, 1000, 1500 mg/L; Cd: 1.5, 3, 5 mg/L)29. The fitting performance of each model was evaluated to determine the kinetic model that best described the experimental data. Table 3 summarizes the fitting parameters of the models, including the coefficient of determination (R2) and standard error (SE). Generally, higher R2 values and lower SE values indicate a better fit to the adsorption process30. As shown in Table 3, the pseudo-second-order model showed the lowest SE and the highest R2 among all tested models, demonstrating its superior ability to describe the adsorption kinetics of SL for Pb and Cd at different ion concentrations. Therefore, the pseudo-second-order model was determined as the optimal model for describing the adsorption kinetics of SL for Pb and Cd. These results indicate that the adsorption process of SL for Pb and Cd is primarily governed by chemisorption mechanisms31.
The pseudo-second-order kinetic curves of SL for Pb and Cd under different ion concentrations are shown in Fig. 5. All experiments were performed at 25℃ and pH 8.6 to maintain consistent adsorption conditions. As shown in Fig. 5a, the adsorption capacity increased rapidly initially, then gradually slowed down, and reached equilibrium after approximately 3–4 h. In addition, the adsorption capacity of Pb was significantly influenced by ion concentration, following the order 1500 mg/kg > 1000 mg/kg > 500 mg/kg. Similarly, as shown in Fig. 5b, the trend of Cd adsorption by SL was similar to that of Pb. During the adsorption period of 0–6 h, the adsorption amount of Cd increased rapidly in the initial stage and then stabilized within 3–4 h. In addition, the ion concentration also significantly influenced the adsorption capacity of Cd, following the order 5 mg/kg > 3 mg/kg > 1.5 mg/kg.
This study was conducted at a fixed pH of 8.6, which represents the actual conditions for the addition of SL (sepiolite and limestone composite) to the soil in the target agricultural area. To investigate the reaction mechanisms, the phase composition and functional groups of SL before and after Pb and Cd adsorption were characterized using XRD and FTIR techniques. The XRD spectrum of SL (Fig. 6) showed diffraction peaks between 10° and 50°. Before the adsorption of Pb and Cd, the diffraction peaks of calcium carbonate were observed at 2θ angles of 29.46°, 39.6°, and 47.68°, while those of marble were observed at 12.16° and 28.6°. Comparison of the XRD patterns of SL before and after adsorption revealed the appearance of diffraction peaks corresponding to PbCO3, CdCO3, Cd(OH)2, and Pb(OH)2. This is attributed to the interaction between surface hydroxyl groups (-OH) and carbonate groups (CO32−) of SL with Pb2+ and Cd2+ ions, resulting in the formation of hydroxide and carbonate precipitates32.
The FTIR spectra of SL before and after adsorption of Pb and Cd are shown in Fig. 7, demonstrating significant changes. As shown in Fig. 7, the characteristic peaks at approximately 1430 cm−1, 869 cm−1, and 450 cm−1 were observed in the spectrum of the original SL. Specifically, the peak at 1430 cm−1 was attributed to the asymmetric stretching vibration of carbonate33, while the peak at 869 cm−1 was characteristic of carbonate absorption, and the peak near 450 cm−1 corresponds to the stretching vibration of the Si–O–Si bond, indicating the presence of a silicate structure in SL34. After the adsorption of Pb and Cd, two new absorption peaks appeared at 1610 cm−1 and 3462 cm−1. The appearance of these two new peaks was attributed to the stretching vibration of the -OH bond, indicating the formation of new -OH chemical bonds on the surface of SL35. This change indicated that SL underwent a chemical reaction during the adsorption process, leading to an increase in surface hydroxyl groups. At the same time, the characteristic peak of carbonate in SL after adsorption (869 cm−1) shifted, and its intensity weakened. This may be due to the interaction of carbonate ions in SL with Cd2+ and Pb2+ ions, leading to the formation of corresponding carbonate precipitates36,37.
Evaluation of Pb and Cd contaminated soil remediation by Pak Choi
The remediation effect of SL on Pb- and Cd-contaminated soil was assessed using pak choi as an indicator plant. During the 30-day stabilization period, pak choi was sown at different time points (0, 6, 12, 18, 24, and 30 days), with each batch grown for 30 days. The Pb and Cd contents, as well as the bioconcentration factor (BCF) in pak choi, were measured at the end of the 30-day growth period for each batch. The control group (CK) was defined as pak choi grown in soil without SL for 30 days, and the results are presented in Fig. 8.
Changes in the contents of Pb (a), Cd (b), and bioconcentration factor (BCF) (c) in pak choi sown at different time points (0, 6, 12, 18, 24, and 30 days) during the 30-day stabilization period. Each batch was grown for 30 days, and the control group (CK) was defined as pak choi grown in soil without SL for 30 days. Different letters indicate significant differences at p < 0.05 (Duncan’s test).
As shown in Fig. 8a, the Pb content and BCF in pak choi planted during the early stabilization phase (0–18 days) decreased rapidly and then stabilized in the later phase (18–30 days). Notably, the Pb content in pak choi sown from day 6 of the stabilization period remained below the safety limit set by Chinese standards. Compared to the CK, the most significant reduction in Pb content occurred in pak choi planted on day 30, with decreases of 98.92%, 97.87%, and 97.59% in low-, medium-, and high-contamination soils, respectively, with statistically significant differences compared to the control group (p < 0.05).
Similarly, as shown in Fig. 8b, the Cd content and BCF in pak choi planted during the initial stabilization phase (0–18 days) exhibited a trend similar to that of Pb. The Cd content and BCF decreased rapidly and stabilized after 18 days. Compared to the CK, the most pronounced reduction in Cd content occurred in pak choi planted on day 30, with decreases of 95.26%, 94.36%, and 94.12% in low-, medium-, and high-contamination soils, respectively, with statistically significant differences compared to the control group (p < 0.05).
Discussion
This study investigated the application of nonmetallic minerals for the remediation of Pb and Cd contaminated agricultural soils, focusing on their remediation effects, mechanisms of action, and evaluation methods. Dose experiments revealed that increasing the stabilizer dosage enhanced the remediation of heavy metal pollution. Specifically, treatments with 2.5% AT, SP, AP, and LS (within the 0–2.5% dosage range) significantly reduced the bioavailability of Pb and Cd. Treatments with AT, SP, AP, and LS reduced the bioavailability of Pb by 35.34%, 39.43%, 42.21%, and 70.92%, respectively, and that of Cd by 24.89%, 44.47%, 33.22%, and 68.27%, respectively.
These findings are consistent with previous studies showing that natural calcium-rich clay minerals, such as calcium-rich sepiolite and calcium-rich attapulgite, significantly reduced the acid-soluble fractions of Cd and Pb. Within the 0–10% dosage range, 10% attapulgite and 10% sepiolite reduced the acid-soluble fractions of Cd by 56.2% and 34.2%, respectively, and those of Pb by 81.5% and 77.4%, respectively38. Similarly, other studies have confirmed the positive correlation between dosage and remediation effectiveness. For example, within the 0–600 kg/ha dosage range, zeolite at 600 kg/ha reduced the available Cd content from 0.030 to 0.006 mg/kg and the available Pb content from 3.15 to 2.51 mg/kg, while pyrophyllite at the same dosage further reduced the available Pb content to 2.06 mg/kg. These results highlight a significant positive correlation between stabilizer dosage and the reduction of available Pb and Cd content39. However, once the dosage exceeds a certain threshold, the rate of improvement in remediation effectiveness gradually slows and stabilizes. This phenomenon occurs as stabilizers preferentially interact with highly active heavy metal ions in the soil through mechanisms such as chemical precipitation and physical adsorption, thereby reducing their bioavailability and mobility. The remaining heavy metal ions, which are chemically more stable and less mobile, are less susceptible to adsorption by stabilizers even with further increases in dosage and thus remain in the soil37.
Soil pH is a key factor influencing the mobility of heavy metals. Under low pH conditions, heavy metals in soil are more likely to be transformed into ionic forms, increasing their solubility and facilitating their transfer or uptake by plants40. In contrast, under high pH conditions, heavy metals in soil are more likely to combine with hydroxyl groups to form insoluble precipitates, thereby reducing their solubility and promoting their transformation from highly bioavailable forms to more stable forms41. The stabilizers used in this study increased soil pH to varying degrees. The Pearson correlation method was used to analyze the relationship between soil pH and the available contents of Pb and Cd. The results indicated a significant negative correlation between soil pH and the available concentrations of Pb and Cd, with correlation coefficients of RPb = − 0.81 and RCd = − 0.77, respectively.
Characterization analysis showed that Pb and Cd interacted with SL to form carbonate and hydroxide precipitates. Sepiolite is a porous mineral with abundant adsorption sites that can effectively absorb heavy metal ions in soil. Additionally, its internal cations (such as K⁺, Mg2⁺, Ca2⁺) can be exchanged with heavy metal ions in the soil to promote the removal of heavy metals42. On the other hand, limestone raises soil pH, decreases heavy metal solubility, transforms them into insoluble precipitates, and immobilizes them in the soil, effectively preventing the migration of heavy metals to plants and groundwater43. When limestone and sepiolite work together, the advantages of the two complement each other to form a more stable heavy metal stabilization system. The alkaline environment created by limestone enhances the adsorption and ion exchange capacity of sepiolite. The adsorption and ion exchange capacity of sepiolite further enhanced the immobilization effect of limestone44. This synergistic effect not only significantly improves the remediation efficiency but also makes the composite material more effective in removing and immobilizing soil heavy metals compared to a single stabilizer, thereby reducing remediation costs and time.
In the pot experiment, compared to the control group (CK), the Pb and Cd contents in pak choi grown in low-, medium-, and high-contamination soils treated with SL decreased by 98.92%, 97.87%, and 97.59%, and 95.26%, 94.36%, and 94.12%, respectively, all of which met Chinese food safety standards. Additionally, the biomass of pak choi increased by 56.58% to 126.94%. This is because, in untreated soil, high concentrations of Pb and Cd significantly inhibited the growth of pak choi, resulting in limited biomass. However, after SL remediation, the concentrations of Pb and Cd in the soil were significantly reduced, thereby alleviating their inhibitory effect on pak choi growth and enabling the plants to return to normal growth conditions. Additionally, it is noteworthy that SL is rich in essential plant nutrients such as K, Mg, and Ca, which promote the growth of pak choi and further increase its biomass22,45. These studies highlight that the evaluation of heavy metal remediation in agricultural land is multifaceted, encompassing changes in the content and forms of heavy metals in soil, as well as the reduction of heavy metal content in agricultural products.
Conclusion
In the remediation of soil Pb and Cd pollution, the individual application of nonmetallic minerals has demonstrated certain effectiveness. However, research on the combined application of two nonmetallic minerals remains relatively limited. This study identified the optimal stabilizer combination (SL) of sepiolite and limestone through screening, evaluated the remediation effects and mechanisms of SL on Pb- and Cd-contaminated soil, and explored its impact on crop safety. Through systematic experiments and analysis, the following conclusions were drawn:
-
1.
The optimal remediation effect was achieved at an SL dosage of 4.0% (composed of 2.5% sepiolite and 1.5% limestone). In low-, medium-, and highly contaminated soils, SL reduced the bioavailability of Pb and Cd by 97.97% and 96.78%, 95.82% and 92.96%, and 91.76% and 91.02%, respectively.
-
2.
The adsorption kinetics of Pb and Cd were best described by the pseudo-second-order model. XRD and FTIR analyses revealed that hydroxyl (-OH) and carbonate (CO₃2⁻) functional groups on the SL surface likely serve as active sites for the binding of Pb and Cd. The interaction between Pb, Cd, and SL is primarily attributed to chemical precipitation reactions, providing a theoretical foundation for the application of SL in heavy metal pollution remediation.
-
3.
In pak choi cultivated in SL-remediated soil, the Pb and Cd concentrations complied with Chinese food safety standards. In low-, medium-, and highly contaminated soils, the Pb and Cd levels in pak choi were reduced by 98.92% and 97.87%, 97.59% and 95.26%, and 94.36% and 94.12%, respectively. These findings confirm that SL remediation technology not only reduces the bioavailability of heavy metals in soil but also minimizes their uptake by crops, ensuring agricultural product safety and promoting sustainable agricultural practices.
-
4.
This study verified the effectiveness of the combined application of sepiolite and limestone (SL) for the remediation of Pb- and Cd-contaminated soil, clarified its mechanism of action, and provided a new technical approach for heavy metal pollution control and theoretical support for sustainable agricultural practices. However, limitations include the lack of long-term field stability data and the need for further research on the effects of environmental factors on SL performance. Future research should focus on field experiments to systematically evaluate the long-term effects of SL on soil ecosystems and agricultural productivity, thereby promoting its practical application in soil remediation.
Data availability
The datasets used and analysed during the current study available from the corresponding author on reasonable request.
References
Dong, H. et al. Research on influencing factors of cultivated land productivity of highstandard farmland projects in Hanzhong city of China—An empirical study based on PLS-SEM. Front. Sustain. Food S. https://doi.org/10.3389/fsufs.2023.1176426 (2023).
Lai, S. Q., Jin, Y., Shi, L. J., Li, Y. P. & Zhou, R. Tailoring multifunctional gel for sensitive naked-eye and fluorescence dual-mode detection and effective adsorption of cadmium(II) and lead(II) ions in water. Chem. Eng. J. https://doi.org/10.1016/j.cej.2021.132367 (2022).
Xia, F., Zhao, Z. F., Niu, X. & Wang, Z. F. Integrated pollution analysis, pollution area identification and source apportionment of heavy metal contamination in agricultural soil. J. Hazard. Mater. 465, 133215–133215. https://doi.org/10.1016/j.jhazmat.2023.133215 (2024).
Xu, D. M., Fu, R. B., Wang, J. X. & An, B. H. The geochemical behaviors of potentially toxic elements in a typical lead/zinc (Pb/Zn) smelter contaminated soil with quantitative mineralogical assessments. J. Hazard. Mater. https://doi.org/10.1016/j.jhazmat.2021.127127 (2022).
Mohammadi, S., Jabbari, F., Cidonio, G. & Babaeipour, V. Revolutionizing agriculture: Harnessing nano-innovations for sustainable farming and environmental preservation. Pestic. Biochem. Phys. 198, 105722. https://doi.org/10.1016/j.pestbp.2023.105722 (2024).
Xiang, M. T. et al. Heavy metal contamination risk assessment and correlation analysis of heavy metal contents in soil and crops. Environ. Pollut. 278, 116911–116911. https://doi.org/10.1016/j.envpol.2021.116911 (2021).
Xiao, J. et al. New strategy for exploring the accumulation of heavy metals in soils derived from different parent materials in the karst region of southwestern China. Geoderma https://doi.org/10.1016/j.geoderma.2022.115806 (2022).
Yang, X. et al. Continuous cropping alters soil hydraulic and physicochemical properties in the karst region of southwestern China. Agronomy 13(5), 1416. https://doi.org/10.3390/agronomy13051416 (2023).
Wahsha, M. et al. Microbial enzymes as an early warning management tool for monitoring mining site soils. CATENA 148, 40–45. https://doi.org/10.1016/j.catena.2016.02.021 (2017).
Xing, W. Q. et al. Effect of soluble phosphate and bentonite amendments on lead and cadmium bioavailability and bioaccessibility in a contaminated soil. Sci. Total. Environ. 900, 166370–166370. https://doi.org/10.1016/j.scitotenv.2023.166370 (2023).
Li, Q. N. et al. Simultaneous immobilization of arsenic, lead and cadmium by magnesium-aluminum modified biochar in mining soil. J. Environ. Manage. 310, 114792–114792. https://doi.org/10.1016/j.jenvman.2022.114792 (2022).
Awad, M. et al. Fractionation of heavy metals in multi-contaminated soil treated with biochar using the sequential extraction procedure. Biomolecules 11(3), 448–448. https://doi.org/10.3390/biom11030448 (2021).
Jiao, L. S. et al. Prediction models for monitoring selenium and its associated heavy-metal accumulation in four kinds of agro-foods in seleniferous area. Front. Nutr. 9, 990628–990628. https://doi.org/10.3389/fnut.2022.990628 (2022).
Khilji Sheza, A. et al. Fulvic acid alleviates paper sludge toxicity in canola (Brassica napus L.) by reducing Cr, Cd, and Pb uptake. Front. Plant. Sci. 13, 874723–874723. https://doi.org/10.3389/fpls.2022.874723 (2022).
Borymski, S., Cycoń, M., Beckmann, M., Mur Luis, A. J. & Piotrowska-Seget, Z. Plant species and heavy metals affect biodiversity of microbial communities associated with metal-tolerant plants in metalliferous soils. Front. Microbiol. 9, 1425. https://doi.org/10.3389/fmicb.2018.01425 (2018).
Wang, X. K. et al. HvPAA1 encodes a P-type ATPase, a novel gene for cadmium accumulation and tolerance in barley (Hordeum vulgare L.). Int. J. Mol. Sci. https://doi.org/10.3390/ijms20071732 (2019).
Zhang, Y. L. et al. Remediation of multiple heavy metals contaminated soils by Mn and Fe-added solid wastes: Effect and mechanisms. Chem. Eng. J. 497, 154649–154649. https://doi.org/10.1016/j.cej.2024.154649 (2024).
Wang, Z. P., Shen, R., Ji, S. B., Xie, L. P. & Zhang, H. B. Effects of biochar derived from sewage sludge and sewage sludge/cotton stalks on the immobilization and phytoavailability of Pb, Cu, and Zn in sandy loam soil. J. Hazard. Mater. 419, 126468–126468. https://doi.org/10.1016/j.jhazmat.2021.126468 (2021).
Ye, S. L., Wang, L. Y. & Liu, T. C. Study of solidification and stabilization of heavy metals by passivators in heavy metal-contaminated soil. Open Chem. 20(1), 1–9. https://doi.org/10.1515/chem-2021-0101 (2022).
Li, H. Y., Guo, X. S. & Ye, X. X. Screening hydroxyapatite for cadmium and lead immobilization in aqueous solution and contaminated soil: The role of surface area. J. Environ. Sci. 52, 141–150. https://doi.org/10.1016/j.jes.2016.04.005 (2017).
Hannan, F. et al. Remediation of Cd-polluted soil, improving Brassica napus L. growth and soil health with Hardystonite synthesized with zeolite, limestone, and green Zinc oxide nanoparticles. J. Clean. Prod. 437, 140737–140737. https://doi.org/10.1016/j.jclepro.2024.140737 (2024).
Bashir, S. et al. Role of sepiolite for cadmium (Cd) polluted soil restoration and spinach growth in wastewater irrigated agricultural soil. J. Environ. Manage. 258, 110020–110020. https://doi.org/10.1016/j.jenvman.2019.110020 (2020).
Ma, G. Y. et al. Effectiveness and potential mechanism of hydrothermal modification of attapulgite for cadmium passivation in soil. Int. J. Environ. Sci. Tech. https://doi.org/10.1007/s13762-023-05124-z (2023).
Wang, W. H. et al. Combined remediation effects of biochar, zeolite and humus on Cd-contaminated weakly alkaline soils in wheat farmland. Chemosphere 302, 134851–134851. https://doi.org/10.1016/j.chemosphere.2022.134851 (2022).
Cui, H. B. et al. Bioavailability and mobility of copper and cadmium in polluted soil after phytostabilization using different plants aided by limestone. Chemosphere 242, 125252–125252. https://doi.org/10.1016/j.chemosphere.2019.125252 (2020).
Zhang, J. Y., Jin, P. X., Lei, N. & Yin, L. Effects of acid modified mineral materials on soil cadmium pollution and plant remediation. Bangl. J. Bot. https://doi.org/10.3329/bjb.v53i30.76680 (2024).
Li, C. Q. et al. The stabilization ability of NaA zeolite derived from fly ash for lead and cadmium in soil: Mechanisms and evaluation of effectiveness. Sci. Total. Environ. 942, 173834–173834. https://doi.org/10.1016/j.scitotenv.2024.173834 (2024).
Ma, C. Y. et al. Impacts of exogenous mineral silicon on cadmium migration and transformation in the soil-rice system and on soil health. Sci Total Environ. 759, 143501. https://doi.org/10.1016/j.scitotenv.2020.143501 (2021).
Zhao, H. H., Li, P. Y. & He, X. D. Remediation of cadmium contaminated soil by modified gangue material: Characterization, performance and mechanisms. Chemosphere 290, 133347–133347. https://doi.org/10.1016/j.chemosphere.2021.133347 (2022).
Hamadeen, H. M., Elkhatib, E. A. & Moharem, M. L. Optimization and mechanisms of rapid adsorptive removal of chromium (VI) from wastewater using industrial waste derived nanoparticles. Sci. Rep. https://doi.org/10.1038/s41598-022-18494-0 (2022).
Li, W. L. et al. MoS2/Bentonite prepared by a facile method for efficient removal of Cd2+ from aqueous solution. J. Alloy. Compd. https://doi.org/10.1016/j.jallcom.2022.164508 (2022).
Abdelbasir, S. M. & Khalek, M. A. A. From waste to waste: Iron blast furnace slag for heavy metal ions removal from aqueous system. Environ. Sci. Pollut. Res. Int. 29(38), 57964–57979. https://doi.org/10.1007/s11356-022-19834-3 (2022).
Simler, T., Mccabe, K. N., Maron, L. & Grégory, N. CO reductive oligomerization by a divalent thulium complex and CO2-induced functionalization. Chem. Sci. https://doi.org/10.1039/d2sc01798a (2022).
Gkiliopoulos, D. et al. SBA-15 mesoporous silica as delivery vehicle for rhBMP-2 bone morphogenic protein for dental applications. Nanomaterials 12(5), 822–822. https://doi.org/10.3390/nano12050822 (2022).
Shen, P. et al. Multi-factors cooperatively actuated photonic hydrogel aptasensors for facile, label-free and colorimetric detection of lysozyme. Biosensors https://doi.org/10.3390/bios12080662 (2022).
Tian, L. et al. Comparison of microscopic adsorption characteristics of Zn(II), Pb(II), and Cu(II) on kaolinite. Sci. Rep. https://doi.org/10.1038/s41598-022-20238-z (2022).
Hu, Y., Wang, L. H., Shen, J., Jiang, G. Q. & Kan, C. Y. Preparation and characterization of porous cationic poly styrene-co-(N,N′-dimethylaminoethyl methacrylate) nanoparticles and their adsorption of heavy metal ions in water. Polym. Int. 67(5), 535–543. https://doi.org/10.1002/pi.5541 (2018).
Yin, H. B. & Zhu, J. W. In situ remediation of metal contaminated lake sediment using naturally occurring, calcium-rich clay mineral-based low-cost amendment. Chem. Eng. J. 285, 112–120. https://doi.org/10.1016/j.cej.2015.09.108 (2016).
Murtić, S., Sijahović, E., Čivić, H., Tvica, M. & Jurković, J. In situ immobilisation of heavy metals in soils using natural clay minerals. Plant. Soil. Environ. 66(12), 632–638. https://doi.org/10.17221/371/2020-pse (2020).
Palansooriya, K. N. et al. Prediction of soil heavy metal immobilization by biochar using machine learning. Environ. Sci. Technol. 56(7), 4187–4198. https://doi.org/10.1021/acs.est.1c08302 (2022).
Yang, L. Y., Wu, P. & Yang, W. T. Characteristics, health risk assessment, and transfer model of heavy metals in the soil—Food chain in cultivated land in karst. Foods 11(18), 2802–2802. https://doi.org/10.3390/foods11182802 (2022).
Chen, D. et al. The effect of sepiolite application on rice Cd uptake—A two-year field study in Southern China. J. Environ. Manage. 254, 109788–109788. https://doi.org/10.1016/j.jenvman.2019.109788 (2020).
Daba, N. A. et al. Long-term fertilization and lime-induced soil pH changes affect nitrogen use efficiency and grain yields in acidic soil under wheat-maize rotation. Agronomy 11(10), 2069–2069. https://doi.org/10.3390/agronomy11102069 (2021).
Shirvani, M., Shariatmadari, H., Kalbasi, M., Nourbakhsh, F. & Bijan, F. Sorption of cadmium on palygorskite, sepiolite and calcite: Equilibria and organic ligand affected kinetics. Colloid. Surface. A. 287(1–3), 182–190 (2006).
Colla, G. et al. Isosmotic macrocation variation modulates mineral efficiency, morpho-physiological traits, and functional properties in hydroponically grown lettuce varieties (Lactuca Sativa L.). Front. Plant. Sci. 12, 678799–678799. https://doi.org/10.3389/fpls.2021.678799 (2021).
Acknowledgements
This research was supported by the Sichuan Science and Technology Program (No. 2023YFG0357), the Ganzi Prefecture Science and Technology Project (220012), the National Key Research and Development Program of China (No. 2023YFC3207300).
Author information
Authors and Affiliations
Contributions
FL: Writing-original draft preparation, writing-review and editing, conceptualization, methodology, software, validation, formal analysis, investigation, resources, and data curation. KF: conceptualization, methodology, validation, resources, supervision, funding acquisition, project administration, and visualization. WZ, YK, HS: writing-review and editing, methodology, software, and validation. ZW: conceptualization, methodology, investigation, and resources. JT: software, validation, formal analysis, and data curation. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
We both declare that the manuscript reports studies that do not involve any human participants, human data, or human tissue. Therefore, it is not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liao, F., Fu, K., Zhang, W. et al. Stabilization mechanism and remediation effectiveness of Pb and cd in agricultural soil using nonmetallic minerals. Sci Rep 15, 12757 (2025). https://doi.org/10.1038/s41598-025-96970-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-96970-z