Abstract
We evaluated the predictive value of radiomics features from different peritumoral ranges for the invasiveness of ground-glass nodular lung adenocarcinoma using various machine learning models. This retrospective study included 317 patients with 323 ground-glass nodules diagnosed as minimally invasive adenocarcinoma (MIA) or invasive adenocarcinoma (IAC) at Benxi Central Hospital (January 2019–December 2023). Radiomic features from tumor margins of 1, 2, 3, 4, and 5 mm were extracted. Eight machine learning models were constructed following dimensionality reduction. The models were evaluated using receiver operating characteristic curves. All models had area under the curve values > 0.75, effectively distinguishing between MIA and IAC. Only the decision tree model showed statistically significant differences (P < 0.05); no differences were found between the other models (P > 0.05). In the training set, the 1-mm margin model achieved the highest ranking, followed by the 2-, 4-, 5-, and 3-mm models. In the validation group, the 3-mm margin model ranked the highest, followed by the 2-, 1-, 4-, and 5-mm models, with no statistically significant differences (P > 0.05). All machine learning models demonstrated good predictive performance for both MIA and IAC. Radiomic features from 1 to 5-mm margins showed strong predictive value, though no optimal margin range was identified.
Similar content being viewed by others
Introduction
Lung cancer is a common malignant tumor, accounting for the highest number of new cases and deaths worldwide1. According to the National Lung Screening Trial in the United States, lung computed tomography (CT) screening in high-risk populations effectively reduces lung cancer mortality2. Most lung cancers appearing as ground-glass nodules on CT scans are adenocarcinomas, and the treatment and prognosis of different invasive adenocarcinomas (IACs) significantly vary. Microinvasive adenocarcinomas (MIAs) are typically treated with segmental resection, whereas IACs often require lobectomy, significantly affecting lung function. The 5-year survival rate after surgery for MIA can reach 100%, while that for IAC is significantly lower3. Therefore, predicting the tumor invasiveness of lung adenocarcinomas that manifest as ground-glass nodules before surgery is highly valuable to physicians.
Radiomics has been widely applied in disease prediction and tumor grading research4,5,6. Studies have developed models with strong classification performance based on tumor imaging omics characteristics7,8,9, confirming that these models can effectively distinguish between microinvasive and invasive adenocarcinomas. Several scholars have established omics models incorporating peritumoral features to predict ground-glass nodules. However, the diagnostic performance of imaging omics models in distinguishing MIA from IAC across different peritumoral ranges remains unclear. In this study, we aimed to evaluate the predictive capability of radiomic features from different peritumoral ranges for the invasiveness of ground-glass nodular lung adenocarcinoma using various machine learning models.
Results
Participant demographics and nodule characteristics
This study enrolled 323 pulmonary ground-glass nodules from 317 patients (239 female and 78 males individuals) aged 24–78 (mean age, 54.90 ± 10.70) years. Of the 323 patients with pulmonary ground-glass nodules, there were 80 male (24.77%) and 243 female individuals (75.23%). There were 157 MIA cases (48.61%) and 166 IAC cases (51.39%). Additionally, 118 nodules were mixed ground-glass (36.53%), while 205 were pure ground-glass (63.47%). The mean diameter of the nodules was 9.99 ± 4.30 mm. Statistical analysis revealed significant differences in age, nodule type, and nodule diameter between the MIA and IAC groups but no significant differences in sex or nodule ___location (Table 1).
Radiomic feature extraction and screening
Z-score normalization was applied to 822radiomic features of the training group, and the T-test, Maximum Relevance Minimum Redundancy (MRMR), and Least Absolute Shrinkage and Selection Operator (LASSO) based on 10-fold cross-validation were used for screening (Fig. 1). Thirteen features were obtained within a 1-mm tumor margin, six within a 2-mm margin, eight within a 3-mm margin, 16 within a 4-mm margin, and eight within a 5-mm margin (Fig. 2).
Selection of least absolute shrinkage and selection operator (LASSO) dimensionality reduction parameters and feature screening. (a, c, e, g, i, and j) shows the lambda value maps of 1-mm, 2-mm, 3-mm, 4-mm, and 5-mm tumors, while (b, d, h and f, and j) present the LASSO algorithm feature screening map based on 10-fold cross-validation.
Weight map and heatmap of peritumoral omics features. (a and b) represent the 1-mm peritumoral features; (c and d) represent the peritumoral 2-mm features; (e and f) represent the peritumoral 3-mm features; (g and h) represent the 4-mm peritumoral features; (i and j) represent the 5-mm peritumoral features.
Machine learning model development and performance
Eight machine learning methods were applied to develop models using the radiomic features extracted from tumor margins of 1 mm, 2 mm, 3 mm, 4 mm, and 5 mm in the training groups. The area under the curve (AUC) values of all the machine learning models were approximately 0.9, except for the decision tree model, which ranged from 0.75 to 0.85. All machine learning models performed well in distinguishing between MIA and IAC (Fig. 3). The optimal model for each set of features was defined as the one with the highest AUC value among the eight machine learning models constructed using radiomic features extracted from the 1 mm, 2 mm, 3 mm, 4 mm, and 5 mm peritumoral ranges. The optimal machine learning models were Naive Bayes for the 1 mm, 2 mm, and 5 mm peritumoral ranges, Neural Network for the 3 mm range, and Logistic Regression model for the 4 mm range. Pairwise comparisons using the DeLong test were performed on the AUC values of the eight machine-learning models for the 1 mm, 2 mm, 3 mm, 4 mm, and 5 mm peritumoral ranges. The results showed statistical differences in AUC values between the decision tree model and other machine learning models (P < 0.05), while no significant differences were found among the other models (P > 0.05).
Model evaluation and statistical analysis
The optimal AUC values and 95% confidence intervals (CIs) for the 1 mm, 2 mm, 3 mm, 4 mm, and 5 mm tumor features in the training group were: 1 M AUC: 0.9265 (95% CI 0.7963–0.9845), 2Ml AUC: 0.9253 (95% CI 0.8357–0.9966), 3 M AUC: 0.9204 (95% CI 0.7998–1.0000), 4 M AUC: 0.9240 (95% CI 0.8160–0.9904), 5Ml AUC: 0.9229 (95% CI 0.7818–0.9981); The optimal AUC values and 95% CIs for each feature group in the validation group are as follows: 1 M AUC: 0.8920 (95% CI 0.7045–1.0000), 2 M AUC: 0.9030 (95% CI 0.7090–1.0000), 3 M AUC: 0.9080 (95% CI 0.8000–1.0000), 4 M AUC: 0.8920 (95% CI 0.6748–1.0000), 5 M AUC: 0.8550 (95% CI 0.6725–1.0000); The AUC values of the receiver operating characteristic (ROC) curves for the 1 mm, 2 mm, 3 mm, 4 mm, and 5 mm peritumoral feature models in both the training and validation sets exceeded 0.8, indicating good discriminatory ability (Fig. 4). Most specificity, accuracy, sensitivity, positive predictive value, and negative predictive value for each model in the training and validation groups exceeded 0.8, further demonstrating the excellent discriminatory capabilities of the models (Table 2). The order of AUC values in the training group was 1M > 2M > 4M > 5M > 3 M, while in the validation group, it was 3M > 2M > 1M > 4M > 5M. All AUC values of the models showed P-values exceeding 0.05 after pairwise comparison using the DeLong test in both the training and validation group, indicating no statistically significant differences in model performance (Tables 3 and 4).
Discussion
This study found no significant differences in sex or nodule ___location between the MIA and IAC groups. However, significant differences were observed in age, nodule type, and nodule diameter, aligning with most previous studies4,7,10. Some studies found no significant differences in age between the two groups11,12. Our findings differ, possibly due to the sample size and selection bias. Nevertheless, we can infer that the nodule type and diameter are relatively stable and reliable features that can be used to develop a multifactorial model to distinguish between MIA and IAC in subsequent studies.
In this study, based on the radiomic features of 1-mm, 2-mm, 3-mm, 4-mm, and 5-mm peritumoral regions on thin-slice CT images, eight different machine learning methods were selected to build models using the same dataset. All models demonstrated AUC values > 0.75, indicating good performance in distinguishing MIA from IAC. While previous studies have shown that machine learning models can effectively classify diseases, most of them selected the optimal model based on AUC values without statistically comparing the diagnostic performance of different models131415. Cho et al. studied the invasiveness of lung adenocarcinoma using three machine-learning models: support logistic regression, vector machine, and free forest. They reported AUC values of 0.7490, 0.7767, and 1.0000 for the training sets and 0.9766, 0.8450, and 0.9211 for the validation sets, respectively. The logistic regression model was identified as the optimal model7. Liu et al. established a prediction model using six machine-learning algorithms to validate the invasiveness of small nodular adenocarcinomas in the lungs. They found that the AUC values of all six models were ≥ 0.914 and ≥ 0.732 in the training and validation groups, respectively. Although the performance of the six machine learning models varied, they all demonstrated good predictive capability for the aggressiveness of pulmonary nodular adenocarcinoma16. These studies show that machine learning models can effectively classify both microinvasive and invasive lung adenocarcinoma; however, none showed statistically significant differences in performance among various models predicting invasiveness.
In this study, eight different machine learning models were compared for their diagnostic performance in distinguishing between microinvasive and invasive lung adenocarcinoma using the DeLong test for pairwise statistical comparisons. The results revealed a statistically significant difference in the AUC values between the decision tree model and the other models. However, no significant differences were observed among the remaining seven models. While the decision tree model demonstrated a statistically significant difference in diagnostic efficacy compared to the other models, it exhibited the weakest overall performance. The AUC values were 0.75–0.85, while the AUC values of the other models were approximately 0.9. This may be related to the higher sample size requirements of the decision tree model. The results indicate that, except for the decision tree model, other machine learning models perform better in distinguishing MIA and IAC features in radiomics.
Microscopic studies have found a transition zone of 0–3.78 mm between lung adenocarcinoma and normal lung tissue, with the width of this zone not significantly correlated with tumor size17, suggesting that useful information may also exist in the surrounding tissue of lung adenocarcinoma. In recent years, some scholars have focused on the radiomic characteristics of the peritumoral tissues. Masquelin et al. investigated radiomic models using tumor tissue, as well as 10-mm and 15-mm peritumoral regions, and found that both tumor and peritumoral models effectively predicted the benign or malignant nature of lung nodules. A statistical difference in performance was observed between the tumor and 10 -mm peritumoral models but not between the tumor and 15-mm peritumoral models18. Zhu et al. reported AUC values of 0.811 and 0.797 for the tumor and peritumoral models in the training set, respectively, and 0.814 and 0.808 in the validation set. The combined model achieved an AUC of 0.828 in bothsets, suggesting that peritumoral features are strong predictors of lung adenocarcinoma invasiveness19.
Yu et al. combined tumor and 15-mm peritumoral radiomic features to construct a three-level random forest model for classifyingatypical adenomatous hyperplasia/adenocarcinoma in-situ, MIA, and IAC. They found that all models had AUC values exceeding 0.9 in both the training and validation sets, suggesting that peritumoral histological features are beneficial for predicting the pathological type of ground-glass nodules20. Similarly, Huang et al. found that a model constructed using the radiomics features of the tumor and a 5-mm tumor periphery could effectively predict the pathological invasiveness of pulmonary nodules. The AUC value of the tumor model was higher than 0.8 in the training, internal validation, and external validation sets, while it was around 0.9 in the training set, internal validation set, and external validation set of the peritumoral set. The AUC of the peritumoral model was slightly higher than that of the tumor model21. These studies confirm the heterogeneity of peritumoral tissues and the valuable information embedded in CT imaging, supporting the effectiveness of peritumoral radiomics models in predicting the invasiveness of lung nodules. However, these studies did not systematically compare different peritumoral ranges.
In this study, the AUC values of the 1-mm, 2-mm, 3-mm, 4-mm, and 5-mm peritumoral models were all above 0.8 in both the training and validation cohorts, indicating strong differentiation between MIA and IAC. These findings are consistent with those of previous studies and validate the diagnostic efficacy of peritumoral radiomic-based models. Other researchers have investigated the ability of 2-mm and 5-mm peritumoral radiomics features to distinguish the invasiveness of ground-glass nodule adenocarcinoma. They found that the AUC values of the 2-and 5-mm peritumoral models ranged from 0.778 to 0.888 in both the training and validation cohorts, all above 0.7, demonstrating good predictive value for the invasiveness of ground-glass nodule-like adenocarcinoma. However, no statistically significant difference was observed between the predictive performance of the two models22.
Further, we aimed to identify the optimal peritumoral range by evaluating five groups with peritumoral margins ranging from 1 to 5 mm. These results indicated that all five peritumoral radiomic features effectively distinguished MIA from IAC. The AUC values of the different peritumoral models followed the order of 1M > 2M > 4M > 5M > 3M in the training set and 3M > 2M > 1M > 4M > 5M in the validation set. Although the AUC values of the five peritumoral models did not show statistical differences after DeLong’s test, this indicates that none of the feature groups has a distinct performance advantage. It also suggests that radiomic models with smaller peritumoral ranges generally yield higher AUC values than those with larger ranges. Therefore, smaller peritumoral ranges appear to be better choices for future multifactorial models.
However, this study had some limitations. First, to verify the performance of the different machine learning models, data were collected under uniform scanning conditions and equipment, reducing variability but limiting the number of eligible cases. Second, the study extracted three-dimensional spatial omics features, which improved model performance; however, external validation may show a significant decrease in performance. Finally, as a single-center retrospective study, future multicenter and prospective studies are required to externally validate the model and enhance the credibility of the results.
In summary, eight machine learning models—including logistic regression, adaptive reinforcement learning, random forest, Naïve Bayes, decision tree, support vector machine, K-nearest neighbor algorithm, and neural networks—demonstrated strong predictive performance for MIA and IAC classification. Peritumoral radiomic models with 1-mm, 2-mm, 3-mm, 4-mm, and 5-mm tumor margins exhibited robust predictive ability for MIA and IAC; however, no single optimal tumor margin range was identified.
Methods
Patient population
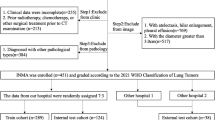
This retrospective study was approved by the Ethics Committee of Benxi Central Hospital in Liaoning Province (approval number: 20230322). Due to the retrospective nature of the study, the Ethics Committee of Benxi Central Hospital in Liaoning Province waived the need of obtaining informed consent. All methods were performed in accordance with the guidelines and regulations of Declaration of Helsinki. Patients with lung nodules confirmed by postoperative pathology between January 2019 and December 2023 were enrolled. The inclusion criteria were as follows: (1) pulmonary nodules with a diameter of ≤ 30 mm presenting as ground-glass; (2) chest CT scan with a thickness of 0.625 mm within 1 month before surgery; and (3) postoperative pathologic confirmation as a MIA or IAC. The exclusion criteria were as follows: (1) biopsy or chemoradiotherapy before CT screening; (2) previous diagnosis of lung cancer or other malignant tumors; and (3) poor CT image quality. Finally, 317 patients with 323 ground-glass density nodules diagnosed as pulmonary MIA and IAC were enrolled and randomly categorized into training and validation groups in a 7:3 ratio. All histopathologies were evaluated and diagnosed by a senior attending physician and a senior associate chief physician with over 15 years of pathology experience according to the 2011 IASLC/ATS/ERS classification.
CT scan and image processing
All high-resolution CT scans were performed using a GE LightSpeed VCT scanner(GE Healthcare, Chicago, IL, USA) with high-resolution computed tomography. The scan parameters were set as follows: detector collimation of 0.625 mm × 64, automatic tube current modulation, Tube voltage of 120 kV, Matrix of 512 × 512, reconstructed section thickness and spacing of 0.625 mm each, and a field of view of 300 mm. All patients underwent a supine examination and completed the scan with the last breath hold during inhalation. The scan covered the entire lung from the apex to the costophrenic angle. Lung window images were reconstructed using bone algorithms.
A senior radiologist (radiologist A) used 3D Slicer 5.2.2 (https://www.slicer.org/, Singapore) to manually delineate the three-dimensional volume of interest (VOI) of all ground-glass nodules and expanded the outer contour of the nodules by 1, 2, 3, 4, and 5 mm. In the peritumoral images, normal tissues such as large blood vessels, bronchioles, pleura, chest wall, and mediastinum were manually delineated and excluded from each VOI (Fig. 5). Another senior radiologist (radiologist B) evaluated the VOI, and any discrepancies in the manually drawn boundaries were resolved by consensus between the two radiologists. After an interval of 3 months, 30 randomly selected nodules were re-delineated by senior radiologist B for VOI assessment. Only the ___location and size of the nodules were provided to the two radiologists; other clinical data and pathological diagnosis results were not available to the radiologists during the segmentation process.
Illustration of the region of interest for pure ground-glass nodules. A 73-year-year-old female patient; (a) The high-resolution computed tomography (HRCT) lung window shows a pure ground glass nodule in the posterior segment of the upper lobe of the right lung; (b) The tumor region of interest (ROI) is manually delineated (green area); (c–g) The software automatically expands the tumor perimeter by 1 mm, 2 mm, 3 mm, 4 mm, and 5 mm; (h) The surrounding larger trachea and blood vessels are manually outlined (red area); (i) A 3D image of the tumor’s surrounding area shows a green hollow sphere.
All images of the three-dimensional VOI of the tumor and tumor margins were resampled to a voxel size of 1 × 1 × 1 mm3. A bandwidth of 25 was selected to extract eight types of features: first-order statistical, grey-level co-occurrence matrix, grey-level region scale matrix, shape, neighborhood grey-level difference matrix, grey-level dependency matrix, wavelet transform, and grey-level run matrix features. Each nodule can be assigned 851 quantitative radiomic features across different tumor margins.
Feature selection and modeling
The data of 30 nodules delineated by Doctor A and Doctor B were subjected to intergroup correlation coefficient (ICC) consistency testing. After excluding 29 features with ICC values < 0.75, the remaining 822 radiomic feature data surrounding the tumors in the training group were processed using Z-score normalization. T-tests, MRMR, and LASSO based on ten-fold cross-validation were applied to remove redundant texture features. Then, eight machine learning models were developed using 10-fold cross-validation on the selected features: k-nearest neighbor, logistic regression, adaptive boosting, support vector machines, random forest, Naive Bayes, decision tree, and Neural Network. The model with the largest area under the ROC curve was selected as the optimal model for the feature set of the training group. This optimal model was validated using standardized features from the selected validation group. Python 3.9 was used to complete both the feature selection and model construction.
Statistical analysis
SPSS (version 23; IBM Corp., Armonk, NY, USA) and Python 3.9 were used for statistical analysis and data processing. All measurement data were expressed by mean ± standard deviation. An independent sample t-test was used for measurement data that conformed to a normal distribution and had uniform variance; when it did not follow a normal distribution, the Mann–Whitney U test was used. Count data were presented as percentages and compared using the chi-square test or Fisher’s exact test, and the difference was considered statistically significant (P < 0.05). Packages, such as pandas, Pingouin, NumPy, roc_curve, and AUC, were utilized to perform ICC testing, generate ROC curves, calculate the AUC, and assess the diagnostic performance of the model. Differences in AUC values among curves were evaluated using the DeLong test, with a two-sided P-value < 0.05 indicating statistical significance.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ferlay, J. et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 136, E359–E386 (2015).
Naidich, D. P. et al. Recommendations for the management of subsolid pulmonary nodules detected at CT: A statement from the Fleischner Society. Radiology 266, 304–317 (2013).
Van Schil, P. E. et al. Surgical implications of the new IASLC/ATS/ERS adenocarcinoma classification. Eur. Respir. J. 39, 478–486 (2012).
Cai, J. et al. A radiomics study to predict invasive pulmonary adenocarcinoma appearing as pure ground-glass nodules. Clin. Radiol. 76, 143–151 (2021).
Kong, C. et al. Prediction of tumor response via a pretreatment MRI radiomics-based nomogram in HCC treated with TACE. Eur. Radiol. 31, 7500–7511 (2021).
Gülbay, M. et al. Evaluation of the models generated from clinical features and deep learning-based segmentations: Can thoracic CT on admission help Us to predict hospitalized COVID-19 patients who will require intensive care? BMC Med. Imaging. 22, 110 (2022).
Cho, H. H., Lee, G., Lee, H. Y. & Park, H. Marginal radiomics features as imaging biomarkers for pathological invasion in lung adenocarcinoma. Eur. Radiol. 30, 2984–2994 (2020).
Wu, G. et al. Preoperative CT-based radiomics combined with intraoperative frozen section is predictive of invasive adenocarcinoma in pulmonary nodules: A multicenter study. Eur. Radiol. 30, 2680–2691 (2020).
Xiong, Z. et al. Use of CT radiomics to differentiate minimally invasive adenocarcinomas and invasive adenocarcinomas presenting as pure ground-glass nodules larger than 10 mm. Eur. J. Radiol. 141, 109772 (2021).
Lv, Y., Ye, J., Yin, Y. L., Ling, J. & Pan, X. P. A comparative study for the evaluation of CT-based conventional, radiomic, combined conventional and radiomic, and delta-radiomic features, and the prediction of the invasiveness of lung adenocarcinoma manifesting as ground-glass nodules. Clin. Radiol. 77, e741–e748 (2022).
Feng, B. et al. Differentiating minimally invasive and invasive adenocarcinomas in patients with solitary sub-solid pulmonary nodules with a radiomics nomogram. Clin. Radiol. 74, 570.e1-570.e11 (2019).
Chen, X. et al. Feasibility of using high-resolution computed tomography features for invasiveness differentiation of malignant nodules manifesting as ground-glass nodules. Can. Respir. J. 2022, 2671772 (2022).
She, Y. et al. The predictive value of CT-based radiomics in differentiating indolent from invasive lung adenocarcinoma in patients with pulmonary nodules. Eur. Radiol. 28, 5121–5128 (2018).
Weng, Q. et al. A radiomics model for determining the invasiveness of solitary pulmonary nodules that manifest as part-solid nodules. Clin. Radiol. 74, 933–943 (2019).
Lee, H. et al. Machine learning approach to identify stroke within 4.5 hours. Stroke 51, 860–866 (2020).
Liu, R. S. et al. The predictive accuracy of CT radiomics combined with machine learning in predicting the invasiveness of small nodular lung adenocarcinoma. Transl. Lung Cancer Res. 12, 530–546 (2023).
Zhang, L. et al. Zone of transition: A potential source of error in tumor volume Estimation. Radiology 256, 633–639 (2010).
Masquelin, A. H. et al. Perinodular parenchymal features improve indeterminate lung nodule classification. Acad. Radiol. 30, 1073–1080 (2023).
Zhu, M. et al. A computerized tomography-based radiomic model for assessing the invasiveness of lung adenocarcinoma manifesting as ground-glass opacity nodules. Respir Res. 23, 96 (2022).
Yu, Z., Xu, C., Zhang, Y. & Ji, F. A triple-classification for the evaluation of lung nodules manifesting as pure ground-glass sign: A CT-based radiomic analysis. BMC Med. Imaging. 22, 133 (2022).
Huang, L. et al. Development and validation of a preoperative CT-based radiomic nomogram to predict pathology invasiveness in patients with a solitary pulmonary nodule: A machine learning approach, multicenter, diagnostic study. Eur. Radiol. 32, 1983–1996 (2022).
Wu, L. et al. The value of various peritumoral radiomic features in differentiating the invasiveness of adenocarcinoma manifesting as ground-glass nodules. Eur. Radiol. 31, 9030–9037 (2021).
Acknowledgements
We thank all the involved physicians, staff, and the clinical laboratory and radiology departments for dedicating their time and skills, which facilitated the completion of this study. Furthermore, we thank all participants for their commitment to data collection.
Author information
Authors and Affiliations
Contributions
WD, XW, and HX conceived the study. XW and HX drafted the manuscript. XW, HX, WD, and FH reviewed and edited the manuscript. FH, YZ, and XP are the guarantors of the study, with full access to all the data, and take responsibility for the integrity and accuracy of the data analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, X., Xue, H., Ding, W. et al. Peritumoral features for assessing invasiveness of lung adenocarcinoma manifesting as ground-glass nodules. Sci Rep 15, 14112 (2025). https://doi.org/10.1038/s41598-025-99180-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-99180-9