Abstract
The outcome of the immune response depends on the content and magnitude of inflammatory mediators, the right time to start, and the duration of inflammatory responses. Patients with coronavirus disease 2019 (COVID-19) represent diverse disease severity. Understanding differences in immune responses in individuals with different disease severity levels can help elucidate disease mechanisms. Here, we serially analyzed the cytokine profiles of 809 patients with mild to critical COVID-19. The cytokine profile revealed an overall increase in IL-1β, IL-1Ra, TNF-α, IL-6, IL-2, IL-8, and IL-18 and impaired production of IFN-α and -β. Only an early rise in IL-1Ra, IL-6, and IL-2 levels was linked to worse disease outcomes. On the other hand, long-term rises in IL-1β, IL-1Ra, TNF-α, IL-6, IL-2, IL-8, and IL-18 levels were linked to worse disease outcomes. Principal component analysis identified a component, including IL-1β, TNF-α, IFN-α, and IL-12, that was associated with disease severity. Spearman analysis revealed that the correlation of IL-1β and IFN-α was entirely different between mild and critical patients. Therefore, the ratio of IL-1β to IFN-α seemed to be a suitable criterion for distinguishing critical patients from mild ones. The higher levels of the IL-1β to IFN-α ratio correlated with improved outcomes. These data point to an imbalance of IL-1β/IFNα, contributing to hyperinflammation in COVID-19.
Similar content being viewed by others
Introduction
Coronavirus disease 2019 (COVID-19), caused by the betacoronavirus SARS-CoV-2, has emerged as a severe pandemic, leading to a significant number of pneumonia cases, acute respiratory distress syndrome (ARDS), and fatalities1,2. SARS-CoV-2 infection can remain asymptomatic for an extended period before some patients experience sudden and severe symptoms, necessitating hospitalization, oxygen support, or intensive care unit (ICU) admission1,2.
Current epidemiological data indicates that COVID-19 has a case fatality rate that is significantly higher than that of seasonal influenza3. Elderly individuals and those with underlying medical comorbidities, including cardiovascular disease, diabetes mellitus, chronic lung disease, chronic renal disease, obesity, hypertension, or cancer, have a much greater death rate compared to healthy young adults4. The primary causes of this disparity are unidentified. However, they could result from a weakened interferon (IFN) response and unregulated inflammatory responses, similar to what has been seen with other zoonotic coronavirus infections, including severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS)5.
COVID-19 is characterized by an excessive inflammatory reaction, referred to a ‘cytokine storm’, which hinders gas exchange and can result in ARDS, multiple organ failure, and death6,7,8. Increased levels of various pro-inflammatory cytokines, including as IL-1β, IL-6, TNF, IL-12, IFN-γ, IL-17, and others, are reported in COVID-19 patients1,9,10, and these elevations are correlated with disease severity and mortality10,11.
The outcome of the immune response depends on the content and magnitude of inflammatory mediators, the right time to start, and the duration of inflammatory responses. Multiple investigations have documented the serum concentrations of various pro-inflammatory cytokines in individuals with COVID-19. Cytokines and chemokines are simultaneously produced in response to infection and can interact in a complex network as either stimulators or inhibitors of inflammation. For example, a study by Sainz et al. showed that treating mice with a combination of IFN-α and IFN-γ offers better protection against a deadly herpes simplex virus type-1 challenge at lower doses than usual12. Additionally, treating primary human fibroblasts with IFN-β alone slows down but does not completely stop myxoma virus (MYXV) replication13. Combining cytokines like IFN-\(\:\beta\:\)/IFN-γ or IFN-β/TNF is necessary to completely stop MYXV replication in these cells14. Therefore, examining cytokines as a component can give a better view of their behavior in COVID-19.
The timing and magnitude of cytokines effectively direct their antiviral or immunomodulatory function. Several studies reveal the dual function of IFNs and pro-inflammatory cytokines15,16,17. For example, persevering IFN production can stimulate the expression of PD-L1 ligands, which can inhibit NK cell antiviral action18. Administration of pegylated IFN-α2 treatment boosted NK cell activation, TRAIL, and CD107a receptor expression in HCV-infected people but also decreased IFN-γ + NK cells in the PBMC compartment19,20. The different effects of type I IFN might depend on when and how much type I IFN is made, or because transcription factors bind to the type I IFN receptor in different ways. In a Listeria monocytogenes infection, exogenous IFN-β delivered earlier during the infection was capable of activate NK cells and increase clearance of the infection. On the contrary, the endogenous IFN-β produced 24 h after infection led to a reduced NK cell response21. Although longitudinal analyses reveal the association of crucial immune mediators with COVID-19 severity and mortality10,22, none of previous studies have used large sample size (1033 patients with 1514 samples) or involved patients with different disease severity (particularly outpatients) at three time points.
In this study, we combined the data of a cross-sectional study with a cohort study conducted at the beginning of the SARS-CoV-2 outbreak. In the cross-sectional study, sampling was done once at different time points after the symptom’s onset, while in the cohort, patients were monitored with weekly blood tests for up to 3 weeks following the onset of symptoms, including mild cases where hospital admission is not necessary. Twelve cytokines were assessed in the blood samples. A longitudinal investigation was conducted to illustrate the changes in cytokine and chemokine production linked to disease progression towards critical illness. Additionally, we use principal component analysis (PCA) to introduce the components of cytokines that collectively contribute to the COVID-19 process at the different time points.
Results
Characteristics of participants
The demographic information of the study population classified based on disease severity is demonstrated in Table 1. By mixing cross-sectional and cohort studies, 392 patients with acute SARS-CoV-2 infection who were admitted to several hospitals in the different provinces of Iran, 417 outpatients, and 224 healthy individuals were included in this study (Fig. 1A). The hospitalized patients were divided into moderate, severe, and critical categories according to their need for supplementary oxygen and admission to the ICU (Fig. 1B). We conducted 1514 collections and follow-up assessments on the patient population, ranging from one to three longitudinal time points occurring 1–50 days after symptom start (Fig. 1C). As presented in Table 1, the age was higher among patients with moderate, severe, and critical diseases (P < 0.001). There was no significant disparity in gender and body mass index (BMI) between the patients and control groups. The median interval from first symptoms on admission was not significantly different between hospitalized patients. The distribution of coexisting disorders, including diabetes, hypertension, cardiovascular disease, renal disease, and cancer, was higher among patients with moderate, severe, and critical diseases (all P < 0.05). Initial presenting symptoms demonstrated a preponderance of fever (39.33-62.16%) and fatigue (39.09-53.69%), with no significant difference between patients with mild to critical disease. The frequency of dry cough and dyspnea was significantly higher in inpatients with moderate, severe, and critical disease compared with patients with mild disease (all P < 0.001). However, myalgia was observed more in mild (42.21%) and moderate patients (40.15%) than in those who developed deleterious clinical manifestations (32.43-37.78%, P < 0.001). Finally, mortality and length of stay in the hospital were significantly higher in patients who were classified into severe and critical groups than in those who were not (P < 0.001).
Study design. (A) The classification of COVID-19 patients assigned according to WHO guidelines as described in EXPERIMENTAL MODEL AND SUBJECT DETAILS. SpO2, saturation of peripheral oxygen; RR, respiratory rate; IMV, intermittent mandatory ventilation; ECMO, extracorporeal membrane oxygenation. (B) Distribution of COVID-19 patients based on the disease severity for Tehran cross sectional study and COVID-19 national cohort. Our first study conducted in Tehran as cross-sectional. Then, the second longitudinal study designed and the samples from five provinces were transferred to Tehran for laboratory examinations. COVID-19 patient cohort overview and sample collection timeline. (C) Frequency of COVID-19 patients with different disease severity at three times interval (1–7, 8–14, and > 14 day after symptom onset).
Cytokine profile in COVID-19 patients
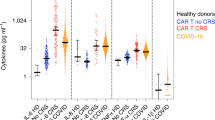
To gain insights into the main differences in the crucial cytokines between COVID-19 patients with diverse disease severity, we analyzed the serum levels of 12 cytokines across all sample collection time points. We observed that the inflammatory cytokines, including IL-1β, IL-1Ra, TNF-α, IL-6, IL-2, IL-8, and IL-18, in all COVID-19 subgroups were significantly elevated compared to the control group. IL-1Ra, IL-6, IL-2, IL-18, and IL-17 displayed more robust associations with illness severity. (Fig. 2). However, the results of other cytokines were different. IFN-γ levels were not significantly different between study groups. IL-12 levels were significantly higher in mild patients than in healthy individuals and hospitalized patients. In patients with moderate and critical disease, we observed an elevation of IFN-β compared to mild patients. In addition, we found that levels of IFN-α were significantly elevated in critical patients as compared to those with mild and moderate conditions (Fig. 2).
Comparison of pro-inflammatory and anti-inflammatory cytokines and type I and II IFN patterns between COVID-19 patients with diverse disease severity. The concentrations of major inflammatory and anti-inflammatory cytokines, as well as interferons type I and II, were quantified and expressed as logarithmically transformed values. Each dot corresponds to an individual time point for each subject (HC, n = 224; mild, n = 559; moderate, n = 521; severe, n = 73; critical, n = 137) and lines show median values. P values were calculated by a two-tailed Mann–Whitney U-test for nonparametric comparisons. P values were computed using a two-tailed Mann-Whitney U-test for nonparametric comparisons. The significance levels of #P < 0.05, ##P < 0.01, and ###P < 0.001 indicate statistical significance compared to the healthy control group. The significance levels between COVID-19 subgroups are *P 0.05, **P 0.01, and ***P 0.001.
Longitudinal analysis of cytokines in COVID-19 patients
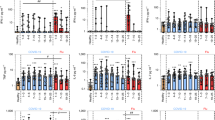
We investigate whether temporal pro-inflammatory cytokine patterns are diverse between the various patient groups. The difference between subgroups is more visible in the case of IL-6 than in other cytokines. This finding is either due to the high importance of the changes and function of this cytokine at different time points of COVID-19 or the fact that they are often measured with in vitro diagnostic (IVD) kits, and due to their high sensitivity, the changes are better displayed. IL-6 levels in all subgroups were higher than the control group at all time points except the mild group at day > 14 (Fig. 3). In the mild and moderate groups, IL-6 concentrations were at their highest level in the first week after the symptom onset and then exhibited a decreasing trend. However, elevated concentrations of IL-6 are linked to disease severity. IL-6 was produced higher in moderate, severe, and critical subgroups compared to the mild group and in moderate compared to the critical group at all studied time points (Fig. 3).
Temporal pro-inflammatory and anti-inflammatory cytokines and type I and II IFN patterns of patients with COVID-19 with diverse states of disease in relation to symptom onset. Serum levels of prominent inflammatory and anti-inflammatory cytokines, interferons type I and II, at various time intervals after symptom onset. Quantifications showed log10-transformed concentrations. The data are displayed as individual data points representing patient measurements, with lines indicating the median values. For mild patients, n = 429, 76, 54; for moderate patients, n = 250, 208, 63; for severe patients, n = 28, 24, 21; and for critical patients, n = 41, 43, 3 for each of the three consecutive time intervals. P values were calculated by a two-tailed Mann–Whitney U-test for nonparametric comparisons. The significance levels of #P < 0.05, ##P < 0.01, and ###P < 0.001 indicate statistical significance compared to the healthy control group. The significance levels between COVID-19 subgroups are *P 0.05, **P 0.01, and ***P 0.001.
It seems that the pattern of changes in IL-1β and IL-1Ra in COVID-19 patients is similar. Remarkably, in the mild group, the amount of these cytokines increased at the day 1–7, reached a peak at the day 8–14, and diminished after > 14 days. While in other groups, from the beginning (day 1–7), they were produced more compared to the mild group, and their levels remained high even after more than 14 days (Fig. 3). However, the variations of TNF-α were the same between subgroups. TNF-α levels elevated at the day 1–7 and 8–14 times intervals, then decreased at the day > 14. We detected that IL-8 was significantly elevated in all COVID-19 subgroups at three studied time intervals compared to the control group. IL-8 was significantly higher in critical patients than moderate subjects (Fig. 3).
Since the amount of IFN-β is undetectable in many samples with COVID-19, no difference between diverse groups has been observed in previous studies9,23,24. However, since the sample size is remarkable in most groups in this study, IFN-β changes have been determined. We found that although there was no difference in the production of IFN-β in all patients with COVID-19 compared to the control group at the day 1–7, IFN-β levels were higher in moderate, severe, and critical subjects at the day 8–14 or > 14 compared to the healthy control and themselves at the day 1–7 (Fig. 3).
Notably, we detected that while patients with mild and moderate COVID-19 produced IFN-α levels that were significantly lower at all time intervals compared to the healthy group, IFN-α levels did not change in those with severe and critical illnesses compared to the control group (Fig. 3). Furthermore, the patients with critical COVID-19 exhibited significantly elevated levels of IFN-α at the day 1–7 time interval compared to mild and moderate patients and at the day 8–14 and > 14 time intervals compared to moderate patients (Fig. 3).
The patients with severe COVID-19 at second- (day 8–14) and third- (day > 14) times intervals and the subjects with critical COVID-19 at the third-time intervals had significantly elevated levels of IFN-γ compared to the healthy control group (Fig. 3). In intragroup comparison, severe patients had higher levels of IFN-γ at the third-time interval compared to the first- and second-time intervals. Furthermore, at the third-time interval, IFN-γ levels were higher in severe individuals compared to moderate patients (Fig. 3). IL-12 reached its maximum level at the day > 14 in mild patients. It was significantly increased in mild COVID-19 at the day 8–14 and > 14 compared to the control and other COVID-19 subgroups. In contrast, there was no difference in serum concentrations of this cytokine between moderate, severe, and critical patients with the control group. Interestingly, the results of IL-18 were opposite those of IL-12. IL-18 was significantly increased in mild COVID-19 patients only at the day 1–7, and in other COVID-19 patients at all time intervals compared to control group. In addition, IL-18 had a significant association with severity at the second and third weeks after symptom onset (Fig. 3).
All patients with varying degrees of COVID-19 severity showed significantly reduced IL-17 induction at all time intervals examined. However, severe and critical patients exhibited significantly higher concentrations of IL-17 than mild and moderate subjects in the first two weeks after symptom onset.
Distinct Temporal cytokine patterns associated with COVID-19 severity
Given that temporal cytokine patterns were diverse between the COVID-19 patients with varying severity, we employed Spearman’s rho and PCA to investigate further crucial overall cytokine profile differences temporally.
Longitudinal cytokine correlations, measured at diverse time points after symptom onset, indicated that correlations of cytokines IL-1β, IL-1Ra, TNF-α, and IL-8 could be seen in many categories, including at all mild and moderate time intervals, at the first week in severe and the second and third weeks in critical, with the difference that there was a simultaneous increase at the first and second week and a simultaneous decrease at the third week in the mild group, while these inflammatory cytokines were increasing together until the third week in the other groups (Fig. 4). The point to consider in this analysis was the opposite correlation of IFN-α with IL-1β, IL-1Ra, and IL-8 in the mild group rather than other groups. In the mild group, IFN-α had a direct correlation with IL-1β and IL-8 at the first week, while in other groups, these correlations were inverse at different time intervals. In addition, our finding demonstrated that IFN-β was strongly associated with IFN-γ in hospitalized patients at different time intervals.
Correlation of pro-inflammatory and anti-inflammatory cytokines and type I and II IFN patterns of patients with COVID-19 with diverse states of disease in relation to symptoms onset. Correlation matrix of 12 cytokines concentration levels in serum at the day 1–7, 8–14, and > 14 time intervals after symptoms onset of patients with COVID-19 with different disease severity. P values for the degree and direction of the relationship between the two variables, as stated in each panel, were calculated using the Spearman rank-order correlation coefficient for nonparametric data. A question mark is displayed when the sample size is insufficient for analysis.
This scenario is consistent with PCA findings. PCA revealed three components with an eigenvalue > 1.0, explaining 50–57% of the variance in the 12 cytokines of COVID-19 patients (Fig. 5). At the day 1–7, the main contributors to PC1 were IL-1β, TNFα, IFN-α, and IL-12. PC1 was significantly higher in individuals with mild disease compared to those with moderate and critical COVID-19 when evaluated across different disease severity categories (both p < 0.001, Fig. 5A). There was no significant difference in PC2 across the study groups, while PC3, including IFN-β and IFN-γ, was higher in those with moderate vs. mild COVID-19.
Distinct cytokine profiles related to COVID-19 severity at different time intervals after symptoms onset. Principal component analysis (PCA) of 12 serum cytokines evaluated in patients with COVID-19 with diverse disease severity at day 1–7, 8–14, and > 14 time intervals. A, B, C, at day 1–7, 8–14, and > 14time intervals, respectively. A-, B-1, C-1, Biplots display the correlation vectors that indicate the two-dimensional projection of the loading for each cytokine included in the specific PCA. A-2, B-2, C-2, PCA tables displaying correlation coefficients for cytokines loaded on the three principal components (PCs) derived from the data analysis. The color of the dots in each cytokine row represents the degree of correlation between that variable and the eigenvector of each principal component. Correlation coefficients with higher absolute values were regarded to be important (dark) in determining the PC. A-3, A-4, A-5, Levels of PC1, PC2, and PC3 are depicted for each respiratory severity group. Dim, dimension. P values were calculated by a two-tailed Mann–Whitney U-test for nonparametric comparisons. *P < 0.05, **P < 0.01 and ***P < 0.001 show significance between COVID-19 subgroups.
At the day 8–14 time interval, PC1 with the greatest contribution of IL-1β, TNFα, IL-1Ra, and IL-8 was significantly higher in mild vs. moderate COVID-19 (Fig. 5B), but PC2 and PC3 did not significantly differ between COVID-19 subgroups. At the day > 14 time interval, IL-2, IL-8, and IL-1Ra contributed in PC1 (Fig. 5C). This component was significantly higher in critical vs. moderate and mild patients (P = 0.003 and P = 0.018, respectively). To better knowledge the results of IFN-α, we divided IFN-α concentrations into three levels and checked the frequency of people based on them (Fig. 6A). Most patients (more than 80%) had a low concentration of IFN-α (0–15 pg/mL). A low percentage of critically ill patients (16%) had moderate levels of IFN-α (15.01-50 pg/mL) at the day time interval > 14, which was significantly higher than other groups (Fig. 6A). According to the obtained results, it seems that delayed increase of type I IFN can impact the disease aggravation.
Wide depletion IFN-α and Imbalance of IL-1β/IFN-α. (A) The serum concentration of IFN-α was categorized into three levels. The frequency of patients with COVID-19 was evaluated based on the levels of IFN-α at day 1–7, 8–14, and > 14 time intervals. Stacked bar graphs showing the percentage of patients with COVID-19 with different levels of IFN-α. P values were calculated by chi-square test. (B) The ratio of IL-1β/IFN-α at various time intervals after symptom onset. Quantifications showed as log10-transformed concentrations. The data are displayed as individual data points representing patient measurements, with lines indicating the median values and dashed line representing first and third quartiles. P values were calculated by a two-tailed Mann–Whitney U-test for nonparametric comparisons.
Based on our previous result that IL-1β and IFN-α were placed in PC1 and had a diverse correlation between mild and critical patients, we assessed the ratio of IL-1β to IFN-α. We observed that this ratio significantly declined in critical patients at the day 1–7 and 8–14 time intervals compared to mild and moderate groups, respectively (Fig. 6B).
To predict illness severity, logistic regression models were employed, adjusting for age and sex. The cytokines that had the highest score on PC1 and were linked to disease severity were input. The logistic regression model reveals that age, Log IL-1β, Log IL-1Ra, Log IL-6, and Log IFNα are significant predictors of hospitalization of COVID-19 patients at the first week after symptom onset. The findings indicate that elevated levels of Log IL-1β were associated with declined odds (OR:0.31 [0.155–0.622]) of hospitalization, whereas higher levels of Log IL-6 (OR: 3.112 [1.749–5.540]), Log IFNα (OR: 2.198 [1.220–3.960]), and Log IL-1Ra (OR: 1.663 [1.160–2.383]) were linked to decreased odds of hospitalization (Table 2). Using the formulation below, the panel of IFN-α2, IL-1β, IL-1Ra, and IL-6 at the first week after symptom onset with the accuracy of 76% and sensitivity of 79% was the suggestive combination to predict hospitalization. However, this combination did not have high sensitivity for invasive mechanical ventilation (33%) and mortality (50%).
= 0.089*sex (male=1)+0.038*age(years: 17-80)-1.171*log (IL-1β(pg/mL:3.9-280)) +0.509*log (IL-1Ra(pg/mL:39-2500))+1.135*log (IL-6(pg/mL:2-1000)) +0.787*log (IFNα2(pg/mL:3.12-200)) - 3.573.
Discussion
Excessive or unregulated inflammatory responses can cause irreparable damage. Finding the harmless and limited damage responses to injury can help us find therapeutic approaches for diseases with uncontrolled inflammation. A prior investigation found that 28% of fatal COVID-19 cases exhibited severe inflammation characterized by the overproduction of pro-inflammatory cytokines25. In this study, we evaluated major cytokine productions in COVID-19 patients with different disease severities to find the deviation from regulation in excessive responses.
Recently, several studies demonstrated abundant data indicating that cytokine storms play a crucial role in severe cases of COVID-1926,27,28,29. Additional research has suggested that immune-modulating cytokines may play a role in the onset of this illness24. As reported in previous studies9,10,23,24,30, in the present study, we observed that the pattern of all inflammatory cytokines among COVID-19 patients is not the same. If we look at cytokine production one by one, we can see that cytokines like TNF, IL-1β, and IL-8 are produced at about the same rate in all patients at the start. After the third week, they keep being produced, which may be related to how the disease starts. In contrast, IL-6, IL-2, IL-1Ra, and IL-18 displayed more robust associations with illness severity. To get a better picture of how cytokines change using PCA, we discovered that the rise of inflammatory cytokines along with IFN-α in the first and second weeks after the onset of symptoms is effective for the antiviral responses in patients with COVID-19, while the continuation of the inflammation centered on IL-2 and IL-8 is detrimental to these patients.
The levels of IL-6 are different from the beginning among patients with different disease severities. The strong predictive role of IL-6 for hospitalization, as evidenced by an odds ratio of 3.112, is consistent with existing literature that links elevated IL-6 levels to increased COVID-19 severity, primarily through its contribution to cytokine storm pathogenesis31,32. This association provides a rationale for the clinical use of IL-6 inhibitors in severe cases, aligning with therapeutic strategies aimed at mitigating excessive inflammatory responses. IL-6 is produced locally from most stromal cells and immune system cells at the site of injury and is distributed throughout the body through the bloodstream, quickly triggering the host defense system to carry out several duties. Excessive or prolonged production of IL-6 contributes to the development of many illnesses. Some hypotheses can be proposed for the pathological function of IL-6 in COVID-19. COVID-19-associated coagulopathy is a severe consequence of SARS-CoV-2 infection that can be life-threatening33. IL-6 has hormone-like characteristics that have an impact on vascular disease34. Treatments of COVID-19 patients with IL-6 signaling inhibitors have resulted in a reduction of inflammatory and coagulation markers35,36. Furthermore, IL-6 trans-signaling promotes the secretion of the chemokine monocyte chemoattractant protein-1 (MCP-1/CCL2)37. Investigators have shown that CCL2 and its receptor (CCR2) play a role in attracting monocytes and causing them to enter the lungs of COVID-19 patients. Additionally, increased concentrations of CCL2 have been documented in the bronchoalveolar lavage fluid (BALF) collected from individuals with severe COVID-19. This leads to a cytokine storm and encourages the influx of CD163 + myeloid cells into the airways, resulting in additional damage to the alveoli38.
Additionally, as with IL-6, we observed that the elevation of IL-2 was linked to the severity of COVID-19 from the first days after symptom onset until three weeks later. Several studies have linked a notable rise in IL-2 levels with critical COVID-19 outcomes39, prolonged hospital stays and lymphopenia in COVID-19 patients40, and poor prognosis and disease severity41. An investigation was conducted on the impact of IL-2 on cellular immunity. IL-2/2R was observed to trigger JAK1–STAT5 signaling, leading to the activation of CD8 + cells42. In a study by Shi et al.43, IL-2 was found to be significantly elevated in the plasma of COVID-19 patients. IL-2R expression and JAK1–STAT5 pathway were reduced in COVID-19 patients with severe symptoms. This group showed that inhibiting IL-2R and increasing IL-2 levels can cause a decrease in CD8 + cells. However, some other studies showed that there was no correlation between IL-2 and the severity of COVID-19. Therefore, further research is required to confirm the functions of IL-2 in response to SARS-CoV-2 infection.
Multiple studies have suggested that immune suppression may possibly play a role in the onset of COVID-1944,45,46. As reported in a previous study22, we found the association of IL-1Ra, as an antagonist of IL-1β, with disease severity. This anti-inflammatory cytokine modulates IL-1, TNF-α47, and type I IFN production and suppresses T cell responses48. The mechanisms that underlie the association of IL-1Ra with COVID-19 severity are unclear but could reflect lymphocytopenia and impaired type I IFN production that are characteristics of severe COVID-1949.
Several studies have demonstrated that viruses such as respiratory influenza virus, syncytial virus, cytomegalovirus, and rotavirus can stimulate the production of IL-850,51,52,53. Consistent with our result, Lucas et al. reported that SARS-CoV-2 induced IL-8 production in patients with moderate and severe COVID-19 up to 25 days after symptom onset10. IL-8 primarily functions to induce chemotaxis in neutrophils. Neutrophils not only exhibit antibacterial action but also contribute to the innate immune system’s ability to resolve and repair by controlling the production of cytokines and growth factors54,55. Considering the lack of correlation between IL-8 and COVID-19 severity, it is possible that the function of neutrophils in COVID-19 patients with diverse severity is different.
The findings of this study indicate that the combination of pro-inflammatory cytokines (IL-1β, TNF-α, IL-12) and IFNα2 during the first week after symptom onset is associated with milder disease outcomes. This pattern may reflect an effective innate immune response that inhibits viral replication through the simultaneous activation of NF-κB and type I interferon pathways. However, the persistence of elevated levels of IL-1β and TNF-α into the second week, along with an increase in IL-1Ra, likely plays a modulatory role in inflammation for mild patients. Dorgham et al. also used PCA to illustrate cytokine profiles linked with COVID-19 severity56. They reported that patients with critical illness exhibit higher levels of inflammatory cytokines (TNF-a, IL-6, IL-8, and IL-10) and lower type-I interferon response (IFN-α and IFN-β). However, they only analyzed the sample of hospitalized patients that were collected on the day of hospital admission or over the first 2 weeks after hospitalization.
In contrast, the sustained elevation of IL-2, IL-8, and IL-1Ra until the third week is associated with progression to critical illness. This finding aligns with studies that have identified IL-8 as a predictive marker for mortality57,58 and linked IL-2 to hyperactivation of T lymphocytes59. The combination of these cytokines may lead to tissue damage through the recruitment of inflammatory neutrophils and macrophages.
The results obtained about IFN-α in different studies are somewhat controversial60. One of the reasons is undetectable levels of IFN-α by common assays among the wide range of patients. Our results revealed that over 80% of all COVID-19 patients, regardless of the disease severity, had undetectable or deficient levels of IFNα. A recent study has shown that in patients with COVID-19 with diverse disease severity, IFN-α is not produced as they could not be secreted in the sera of patients with influenza30. Due to this sharp decline in IFN-α in patients with COVID-19, ultrasensitive assays must be utilized to compare IFN-α between patients with different disease severity. The recent demonstration in a study using digital ELISA has identified higher levels of plasma IFN-α2 in outpatients compared to critical patients with COVID-19. Despite the high sensitivity of the assay used in this study, IFN-α2 dispersion is high among the patients, and the concentration of IFN-α2 in some outpatients is equal to the median of critical patients61. These findings imply that the reduction of IFN-α alone is not involved in the pathogenicity of COVID-19. Indeed, our finding by Spearman correlation and PCA uncovered that an early increase of IFN-α along with the production of inflammatory cytokines (especially IL-1β) can help antiviral responses. In contrast, an early or delayed decrease of IFN-α in conjunction with the rise of IL-1β is detrimental to the patient. A study by Karim Dorgham and colleagues revealed that inflammatory cytokines and type-I interferons (IFN-α and IFN-β) contributed to the first and second principal components, respectively. They reported that the increase of inflammatory cytokines and reduction of type-I interferons had an association with COVID-19 severity. However, they did not separate the samples based on time intervals, and the samples collected until 21 days after symptom onset were analyzed together56.
The available information indicates that the interaction between IL-1 and IFNs plays a vital role in preserving a delicate balance in the innate inflammatory response. However, there is complex evidence about IL-1 and IFNs cross-talk. Notably, both IFN-α and IFN-β have the ability to inhibit the transcription and translation of IL-1α and IL-1β in different types of cells62,63,64. Therefore, it could be suggested that impaired IFN-α production in critical individuals leads to unregulated prolonged production of IL-1β and acute consequences. On the other hand, a study has demonstrated that IL-1β interacts with IFN-α to increase the production of genes that fight against viruses by influencing the activation of STAT1 induced by IFN-α65. Considering that the prolonged increase of inflammatory cytokines and impaired production of IFN-α are the hallmarks of SARS-CoV-2 infection, precise investigations of the crosstalk of IFN-α and IL-1β can help to clarify the disease mechanism and appropriate treatment strategies.
Our study is not without caveats. The population of severely ill patients was smaller than that of other groups, which made it difficult to draw conclusions. Furthermore, the detection limit of our kits was in the range of picograms per milliliter (pg/mL), whereas employing more sensitive assays with detection limits in the femtogram per milliliter (fg/mL) range—particularly for IFNα2 and IFNβ—could have yielded more precise measurements and enhanced the detection of low-level cytokines. Additionally, the data primarily originate from blood samples and partially reflect immune responses within the lung. However, considering that local investigation can be invasive, examination of immune responses in peripheral blood is helpful.
Collectively, Due to the vast quantity of clinical samples included in our integrated study, we were able to thoroughly evaluate a longitudinal comparison of the cytokine profile in COVID-19 patients with different severity. Our findings support the idea that an excessive, persistent, and unbalanced production of a specific group of cytokines occurs after SARS-CoV-2 infection and is linked to the severity of respiratory symptoms. Consequently, we support the enhancement of individualized patient care by utilizing cytokine profiling.
Methods
Study population
COVID-19 patient samples were gathered as part of a cross-sectional and cohort study aimed at documenting clinical, immunological, and hematological abnormalities in these individuals (Fig. 1A). The COVID-19 diagnosis was made based on the recommendations provided by the World Health Organization (WHO) and confirmed with a positive SARS-CoV-2 PCR test using reverse transcription (RT–PCR) conducted on a sample taken from the nasopharynx. The classification of COVID-19 patients was determined according to the guidelines provided by the WHO66. The patients with mild complications were symptomatic and met the criteria for COVID-19 without any signs of viral pneumonia or low oxygen levels. The hospitalized adults with moderate illness exhibited clinical manifestations of pneumonia such as fever, cough, dyspnea, and rapid breathing. However, they did not display any indications of severe pneumonia, including a blood oxygen saturation level (SpO2) of 90% or more while breathing normal air. The hospitalized individuals with a severe illness exhibited clinical symptoms of pneumonia, including fever, cough, and difficulty breathing, along with one of the following: a respiratory rate above 30 breaths per minute, severe respiratory distress, or an oxygen saturation level below 90% when breathing normal air. The hospitalized individuals with a severe illness exhibited clinical symptoms of pneumonia, including fever, cough, and difficulty breathing, along with one of the following: a respiratory rate above 30 breaths per minute, severe respiratory distress, or an oxygen saturation level below 90% when breathing normal air. And finally, the patients with critical implications required the life support of mechanical ventilation (Fig. 1B). None of the participants had received any COVID-19 vaccine at the time of the study. The days from symptom onset were calculated, with the utmost priority given to explicit onset dates reported by patients. Clinical treatment information is demonstrated in Supplementary Material.
Cross-sectional/Tehran study
This study was conducted by Immunoregulation Research Center of Shahed University and approved by the National Ethics Committee on Research in Medical Sciences of the Iranian Ministry of Health (IR.NIMAD.REC.1398.411). All enrolled people provided informed consent. The study included a total of 72 control donors and 144 COVID-19 patients, as shown in Fig. 1A. Demographic and medication information are listed in Table 1. Hospitalized patients were recruited between March and April 2020 from the Imam Khomeini Hospital Complex in Tehran, Iran. Healthy, asymptomatic individuals were considered as the control group. SARS-CoV-2 PCR test was not performed for the control group, while individuals with positive results for SARS-CoV-2 IgM or IgG were excluded. Blood was drawn once. The serum samples were collected and stored until further use.
COVID-19 National cohort study
The national COVID-19 study comprises a subset of patients who were registered between May and August of 2020. This study conducted a prospective observational cohort analysis to assess the clinical, immunological, and hematological features of patients with COVID-19 in six provinces of Iran, namely Tehran, Razavi Khorasan, Isfahan, Khuzestan, Sistan and Baluchestan, and Mazandaran (Fig. 1A). The study is approved by the National Ethics Committee on Research in Medical Sciences the Iranian Ministry of Health (IR.NIMAD.REC.1399.041). Written informed consent was obtained from each patient or their legal representative in order to take part in the study. The number of control subjects and patients in each subgroup in each province was approximately equal. In total, 152 healthy controls with serological negative results for SARS-CoV-2 and 665 Covid-19 patient samples were received. (Fig. 1A). The collected samples were classified into three time periods: one to seven days, eight to fourteen days, and more than fourteen days after the symptom onset, which provided a unique opportunity to recreate the temporal cytokine profile by combining data from multiple patients (Fig. 1C). Data regarding age, gender, body mass index, smoking status, medication, symptoms, and co-morbidities is listed in Table 1. All methods of both studies were performed in accordance with Ethical Guidance for Research on Human Tissues and Organs in the Islamic Republic of Iran.
Cytokine analysis
The cytokine assessment was conducted on serum samples that had been frozen and stored at a temperature of -80 °C. Human IFN-α (#DY9345), IFN-β (#DY814), IFN-γ (#DY285), TNF (#DY210), IL-1β (#DY201), IL-1Ra (#DY280), IL-8 (#DY208-05), IL-17 A (#DY317), and IL-18 (#DY318) DuoSet ELISA kits were purchased from R&D Systems (Wiesbaden-Nordenstadt, Germany). The assay was conducted in accordance with the manufacturer’s procedure for serum samples. All procedures were performed with half the recommended volume of the kit. Briefly, the diluted capture antibody was applied to 96-well microplates, which were then incubated overnight at room temperature (RT). To block the plates, a 1% solution of bovine serum albumin in phosphate-buffered saline (PBS) was added. The plates were then incubated at RT for 2–3 h. Then, the samples or standards were added and incubated for 2 h at RT. After washing, the detection antibody was surcharged, and incubation was repeated. The plates were incubated with a working dilution of Streptavidin-HRP B in a dark place for 20 min. Then, each well was left with tetramethylbenzidine as the substrate. In this step, the incubation time was different for each cytokine. The reaction was stopped with H2SO4 (2 N). Finally, the plates were read using Biotek Elisa reader at 450 nm. The data were obtained and processed using Biotek Gen5 v.1.05 software.
We used an automated immunoassay to check the amounts of IL-6 (L2K6P2) and IL-2 (L2KIP2, IMMULITE 2000 Immunoassay System, Siemens Healthcare Diagnostics Inc.) in the serum. The measurable range of IL-6 and IL-2 concentrations for these assays are 2-1000 pg/mL and 5-1000 pg/mL, respectively.
Statistical analysis
Data were analyzed on GraphPad Prism software v8.0. Basic demographic characteristics between healthy controls and COVID-19 patients with different disease severity (mild/moderate/severe/ critical), were analyzed using ANOVA and chi-square tests as appropriate. The cytokine data was log transformed. For comparison of the cytokine’s levels across study groups, Kurskal-Wallis test was used followed with Mann-Whitney. For correlational analysis, Spearman rank tests were used. The correlation results visualized using the corrplot R package. After that, PCA was run on all cytokines that a significant different between severity groups for COVID-19 patients. For this analysis, log transformed data was reported as z-scores of the overall cohort due to the departed distribution from normal. The missing data for cytokines were replaced via regression with other cytokines. The Kaiser-Meyer-Olkin measure and the Bartlett test of sphericity were used to assess the data’s appropriateness for the PCA dataset in the initial analysis. individual scores for components with an Eigenvalue > 1.0 that remained constant after varimax rotation were allocated to individuals with acute COVID-19. Principal component scores for these components were kept.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Huang, C. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395(10223), 497–506. https://doi.org/10.1016/s0140-6736(20)30183-5 (2020).
Yue, H. et al. Clinical characteristics of coronavirus disease 2019 in Gansu Province, China. Ann. Palliat. Med. 9(4), 1404–1412. https://doi.org/10.21037/apm-20-887 (2020).
Wu, Z. & McGoogan, J. M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. Jama 323(13), 1239–1242. https://doi.org/10.1001/jama.2020.2648 (2020).
Preliminary Estimates of the Prevalence of Selected Underlying Health Conditions Among Patients with Coronavirus. Disease 2019 - United States, February 12-March 28, 2020. MMWR Morb Mortal. Wkly. Rep. 69(13), 382–386. https://doi.org/10.15585/mmwr.mm6913e2 (2020).
Channappanavar, R. & Perlman, S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 39(5), 529–539. https://doi.org/10.1007/s00281-017-0629-x (2017).
Mehta, P. et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 395(10229), 1033–1034. https://doi.org/10.1016/s0140-6736(20)30628-0 (2020).
Wu, C. et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 180(7), 934–943. https://doi.org/10.1001/jamainternmed.2020.0994 (2020).
Yang, X. et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 8(5), 475–481. https://doi.org/10.1016/s2213-2600(20)30079-5 (2020).
Hadjadj, J. et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 369(6504), 718–724. https://doi.org/10.1126/science.abc6027 (2020).
Lucas, C. et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 584(7821), 463–469. https://doi.org/10.1038/s41586-020-2588-y (2020).
Arunachalam, P. S. et al. Systems biological assessment of immunity to mild versus severe COVID-19 infection in humans. Science 369(6508), 1210–1220. https://doi.org/10.1126/science.abc6261 (2020).
Sainz, B. Jr. & Halford, W. P. Alpha/Beta interferon and gamma interferon synergize to inhibit the replication of herpes simplex virus type 1. J. Virol. 76(22), 11541–11550. https://doi.org/10.1128/jvi.76.22.11541-11550.2002 (2002).
Bartee, E., Mohamed, M. R., Lopez, M. C., Baker, H. V. & McFadden, G. The addition of tumor necrosis factor plus beta interferon induces a novel synergistic antiviral state against poxviruses in primary human fibroblasts. J. Virol. 83(2), 498–511. https://doi.org/10.1128/jvi.01376-08 (2009).
Kerr, P. J. et al. Evolutionary history and Attenuation of Myxoma virus on two continents. PLoS Pathog. 8(10), e1002950. https://doi.org/10.1371/journal.ppat.1002950 (2012).
Lee, A. J. & Ashkar, A. A. The dual nature of type I and type II interferons. Front. Immunol. 9, 2061. https://doi.org/10.3389/fimmu.2018.02061 (2018).
Hunter, C. A. & Jones, S. A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 16(5), 448–457. https://doi.org/10.1038/ni.3153 (2015).
Liu, J. et al. TNF is a potent anti-inflammatory cytokine in autoimmune-mediated demyelination. Nat. Med. 4(1), 78–83. https://doi.org/10.1038/nm0198-078 (1998).
Teijaro, J. R. et al. Persistent LCMV infection is controlled by Blockade of type I interferon signaling. Science 340(6129), 207–211. https://doi.org/10.1126/science.1235214 (2013).
Ahlenstiel, G. et al. Early changes in natural killer cell function indicate virologic response to interferon therapy for hepatitis C. Gastroenterology. 141(4), 1231-9, 1239.e1-2, https://doi.org/10.1053/j.gastro.2011.06.069 (2011).
Werner, J. M. et al. Ribavirin improves the IFN-γ response of natural killer cells to IFN-based therapy of hepatitis C virus infection. Hepatology 60(4), 1160–1169. https://doi.org/10.1002/hep.27092 (2014).
Pontiroli, F. et al. The timing of IFNβ production affects early innate responses to Listeria monocytogenes and determines the overall outcome of lethal infection. PLoS ONE. 7(8), e43455. https://doi.org/10.1371/journal.pone.0043455 (2012).
Zhao, Y. et al. Longitudinal COVID-19 profiling associates IL-1RA and IL-10 with disease severity and RANTES with mild disease. JCI Insight. https://doi.org/10.1172/jci.insight.139834 (2020).
Lee, J. S. et al. Immunophenotyping of COVID-19 and influenza highlights the role of type I interferons in development of severe COVID-19. Sci. Immunol. https://doi.org/10.1126/sciimmunol.abd1554 (2020).
Blanco-Melo, D. et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 181(5), 1036–1045e9. https://doi.org/10.1016/j.cell.2020.04.026 (2020).
Zhang, B. et al. Clinical characteristics of 82 cases of death from COVID-19. PLoS ONE. 15(7), e0235458. https://doi.org/10.1371/journal.pone.0235458 (2020).
Del Valle, D. M. et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 26(10), 1636–1643. https://doi.org/10.1038/s41591-020-1051-9 (2020).
Hu, B., Huang, S. & Yin, L. The cytokine storm and COVID-19. J. Med. Virol. 93(1), 250–256. https://doi.org/10.1002/jmv.26232 (2021).
Jose, R. J. & Manuel, A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir. Med. 8(6), e46–e47. https://doi.org/10.1016/s2213-2600(20)30216-2 (2020).
Kox, M., Waalders, N. J. B., Kooistra, E. J., Gerretsen, J. & Pickkers, P. Cytokine levels in critically ill patients with COVID-19 and other conditions. Jama 324(15), 1565–1567. https://doi.org/10.1001/jama.2020.17052 (2020).
Galani, I. E. et al. Untuned antiviral immunity in COVID-19 revealed by temporal type I/III interferon patterns and flu comparison. Nat. Immunol. 22(1), 32–40. https://doi.org/10.1038/s41590-020-00840-x (2021).
Liu, F. et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J. Clin. Virol. 127, 104370. https://doi.org/10.1016/j.jcv.2020.104370 (2020).
Mandel, M., Harari, G., Gurevich, M. & Achiron, A. Cytokine prediction of mortality in COVID19 patients. Cytokine 134, 155190. https://doi.org/10.1016/j.cyto.2020.155190 (2020).
Conway, E. M. et al. Understanding COVID-19-associated coagulopathy. Nat. Rev. Immunol. 22(10), 639–649. https://doi.org/10.1038/s41577-022-00762-9 (2022).
Schett, G., Elewaut, D., McInnes, I. B., Dayer, J. M. & Neurath, M. F. How cytokine networks fuel inflammation: toward a cytokine-based disease taxonomy. Nat. Med. 19(7), 822. https://doi.org/10.1038/nm.3260 (2013).
Hafez, W. et al. Treatment outcomes of tocilizumab in critically-Ill COVID-19 patients, single-centre retrospective study. Antibiot. (Basel). https://doi.org/10.3390/antibiotics11020241 (2022).
Thoms, B. L., Gosselin, J., Libman, B., Littenberg, B. & Budd, R. Efficacy of combination therapy with the JAK inhibitor baricitinib in the treatment of COVID-19. Res. Sq. https://doi.org/10.21203/rs.3.rs-835734/v1 (2021).
Hurst, S. M. et al. Il-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity 14(6), 705–714. https://doi.org/10.1016/s1074-7613(01)00151-0 (2001).
Ranjbar, M., Rahimi, A., Baghernejadan, Z., Ghorbani, A. & Khorramdelazad, H. Role of CCL2/CCR2 axis in the pathogenesis of COVID-19 and possible treatments: all options on the table. Int. Immunopharmacol. 113(Pt A), 109325. https://doi.org/10.1016/j.intimp.2022.109325 (2022).
Torres-Ruiz, J. et al. Clinical and immunological features associated to the development of a sustained immune humoral response in COVID-19 patients: results from a cohort study. Front. Immunol. 13, 943563. https://doi.org/10.3389/fimmu.2022.943563 (2022).
Singh, A. K. et al. Opposing roles for sMAdCAM and IL-15 in COVID-19 associated cellular immune pathology. J. Leukoc. Biol. 111(6), 1287–1295. https://doi.org/10.1002/jlb.3covbcr0621-300r (2022).
Khan, M. et al. Gut dysbiosis and IL-21 response in patients with severe COVID-19. Microorganisms https://doi.org/10.3390/microorganisms9061292 (2021).
Fu, X. et al. Enhanced interaction between sect. 2 mutant and TCR Vβ induces MHC II-independent activation of T cells via PKCθ/NF-κB and IL-2R/STAT5 signaling pathways. J. Biol. Chem. 293(51), 19771–19784. https://doi.org/10.1074/jbc.RA118.003668 (2018).
Shi, H. et al. The Inhibition of IL-2/IL-2R gives rise to CD8(+) T cell and lymphocyte decrease through JAK1-STAT5 in critical patients with COVID-19 pneumonia. Cell. Death Dis. 11(6), 429. https://doi.org/10.1038/s41419-020-2636-4 (2020).
Reyes, M. et al. Induction of a regulatory myeloid program in bacterial sepsis and severe COVID-19. bioRxiv. https://doi.org/10.1101/2020.09.02.280180 (2020).
Tian, W. et al. Immune suppression in the early stage of COVID-19 disease. Nat. Commun. 11(1), 5859. https://doi.org/10.1038/s41467-020-19706-9 (2020).
Liu, N. et al. Single-cell analysis of COVID-19, sepsis, and HIV infection reveals hyperinflammatory and immunosuppressive signatures in monocytes. Cell. Rep. 37(1), 109793. https://doi.org/10.1016/j.celrep.2021.109793 (2021).
Oleksowicz, L. & Dutcher, J. P. A review of the new cytokines: IL-4, IL-6, IL-11, and IL-12. Am. J. Ther. 1(2), 107–115. https://doi.org/10.1097/00045391-199408000-00002 (1994).
Theofilopoulos, A. N., Baccala, R. & Beutler, B. Kono. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu. Rev. Immunol. 23, 307–336. https://doi.org/10.1146/annurev.immunol.23.021704.115843 (2005).
Wang, Z., Pan, H. & Jiang, B. Type I IFN deficiency: an immunological characteristic of severe COVID-19 patients. Signal. Transduct. Target. Ther. 5(1), 198. https://doi.org/10.1038/s41392-020-00306-4 (2020).
Choi, A. M. & Jacoby, D. B. Influenza virus A infection induces interleukin-8 gene expression in human airway epithelial cells. FEBS Lett. 309(3), 327–329. https://doi.org/10.1016/0014-5793(92)80799-m (1992).
Fiedler, M. A. & Wernke-Dollries, K. and J. M. Stark. Respiratory syncytial virus increases IL-8 gene expression and protein release in A549 cells. Am. J. Physiol. 269(6 Pt 1), L865-72. https://doi.org/10.1152/ajplung.1995.269.6.L865 (1995).
Murayama, T. et al. Human cytomegalovirus induces interleukin-8 production by a human monocytic cell line, THP-1, through acting concurrently on AP-1- and NF-kappaB-binding sites of the interleukin-8 gene. J. Virol. 71(7), 5692–5695. https://doi.org/10.1128/jvi.71.7.5692-5695.1997 (1997).
Sheth, R. et al. Rotavirus stimulates IL-8 secretion from cultured epithelial cells. Virology 221(2), 251–259. https://doi.org/10.1006/viro.1996.0374 (1996).
Wang, J. et al. Visualizing the function and fate of neutrophils in sterile injury and repair. Science 358(6359), 111–116. https://doi.org/10.1126/science.aam9690 (2017).
Yang, W. et al. Neutrophils promote the development of reparative macrophages mediated by ROS to orchestrate liver repair. Nat. Commun. 10(1), 1076. https://doi.org/10.1038/s41467-019-09046-8 (2019).
Dorgham, K. et al. Distinct cytokine profiles associated with COVID-19 severity and mortality. J. Allergy Clin. Immunol. 147(6), 2098–2107. https://doi.org/10.1016/j.jaci.2021.03.047 (2021).
Iskandar, A. et al. Correlation between IL-8, C-reactive proteins (CRP) and neutrophil to lymphocyte ratio (NLR) as predictor of mortality in COVID-19 patients with diabetes mellitus comorbidity. Int. J. Gen. Med. 16, 2349–2354. https://doi.org/10.2147/ijgm.S412070 (2023).
Uno, K. et al. Predictive biomarkers of COVID-19 prognosis identified in Bangladesh patients and validated in Japanese cohorts. Sci. Rep. 14(1), 12713. https://doi.org/10.1038/s41598-024-63184-8 (2024).
Ross, S. H. & Cantrell, D. A. Signaling and function of interleukin-2 in T lymphocytes. Annu. Rev. Immunol. 36, 411–433. https://doi.org/10.1146/annurev-immunol-042617-053352 (2018).
Eskandarian Boroujeni, M. et al. Dysregulated interferon response and immune hyperactivation in severe COVID-19: targeting stats as a novel therapeutic strategy. Front. Immunol. 13, 888897. https://doi.org/10.3389/fimmu.2022.888897 (2022).
Smith, N. et al. Defective activation and regulation of type I interferon immunity is associated with increasing COVID-19 severity. Nat. Commun. 13(1), 7254. https://doi.org/10.1038/s41467-022-34895-1 (2022).
Novikov, A. et al. Mycobacterium tuberculosis triggers host type I IFN signaling to regulate IL-1β production in human macrophages. J. Immunol. 187(5), 2540–2547. https://doi.org/10.4049/jimmunol.1100926 (2011).
Mayer-Barber, K. D. et al. Caspase-1 independent IL-1beta production is critical for host resistance to mycobacterium tuberculosis and does not require TLR signaling in vivo. J. Immunol. 184(7), 3326–3330. https://doi.org/10.4049/jimmunol.0904189 (2010).
Mayer-Barber, K. D. et al. Host-directed therapy of tuberculosis based on interleukin-1 and type I interferon crosstalk. Nature 511(7507), 99–103. https://doi.org/10.1038/nature13489 (2014).
Ichikawa, T. et al. Involvement of IL-1beta and IL-10 in IFN-alpha-mediated antiviral gene induction in human hepatoma cells. Biochem. Biophys. Res. Commun. 294(2), 414–422 https://doi.org/10.1016/s0006-291x(02)00502-8 (2002).
World Health Organization. Clinical management of COVID-19: Living guideline. https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2023.1 (Accessed 13 Jan 2023).
Acknowledgements
This study was funded by the Ministry of Health and Medical Education of Iran and the Immunoregulation Research Centre of Shahed University. As a final note, we would like to thank all of the participants and medical professionals who helped with COVID-19 diagnosis and treatment.
Author information
Authors and Affiliations
Contributions
T.G., S.KA., and S.G. conceived, designed the study. S.G. analyzed results and wrote the manuscript. S.G., A.M.M.M., and A.R. performed most of the experiments. M.S., and M.R.V.M., M.R.C., A.M., A.R., A.K., S.I., and H.A.K. facilitated with some experiments. M.M.N. and S.G. assisted with statistical analysis. T.G. and S.K.A. proofread the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ghaffarpour, S., Ghazanfari, T., Ardestani, S.K. et al. Cytokine profiles dynamics in COVID-19 patients: a longitudinal analysis of disease severity and outcomes. Sci Rep 15, 14209 (2025). https://doi.org/10.1038/s41598-025-98505-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-98505-y
Keywords
This article is cited by
-
A multiplexed LC–MS/MS method to reveal changes in inflammatory and coagulation cascades induced by host infection
Analytical and Bioanalytical Chemistry (2025)