Abstract
To explore the influencing factors of spinal mixed infection under mNGS-assisted detection. A retrospective analysis was conducted on the general clinical data of patients diagnosed with spinal infections at Guilin People’s Hospital, covering the period from October 2022 to October 2024, to evaluate the effectiveness of different treatment modalities including conservative, pharmacological, and surgical interventions. In the end, a total of 45 cases were included, including 18 cases of mixed infection and 27 cases of single infection. The receiver operating characteristic (ROC) curve was utilized to evaluate the predictive efficacy of various indices for the occurrence of mixed infection in patients with spinal infections, with the curve’s proximity to the top left corner indicating higher diagnostic accuracy. Multivariate Logistic regression was used to analyze the independent risk factors affecting the development of mixed infection in patients with spinal infection. No significant differences were found between the two groups regarding gender, smoking, alcohol consumption, hypertension, albumin levels, liver function, malignancy, or rheumatic immune disease history (P > 0.05). However, the mixed infection group had significantly higher proportions of patients aged > 60 years (78% (14/18) vs. 48% (13/27)), diabetes mellitus (44% (8/18) vs. 15% (4/27)), chronic kidney disease (17% (3/18) vs. 0.00 (0/27)), and previous spinal surgery (39% (7/18) vs. 11% (3/27)), along with lower BMI (20.70 ± 2.15 vs. 24.04 ± 3.76) and hemoglobin levels (105.17 ± 14.05 g/L vs. 117.48 ± 18.08 g/L). The results of the ROC curve analysis showed that the area under the curve for BMI and hemoglobin in predicting the occurrence of mixed infection in patients was 0.787 and 0.704, respectively, with optimal cutoff values of 21.12 kg/m2 and 119 g/L. Multivariate logistic regression identified BMI < 21.12 kg/m2, hemoglobin < 119 g/L, and diabetes as independent risk factors. Lower BMI, diabetes and hemoglobin are independent risk factors for spinal mixed infection. Increasing BMI, effectively controlling blood glucose and improving anemia are helpful to reduce the occurrence of spinal mixed infection.
Similar content being viewed by others
Introduction
Mixed infection refers to the simultaneous infection of more than two pathogens in the body. Most of the clinical “mixed infections” are the combination of viruses, acteriab and mycoplasma. The incidence of spinal infections is increasing year by year with the aging of the population, increased use of immunosuppressive drugs and other factors. The morbidity of spinal and spinal cord infections is closely linked to a high mortality risk, necessitating prompt clinical identification and management effective diagnostic evaluation and multidisciplinary treatment1. Currently, The gold standard for diagnosing spinal infections typically includes microbiological culture and pathological examination. Microbiological culture is capable of identifying the specific pathogenic microorganisms responsible for the infection, while pathological examination allows for the observation of histological changes associated with the infection. By integrating the results from both methods, the accuracy and reliability of the diagnosis can be significantly enhanced2. mNGS technology is a recently developed emerging diagnostic tool. Based on the nucleic acid level, it can cover all pathogens involved in the sample submitted for inspection at one time without bias, including bacteria, fungi, viruses and even parasites. This technology has the characteristics of rapidity, high accuracy and wide coverage of pathogenic microorganisms, and has been widely used in the etiological diagnosis of multi-system infections3.
Spinal infection frequently exhibits a subtle onset, untypical clinical presentations, and a non-specific appearance in imaging and laboratory tests, posing significant challenges to accurate diagnosis and treatment, frequently resulting in missed, incorrect diagnoses, or even inappropriate therapies4. However, with the application of mNGS in spinal surgery, more and more patients with spinal infection have obtained exact etiological evidence, and some cases of spinal mixed infection have also been reported in some studies5,6. However, the influencing factors of spinal mixed infection are currently unclear, and there are few related studies. This study explores the influencing factors of spinal mixed infection based on mNGS-assisted detection and provides theoretical reference for clinical prevention or reduction of mixed spinal infection.
Materials and methods
General information
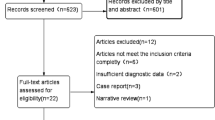
A retrospective analysis was conducted on the clinical data of 45 patients diagnosed with spinal infections, who were hospitalized in the Department of Spinal Surgery at Guilin People’s Hospital between October 2022 and October 2024. The study encompassed the diagnosis, treatment, and outcomes of these patients, with a focus on understanding the progression and management of spinal infections.Inclusion criteria: (1) The patient exhibited clinical manifestations, signs, laboratory test results, and imaging findings that were indicative of spinal infection; (2) The patient had no significant neurological impairments upon admission; (3) All patients underwent either lesion puncture or open surgical intervention, Specimens were obtained with the assistance of a C-arm X-ray machine, To minimize the risk of specimen contamination caused by open surgery, we adhered to strict aseptic operating protocols and collected specimens from the depths of the lesion. Additionally, negative control samples were used during laboratory processing to detect potential contamination; (4) All patients underwent mNGS testing to identify the pathogenic microorganisms they were infected with. Exclusion criteria: (1) patients who did not complete all required routine examinations and mNGS tests; patients with obvious exogenous contamination during the specimen submission process; (2) patients with incomplete data or who were lost to follow-up. Based on the above inclusion and exclusion criteria, a total of 45 patients were included in this study, including 23 men and 22 women; The age ranged from 17 to 91 years, with an average of 63.2 ± 14.4 years.
This study has been approved by the Ethics Committee of Guilin People’s Hospital, and informed consent was exempted for the patients involved.
Research methods
When the patient is admitted, the specific laboratory tests, such as Brucella antibody and serum agglutination test, T cell spot test for tuberculosis infection, galactomannan antigen test, 1, 3-β-D-glucan test, and the blood routine, erythrocyte sedimentation rate, high-sensitivity C-reactive protein, procalcitonin and other infection indicators are regularly monitored. Blood cultures are drawn after admission and during fever. Patients with conservative treatment regimen were biopsied by Under the guidance of a C-arm X-ray machine, a spinal surgeon performs procedures. Specimens acquired through needle biopsy and open surgery are then subjected to a comprehensive diagnostic approach, including bacterial culture, pathological examination, and mNGS detection, to identify a broad spectrum of pathogens.
In this study, specimens obtained from patients included pus and tissue samples. Pus samples were collected through lesion puncture or open surgery, while tissue samples were acquired via open surgery. To minimize the interference of blood on mNGS results, standardized measures were implemented during specimen collection and laboratory processing. These included prioritizing the collection of non-bloody pus, removing surface blood from tissue samples, and performing centrifugation pretreatment prior to mNGS testing. Among similar microorganisms, the term ‘relative abundance’ refers to the percentage of those with the lowest detection level. Referring to similar literatures, the standard of mNGS detection results is formulated7. During the actual detection process, the results can also be influenced by background microorganisms. Common sources of background microorganisms include sample collection tools, experimental consumables, and reagents utilized in library construction, examples of which are Burkholderia, Acinetobacter, and Bradyrhizobium. Ralstonia, Sphingomonas, Desulfocarbo, Moraxella and other bacteria, as well as circovirus, human herpesvirus, etc. In interpreting mNGS test results, most background bacteria are not classified as pathogenic microorganisms.
Statistical analysis
Statistical analysis was performed using SPSS 25.0 software. Normally distributed continuous data were expressed as mean ± standard deviation ( x̄ ± s), and intergroup comparisons were analyzed using independent-samples t-test. Categorical data were presented as numbers (percentages) and compared using Chi-square (χ²) test. ROC curves were constructed using MedCalc 16.4.3 software (MedCalc Software bv, https: //whttp://ww.medcalc.org; 2016), with the area under the curve (AUC) calculated to quantify diagnostic accuracy. Multivariate logistic regression analysis was applied to identify general clinical data influencing infection type (mixed spinal infection vs. single-pathogen infection) and evaluate the impact of variables on infection classification. A two-tailed P < 0.05 was considered statistically significant.
Results
Analysis of risk factors for spinal mixed infection
Univariate analysis showed that age (> 60 (78% (14/18) vs. 48% (13/27))), low BMI (20.70 ± 2.15 vs. 24.04 ± 3.76), diabetes mellitus (44% (8/18) vs. 15% (4/27)), hemoglobin levels (105.17 ± 14.05 g/L vs. 117.48 ± 18.08 g/L), chronic kidney disease (17% (3/18) vs. 0.00 (0/27)), and history of previous spinal surgery (39% (7/18) vs. 11% (3/27)) were all risk factors for spinal mixed infection, and the difference was statistically significant (P < 0.05). See Table 1 for details.
Efficacy of each index in predicting mixed infection in patients with spinal infection
The results of ROC curve analysis showed that the AUC of BMI and hemoglobin in predicting mixed infection of the spine was 0.787 and 0.704, respectively, and the best cutoff values were 21.12 kg/m2, 119 g/L. See Table 2; Fig. 1.
Independent risk factors affecting spinal mixed infection
Multivariate Logistic regression analysis indicated that a lower BMI (less than 21.12 kg/m2), reduced hemoglobin levels (< 119 g/L), and the presence of diabetes were independently associated with an increased risk of spinal mixed infection (P < 0.05) See Table 3.
Discussion
Spinal infections include primary infections of the spine, infections secondary to another primary source, and postoperative infections including vertebral body infections, spondylodiscitis, epidural abscesses, paravertebral soft tissue infections, or any combination of these8. With the global spread of the novel coronavirus, the incidence of spinal infections has increased. Using the keywords ‘spinal infection’ and ‘spine infection’, we searched on PubMed and found that as of December 2024, the total number of publications on spinal infections is approximately 42,000+. Since 2020, the total number of publications has reached over 8,000+, accounting for more than one-sixth of the total literature on spinal infections in the post-COVID era. Although there is still no clinically evidence-based medical evidence to establish a definitive correlation between spinal infection and the COVID-19 epidemic, The number of patients with mixed infection also increased significantly9. Currently, there is a lack of clear treatment options and long-term follow-up for spinal infections, with medical and surgical departments often disagreeing on the outcomes and effectiveness of various treatment strategies8. At the same time, Chaotic clinical diagnosis and treatment procedures, diverse methods, and irregular medication schedules still impact the prognosis of spinal infections. Therefore, it is essential to focus on analyzing the risk factors affecting spinal infections, especially for patients with mixed spinal infection, which can significantly reduce the morbidity and mortality of patients, improve the quality of life of patients, and reduce the economic burden of the population and society.
In this study, we compared the general clinical data of patients with mixed spinal infection and single infection by Logistic regression analysis, and found that low BMI, diabetes mellitus and decreased hemoglobin were independent risk factors for mixed spinal infection. Obesity is one of the predisposing factors of various infections. Obesity is closely related to the increase of sepsis mortality in obese patients. The increase in fat infiltration rates among obese patients, specifically the elevation off The paraspinal muscle fat infiltration rate is an independent risk factor for deep infection in posterior lumbar surgery10. Additionally, studies have indicated that a BMI greater than 25 kg/m2 can influence the mortality rates of patients suffering from spinal infections11. Our findings indicate that patients with mixed spinal infections often exhibit a low BMI, which is a risk factor for poorer health outcomes and can contribute to a delayed or hidden onset of the infection. Most infected patients are admitted to hospital with severe pain or nerve function impairment in the later stage, which leads to limb movement disorder. The prolonged presence of pathogenic bacteria in the body may cause gradual weight loss, particularly in individuals with mixed infections. This weight loss and subsequent reduction in BMI are attributed to the metabolic burden of multiple infectious agents, suggesting the presence of a mixed spinal infection.
Diabetes is another risk factor that may lead to spinal mixed infection12. Diabetes can lead to spinal mixed infection due to various factors. First, diabetic patients generally have low white blood cells and low immune function, and there are bacteria in the intervertebral disc itself13. When the autoimmune system fails to resist endogenous bacteria, it readily leads to various infections. Secondly, diabetes causes local blood circulation disturbances and reduced blood flow. The blood supply to spinal vertebral bodies and intervertebral discs mainly consists of terminal arterioles. Diabetes exacerbates the decrease in blood flow to these areas, leading to tissue hypoxia and increased susceptibility to infection. Infection can aggravate metabolic disorders, and the two affect each other. At the same time, hypoxia can easily induce the growth of anaerobic bacteria, so this may also be the cause of spinal mixed infection in patients. Furthermore, anemia is frequently accompanied by infections of varying degrees and different forms. Inflammatory anemia is the most common and is also not specific to the type of infection. Our study shows that decreased hemoglobin is an independent risk factor for spinal mixed infection, and the increased degree of anemia in patients before spinal surgery increases the risk of infectious complications14. Concurrently, studies have demonstrated that the hemoglobin level at admission is associated with the The prognosis of sepsis patients is influenced by the decrease in hemoglobin levels, which is associated with an increased mortality rate among these patients15. In summary, the reduction in hemoglobin levels not only exacerbates infection but also contributes to mixed infections in patients; however, additional high-quality studies are required to further validate this finding.
Furthermore, single factor analysis reveals significant disparities in age, chronic kidney disease, and prior spinal surgery histories between patients suffering from mixed and single spinal infections. Spinal infections are prevalent among adults aged over 50, with age serving as a predictable factor for infection occurrence. Age-related immune system impairments in the elderly often result in non-specific symptoms. As age increases, the immunity of the populationulation gradually decreases, and the probability of infection in the body also gradually increases16. The study reveals that infected patients over 60 years old have a higher probability of mixed infection; Surgical site infection is a common and costly complication after spinal surgery17. Overall, spinal surgery patients experience a 3.1% incidence of surgical site infection18. Patients with spinal infection often need a long and sufficient course of antibiotics, which has a huge impact on patients. Furthermore, our study reveals a significant difference in the incidence of chronic kidney disease between the two patient groups, attributed to a single factor. Patients with chronic kidney disease suffer from renal insufficiency and are at a higher risk of co-infection. Regarding hemodynamics, kidney infections can lead to immobilization of the spine, particularly the lumbar spine. Therefore, there is a theoretical basis for the higher incidence of chronic kidney disease in patients with mixed infection compared with patients with single infection in our study.
In conclusion, by comparing the general data of patients with spinal infection, this study concluded that patients with mixed infection were significantly different from those with simple infection in advanced age (> 60), chronic kidney disease and previous history of spinal surgery. Lower BMI (< 21.12 kg/m²), low hemoglobin levels (< 119 g/L), and diabetes mellitus emerged as independent risk factors for patients with mixed spinal infections. Increasing BMI, effective blood glucose control and improving hemoglobin were all helpful to reduce the occurrence of mixed spinal infection. The limitations of this study are as follows: (1) While this retrospective study with a limited number of patients provides preliminary insights, the findings require validation through larger-scale, multi-center prospective studies to establish more definitive conclusions. (2) However, the variables considered in this study are limited, and there may be some influencing factors beyond the scope of this study, so further research needs to be verified.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Bhattacharyya, S. & Bradshaw, M. J. Infections of the spine and spinal cord. Continuum (Minneap Minn). 27 (4), 887–920. https://doi.org/10.1212/CON.0000000000001031 (2021).
Tsantes, A. G. et al. Spinal infections: an update. Microorganisms 8 (4), 476. https://doi.org/10.3390/microorganisms8040476 (2020). Published 2020 Mar 27.
Jiang, X. W., Liang, Z. K., Zeng, L., Yuan, Y. L. & Zhonghua Yu Fang Yi Xue Za Zhi ;57(7):1124–1130. doi:https://doi.org/10.3760/cma.j.cn112150-20220824-00836 (2023).
Yokota, H. & Tali, E. T. Spinal infections. Neuroimaging Clin. N Am. 33 (1), 167–183. https://doi.org/10.1016/j.nic.2022.07.015 (2023).
Enríquez-Ruano, P., Navarro, C. E., Ariza-Varón, M. & Calderón-Castro, A. D. P. Myelopathy secondary to human T-lymphotropic virus and Treponema pallidum infection: case report. Spinal Cord Ser. Cases. 5, 93. https://doi.org/10.1038/s41394-019-0238-0 (2019). Published 2019 Nov 6.
Zhao, X., Sun, F., Li, H. X. & Li, Y. P. Tuberculosis complicated by spinal cord cryptococcosis: a case report and literature review. Eur. Rev. Med. Pharmacol. Sci. 27 (1), 411–416. https://doi.org/10.26355/eurrev_202301_30896 (2023).
Yin, C. et al. Pathogenic detection by metagenomic Next-generation sequencing in spinal infections. Spine (Phila Pa. 1976). 50 (4), E70–E75. https://doi.org/10.1097/BRS.0000000000005148 (2025).
Kleck, C. C. J., Damioli, L. & Ou-Yang, D. Treatment of spinal infections. Instr Course Lect. 73, 675–687 (2024).
Li, C., Xiao, N. S., Ke, B. Y., Li, S. & Lin, Y. Application of metagenomic Next-Generation sequencing in suspected spinal infectious diseases. World Neurosurg. 185, e542–e548. https://doi.org/10.1016/j.wneu.2024.02.071 (2024).
Gupta, V. K., Zhou, Y., Manson, J. F. & Watt, J. P. Radiographic spine adipose index: an independent risk factor for deep surgical site infection after posterior instrumented lumbar fusion. Spine J. 21 (10), 1711–1717. https://doi.org/10.1016/j.spinee.2021.04.005 (2021).
Lener, S., Hartmann, S. & Thomé, C. Reply to the letter to editor regarding, A scoring system for the preoperative evaluation of prognosis in spinal infection: the MSI-20 score. Spine J. 22 (8), 1419–1420. https://doi.org/10.1016/j.spinee.2022.04.017 (2022).
Thurnher, M. M. & Olatunji, R. B. Infections of the spine and spinal cord. Handb. Clin. Neurol. 136, 717–731. https://doi.org/10.1016/B978-0-444-53486-6.00035-1 (2016).
Jiao, Y. et al. The bacteria-positive proportion in the disc tissue samples from surgery: a systematic review and meta-analysis. Eur. Spine J. 28 (12), 2941–2950. https://doi.org/10.1007/s00586-019-06062-6 (2019).
Mo, K. et al. Increased severity of Anemia is associated with postoperative complications following a adult spinal deformity surgery. World Neurosurg. 167, e541–e548. https://doi.org/10.1016/j.wneu.2022.08.045 (2022).
Zhu, J. et al. Prognostic value of hemoglobin in patients with sepsis: A systematic review and meta-analysis. Heart Lung. 64, 93–99. https://doi.org/10.1016/j.hrtlng.2023.12.001 (2024).
Scott, M. M. & Liang, S. Y. Infections in older adults. Emerg. Med. Clin. North. Am. 39 (2), 379–394. https://doi.org/10.1016/j.emc.2021.01.004 (2021).
Stewart, K. E. et al. Trends and prediction of surgical site infection after elective spine surgery: an analysis of the American college of surgeons National surgical quality improvement project database. Surg. Infect. (Larchmt). 24 (6), 506–513. https://doi.org/10.1089/sur.2023.067 (2023).
Zhou, J. et al. Incidence of surgical site infection after spine surgery: A systematic review and Meta-analysis. Spine (Phila Pa. 1976). 45 (3), 208–216. https://doi.org/10.1097/BRS.0000000000003218 (2020).
Funding
This work was supported by the Guilin Science and Technology Development Program (Project) (grant nos. 20230135-9-9).
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The study was approved by the licensing ethical committee of Guilin People’s Hospital, and all patients provided signed informed consent. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, C., Zhu, J., Wang, Y. et al. Metagenomic NGS reveals determinants of polymicrobial spinal infection pathogenesis. Sci Rep 15, 13959 (2025). https://doi.org/10.1038/s41598-025-99283-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-99283-3