Abstract
Sepsis is characterized by severe organ failure due to an impaired response to infection. The underlying pathophysiology of sepsis is characterized by concurrent unbalanced hyperinflammatory and immunoparalysis. This study aimed to identify new key biomarkers that could predict outcomes in sepsis patients and explore theirunderlying molecular mechanisms. Bulk transcriptome data (GSE65682, GSE28750, GSE57065, GSE95233) and scRNA-seq data (GSE167363) of sepsis were obtained from the GEO database. Data for MR analysis were sourced from the eQTLGen Consortium and IEU OpenGWAS project. Prognostic biomarkers and potential drug targets for sepsis were identified through univariate Cox regression and MR analysis. The expression of these biomarkers was further validated using scRNA-seq data to investigate the underlying molecular mechanisms. Significantly higher expression of CHIT1 was found at sepsis non-survivor and associated with 28-day mortality of sepsis. scRNA-seq data of septic samples found that CHIT1 mainly expressed in neutrophils, which was also higher in sepsis non-survivors. The CHIT1 + neutrophils expressed higher inflammation related genes of S100A8, S100A9, S100A11, S100A12, IL1R2, IFNGR2, TLR2 and CXCL8 and reduced expression of HLA related genes of HLA-DMA, HLA-DPA1, HLA-DPB1, HLA-DRA, HLA-DRB1 and HLA-DRB5. Moreover, cell-chat analysis also showed that CHIT1 + neutrophils could interact with other immune cell types, including NK cells, erythroid cells, monocytes/macrophages, and DC by the way of ICAM1-(ITGAM + ITGB2) pathway. We identified CHIT1 as new biomarker and potential drug target for sepsis, which may intensify hyperinflammation and immune suppression of neutrophils. Developing immunotherapeutic strategies aimed at targeting CHIT1 would help to enhance sepsis outcomes.
Similar content being viewed by others
Introduction
Sepsis is a severe condition involving organ dysfunction due to an abnormal response to infection. It has evolved from earlier definitions based on systemic inflammatory response syndrome (SIRS) criteria. More than 20 million cases are reported annuallywith a mortality rate of approximately 26%. In the U.S., around 850,000 emergency department visits are linked to sepsis each year1. Despite extensive researches and over 200 randomized controlled trials, no single treatment has proven reliably effective for sepsis2. Current management focuses on supportive care, including infection control, antibiotic administration, resuscitation, and organ dysfunction management3. This highlights the need for continued research into its biological mechanisms and identification of potential biomarkers.
Sepsis triggers complex and often maladaptive responses in the immune, vascular, and metabolic systems4. It is marked by an imbalanced immune reaction, where pathogens evade defense mechanisms, causing ongoing cellular damage without restoring balance. This results in harmful excessive inflammation and immune suppression5. Understanding these dual responses and their long-term effects in critically ill patients is crucial for discovering new therapeutic targets6,7.
Recent advancements in mRNA and single-cell RNA sequencing (scRNA-seq) have significantly enhanced our understanding of sepsis mechanisms by providing detailed insights into gene expression and cellular diversity8,9. Mendelian randomization (MR) analysis helps overcome limitations of observational studies, revealing causal relationships between molecular traits and outcomes. MR analysis is more cost-effective and ethical compared to randomized controlled trials (RCTs) and, when combined with eQTL and GWAS data, can identify variants linked to disease risk and molecular traits10. For instance, MR analysis has been used to study the link between HMGCR inhibition and reduced COVID-19 hospitalization risk11.
In this study, we utilized bulk RNA-seq data from the gene expression omnibus (GEO) to examine gene expression patterns in sepsis, particularly among non-survivors. Using cox regression analysis, we identified key prognostic genes related to sepsis outcomes. MR analysis further pinpointed chitinase 1 (CHIT1); as a causal gene and potential drug target associated with 28-day sepsis mortality. Validation with scRNA-seq data showed that CHIT1 was predominantly expressed in neutrophils, with higher levels in non-survivors. CHIT1 + neutrophils may drive sepsis progression through increased expression of pro-inflammatory genes, reduced HLA-DR, and enhanced recruitment of other immune cells via the ICAM1-(ITGAM + ITGB2) pathway.
Materials and methods
Datasets acquirement
Bulk transcriptome data for sepsis patients were acquired from the GEO database under accession number GSE65682. Following the exclusion of samples with incomplete prognostic information, 42 healthy controls (HCs) -, 114 samples from sepsis non-survivors, and 365 samples from sepsis survivors were analyzed12. This dataset, which was produced using the Affymetrix Human Genome U219 Array, contained expression profiles with 49,386 probes. Additional independent cohort of GSE28750 cohort (20 HCs and 10 sepsis patients), GSE57065 cohort (20 HCs and 28 sepsis patients) and GSE95233 cohort (22 HCs and 51 sepsis patients) were used to strengthen the findings. In addition, peripheral blood single-cell RNA sequencing data were retrieved from GEO with accession number GSE167363, which included samples from two HCs, two sepsis non-survivors, and three sepsis survivors13. The samples were processed using the Illumina NovaSeq 6000, with the raw gene expression matrix being filtered, normalized, and clustered to concentrate on high-quality cells characterized by 200–6000 detected genes and mitochondrial gene counts below 10%.
Differentially expressed genes (DEGs) analysis
DEGs between sepsis patients and HCs were identified through the application of the limma package. Significance was determined by applying thresholds where log2FC was set to greater than 1 and the adjusted p-value was required to be less than 0.01. We implemented an appropriate multiple testing correction method to control the false discovery rate (FDR). Specifically, we had applied the Benjamini-Hochberg procedure, which was widely recognized for its balance between controlling the FDR and maintaining statistical power. This method adjusted the p-values of our statistical tests to account for the multiple comparisons made. These genes were enriched using the DAVID database, with focus on gene ontology aspects such as biological processes, cellular components, and molecular functions. KEGG pathway analyses were also conducted, and visualization was achieved using the ggplot2 R package14,15,16.
Survival analysis
Kaplan-Meier survival curves were generated using data sourced from the GSE65682 database, and the log-rank test was applied for assessment. The goal of this analysis was to pinpoint genes associated with 28-day mortality in sepsis patients. Statistical analysis was conducted using the R survival package, which included the calculation of hazard ratios (HR) and determination of log-rank p-values within a 95% confidence interval (CI), to evaluate the influence of gene expression on survival.
MR analysis
MR analysis, similar to RCTs, was utilized to overcome the limitations inherent in observational studies and to evaluate causal relationships between molecular traits and phenotypes. European descent cohorts were employed, with eQTL data obtained from the eQTLGen Consortium (https://www.eqtlgen.org/cis-eqtls.html)17. This dataset included information on 10,317 single nucleotide polymorphisms (SNPs) and 19,942 gene expressions from 31,684 blood samples. Summary data for sepsis genome-wide association study (GWAS) were acquired from the integrative epidemiology unit (IEU) OpenGWAS project (https://gwas.mrcieu.ac.uk/), encompassing 1,896 cases and 484,588 controls18.
Instrumental variables (IVs) selection criteria: In this study, the selection criteria for SNPs as IVs included: (1) SNPs reaching genome-wide significance 5 × 10⁻⁸ for association with circulating CHIT1 levels in the largest publicly available GWAS (encompassing 1,896 cases and 484,588 controls); (2) Identification of independent SNPs using a linkage disequilibrium (LD) threshold of r²<0.001 within a 10,000 kb window based on the 1000 Genomes European reference panel; and (3) Exclusion of pleiotropic: SNPs potentially associated with sepsis risk factors or inflammatory traits using PhenoScanner v2.0, with further assessment of horizontal pleiotropy through MR-Egger intercept tests and MR-PRESSO outlier correction. The strength of the IVs was confirmed by a mean F-statistic > 10, thereby minimizing weak instrument bias. To address pleiotropy, several statistical methods were employed. MR-Egger regression tested for directional pleiotropy through the intercept term, with a non-significant intercept indicating no genome-wide pleiotropic bias. MR-PRESSO identified and removed one outlier SNP showing evidence of horizontal pleiotropy global test. Additionally, weighted median and mode estimators provided consistent effect estimates HR per standard deviation increase in CHIT1, supporting robustness against invalid IVs. Biologically, all retained SNPs were located in or near the CHIT1 gene or its regulatory regions, which reduced the likelihood of pleiotropy via unrelated pathways.
The rationale for selecting Inverse Variance Weighting (IVW) as the primary estimator was based on its statistical efficiency, which provided the most accurate estimate when all instrumental variables IVs were valid and free from pleiotropy, in line with recommendations from Burgess. et al.19. Given potential heterogeneity Cochran’s Q, a random-effects IVW approach was used to account for balanced pleiotropy, offering a conservative analysis. Consistency across multiple methods, including IVW, MR-Egger, and weighted median, further strengthened the confidence in the causal inference.
Our research results were disseminated in compliance with the MRSTROBE (MR-Strengthening the Reporting of Observational Studies in Epidemiology) guidelines (Supplementary material of STROBE-MR checklist), as recommended in the field.
scRNA-seq data processing
A detailed atlas of sepsis was constructed using the Seurat R package for the processing of scRNA-seq data, including quality control, normalization, dimensionality reduction, and clustering. Cells were excluded if they had fewer than 200 unique molecular identifiers (UMIs), more than 10% mitochondrial content, or over 6000 UMIs. Normalization was carried out using techniques such as LogNormalize and Harmony to correct for batch effects. Dimensionality reduction was achieved through RunPCA, and clustering was conducted using FindClusters. Unsupervised clustering and visualization were performed with UMAP based on principal components. Initial cell annotations were done using SingleR and were subsequently refined according to marker gene expression profiles.
Cell pseudotime trajectory analysis
The Monocle2 R package was employed to infer cell lineage trajectories and to visualize changes in gene expression along pseudotime using UMAP plots20.
Cell-cell interaction analysis
The CellChat tool was used to analyze intercellular communication networks derived from scRNA-seq data. Communication probabilities at the signaling pathway level were calculated using the computeCommunProbPathway function, and ligand-receptor interactions were also visualized21.
Experimental validation
We enrolled 5 healthy controls and 5 patients with sepsis and isolated their PBMCs for analysis of CHIT1 in sepsis. Sepsis was characterized by the presence of organ dysfunction necessitating mechanical ventilation due to an imbalanced immune reaction stemming from a suspected or confirmed infection, specifically pneumonia. Blood samples obtained more than 24 h after intubation were not included in the analysis. Additionally, individuals who had undergone organ transplantation, those with active cancer, or those receiving systemic immunosuppressive therapy or systemic glucocorticoids were excluded from the study. The ethical approval for our study was obtained from the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University (Protocol license number: 2025-E0219), and adhered to the principles of the Declaration of Helsinki. We acquired informed consent from patients or their relatives. All procedures performed in this study were in accordance with the ethical standards of the institutional and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Blood was collected into a tube containing acid citrate dextrose ACD and subsequently transferred to a 50 mL Falcon tube. The blood was then diluted approximately 11 with sterile PBS that had been tested for LPS. Following this, the samples were layered over Ficoll Paque PLUS and centrifuged at 450 g for 30 min at 25 °C using a swinging-bucket rotor without applying the brake. The mononuclear cell layer at the interface was carefully removed and washed three times with 15 mL of PBS. The peripheral blood mononuclear cells (PBMCs) were then resuspended in a cold cryopreservation medium composed of 10%dimethyl sulfoxide,40%fetal bovine serum FBS, and 50%X-VIVO15 medium.
During the quantitative real-time PCR experiment (qRT-PCR), we immediately placed the excised tissues into RNAlater to preserve RNA integrity. Subsequently, total RNA was isolated using the TRIzol reagent method. The extracted RNA was converted into cDNA using a reverse transcription kit from Thermo Fisher Scientific. The qRT-PCR was performed with the SYBR Green PCR Master MixThermo Fisher Scientific. Throughout this process, GAPDH was used as the endogenous control gene. The primer sequences used were as follows for CHIT1, the forward primer was 5’- ACCGTCAGACCTTTGTCAACT-3’, and the reverse primer was 5’- CCTGTACCAGGGTTGTGAAGC-3’; for GAPDH, the forward primer was 5’-GGAGCGAGATCCCTCCAAAAT-3’, and the reverse primer was 5’-GGCTGTTGTCATACTTCTCATGG-3’. For each sample, the cycle threshold Ctvalue was recorded. The relative expression levels were calculated using the Eq. 2^-ΔCt, where ΔCt represents the difference between the average Ct value of the target gene and the average Ct value of the reference gene.
Statistical analysis
GraphPad Prism 5.01 and R (version 4.1.2) were used to perform statistical analysis and generate graphics. Data were presented as mean ± standard deviation or median based on distribution. Wilcoxon tests or Student’s t-tests were applied for comparisons between two groups, while ANOVA was utilized for multiple group comparisons. Spearman correlation was employed for assessing correlations. Univariate Cox regression was conducted to evaluate prognostic indicators and the effect of CHIT1 expression on 28-day sepsis mortality. Statistical significance was set at P < 0.05.
Results
Identification of DEGs between sepsis and HCs
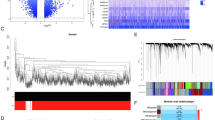
In the GSE65682 dataset, bulk RNA expression data were available for 42 HCs, 114 sepsis non-survivors, and 365 sepsis survivors. UMAP analysis of this dataset revealed a clear separation between sepsis and HCs, indicating notable differences in gene expression profiles between these conditions (Fig. 1A). Compared to HCs, 460 genes were identified as up-regulated, and 853 genes were found to be down-regulated in sepsis (log2FC > 1 and adjusted p-value < 0.01, Fig. 1B). Ten genes with the most pronounced up-regulation and ten with the most significant down-regulation were selected for display. To further explored the biological significance of these DEGs, gene ontology (GO) annotation and kyoto encyclopedia of genes and genomes (KEGG) pathway enrichment analyses were conducted. In the biological process (BP) analysis, DEG-related genes were implicated in immune cell differentiation, activation, migration, and chemotaxis; including positive regulation of cytokine production, regulation of cell-cell adhesion, leukocyte adhesion, mononuclear cell differentiation, lymphocyte differentiation, and regulation of leukocyte adhesion (Fig. 1C). KEGG analysis highlighted the top five significantly enriched pathways: human T-cell leukemia virus 1 infection, hematopoietic cell lineage, Th17 cell differentiation, Epstein-Barr virus infection, and Th1 and Th2 cell differentiation (Fig. 1D). Collectively, these findings suggested that the dysregulated genes in sepsis were significantly associated with immune cell functions, which may contribute to the development and progression of sepsis.
Identification of differentially expressed genes (DEGs) between sepsis and healthey controls (HCs). (A) UMAP analysis of the GSE65682 dataset revealed a clear distinction between sepsis and HCs. (B) A volcano plot illustrated the DEGs between sepsis and HCs, with a threshold of log2FC > 1 and an adjusted p-value < 0.01. Red dots signified upregulated genes, green dots indicated downregulated genes, and gray dots represented genes without significant changes. The plot highlighted the 10 most significantly upregulated and 10 most significantly downregulated genes. (C) Gene ontology (GO) enrichment analysis of the DEGs identified in sepsis. (D) Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis for the DEGs associated with sepsis.
Identification of genes associated with 28-day mortality in sepsis patients
To identify potential hub genes among the DEGs related to sepsis, survival analysis was performed using the GSE65682 dataset. This dataset included data from 114 sepsis non-survivors and 365 sepsis survivors, along with key clinical parameters such as 28-day survival status and time to 28-day survival status. Univariate survival analysis revealed 90 genes classified as high-risk and 397 genes classified as low-risk in relation to 28-day mortality in sepsis patients (all p-values < 0.05). From these, 30 genes with the highest risk and 30 genes with the lowest risk were selected for detailed display (Fig. 2A and B). Furthermore, by intersecting these findings with DEGs, 86 genes were identified as both high-risk for 28-day mortality and upregulated in sepsis, while 345 genes were identified as both low-risk for 28-day mortality and downregulated in sepsis (Fig. 2C and D).
Identification of genes associated with 28-day mortality of sepsis patient. (A) High-risk genes associated with sepsis-related death. The forest plot highlighted the top 30 most high-risk genes associated with sepsis-related death. (B) Low-risk genes associated with sepsis-related death. The forest plot highlighted the top 30 most low-risk genes associated with sepsis-related death. (C) Venn diagram illustrating the overlap genes between high-risk genes associated with 28-day mortality of sepsis and upregulated DEGs compared to HCs. The diagram revealed 86 genes that were both high risk genes for 28-day mortality and upregulated in sepsis. (D) Venn diagram showed the overlap between low-risk genes associated with 28-day mortality of sepsis and downregulated DEGs in sepsis versus HCs. The diagram identified 345 genes that were both low risk genes for 28-day mortality and downregulated in sepsis.
MR analysis further identified CHIT1 as causal gene and potential drug target associated with 28-day mortality of sepsis
Following the identification of 86 genes that were both high-risk for 28-day mortality and upregulated in sepsis, as well as 345 genes that were both low-risk for 28-day mortality and downregulated in sepsis, a further analysis was conducted to identify causal genes and potential drug targets associated with 28-day mortality in sepsis patients using MR analysis. Among the 86 high-risk, upregulated genes, four (IFIT1B, CHPT1, CHIT1, and G0S2) were found to be significantly associated with 28-day mortality. However, only CHIT1 was identified as both a high-risk gene associated with death, upregulated in sepsis, and a significant susceptibility gene for 28-day mortality from sepsis (Fig. 3A). Similarly, among the 345 low-risk, downregulated genes, four (MGMT, SPON2, RABLA2, and PYHIN1) were significantly associated with 28-day mortality, but none were both low-risk genes associated with death, downregulated in sepsis, and significant susceptibility genes for 28-day mortality from sepsis (Fig. 3B). Consequently, CHIT1 was selected for further analysis. As illustrated in Fig. 3C, the forest plot, leave-one-out sensitivity analysis, and scatter plot strongly indicated that CHIT1 levels were positively associated with 28-day mortality in sepsis patients.
Mendelian randomization (MR) analysis further identified causal genes and potential drugable targets associated with 28-day mortality of sepsis (A) Forest plot summarizing the results of MR analysis for genes (including IFIT1B, CHPT1, CHIT1, and G0S2) significantly associated with 28-day mortality in sepsis, which were derived from the 86 genes that were both high risk genes for 28-day mortality and upregulated in sepsis. Among these four genes, only CHIT1 was both a high-risk gene associated with death, upregulated in sepsis, and a high-risk susceptibility gene associated with 28-day mortality from sepsis (B) Forest plot summarizing the results of MR analysis for genes (including MGMT, SPON2, RABLA2, and PYHIN1) significantly associated with 28-day mortality in sepsis, which were derived from the 345 genes that were both low risk for 28-day mortality and downregulated in sepsis. Among these four genes, none of them were both a low-risk gene associated with death, downregulated in sepsis, and a low-risk susceptibility gene associated with 28-day mortality from sepsis. (C) Forest plot, leave-one-out sensitivity analysis and scatter plot for MR analysis of eQTL for CHIT1 (ENSG00000133063) in relation to 28-day sepsis mortality.
Expression of CHIT1 in sepsis and its association with 28-day mortality of sepsis
Compared to HCs, a significantly higher expression of CHIT1 was observed in sepsis patients (p < 0.001, Fig. 4A). Additionally, sepsis non-survivors exhibited a significantly greater expression of CHIT1 compared to sepsis survivors (p < 0.05, Fig. 4B). The receiver operating characteristic (ROC) curve analyses, utilizing CHIT1 gene expression data from the GSE65682 dataset, were performed to assess its diagnostic performance in sepsis. The area under the curve (AUC) of the ROC curve was determined to be 0.872, indicating a high diagnostic accuracy as shown in Fig. 4C. Furthermore, an association was found between higher CHIT1 expression and poorer survival outcomes in sepsis patients, with a HR of 1.38 (95% CI: 1.08–1.76, p = 0.007, Fig. 4D). Additional independent cohort of GSE28750 cohort, GSE57065 cohort and GSE95233 cohort were used to strengthen the findings. In all three additional independent cohort, significantly higher expression of CHIT1 were all found in sepsis patients compared to HC (Supplementary Fig. 1A-1 C). Moreover, we enrolled 5 HC and 5 patients with sepsis and isolated their PBMCs for analysis of CHIT1 in sepsis. Similar to our findings of bioinformatics analyses, significantly elevated expression of CHIT1 was also found in sepsis patients (Supplementary Fig. 1D). Subgroup analysis also showed that there were no difference in the expression of CHIT1 between female and male, patients with age ≤ 60 and patients with age > 60, patient with community acquired pneumonia and patients with hospital acquired pneumonia, patients with no intensive care unit (ICU) acquired infection and patients with ICU acquired infection (all p > 0.05, Supplementary Fig. 2A-D), these results undoubtedly strengthened our findings and confirmed the reproducibility of CHIT1 as a sepsis biomarker.
Expression of CHIT1 in sepsis and its association with sepsis outcomes. (A) Comparison of CHIT1 expression between HCs and sepsis patients. Sepsis patients exhibited significantly higher CHIT1 expression compared to HCs (p < 0.001). (B) CHIT1 expression in sepsis patients stratified by survival status. Patients who died within 28 days showed significantly higher CHIT1 expression compared to survivors (p < 0.05). (C) Receiver operating characteristic (ROC) curve analysis for CHIT1 expression as a predictor of sepsis patients. The area under the curve (AUC) was 0.872, indicating good predictive accuracy. (D) Kaplan-Meier survival curve comparing 28-day survival rates between sepsis patients with high versus low CHIT1 expression. Higher CHIT1 expression was associated with worse survival outcomes, with a hazard ratio (HR) of 1.38 (95% CI 1.08–1.76, p = 0.007).
sc-RNA analysis showed expression of CHIT1 mainly enriched in myeloid cells of peripheral blood of sepsis
The sc-RNA analysis data of peripheral blood from sepsis patients were obtained from the GSE167363 dataset. After performing quality control and removing batch effects, a total of 33,409 cells were analyzed. Nine distinct cell clusters were identified and visualized through UMAP plots (Fig. 5A). Using SingleR, these cells were classified into six types: T cells, NK cells, B cells, platelets, erythroid cells, and myeloid cells (Fig. 5B). A heatmap illustrated the specific gene clusters expressed by each cell subpopulation (Fig. 5C), while a bar plot displayed the varying compositions of immune cell subsets across different sepsis statuses (Fig. 5D). Additionally, CHIT1 expression was found to be significantly higher in myeloid cells compared to other immune cell types (Fig. 5E). These findings indicated that CHIT1 was predominantly enriched in the myeloid cells of sepsis patients’ peripheral blood.
Single-cell RNA sequencing (scRNA-seq) analysis found CHIT1 mainly expressed in myeloid cells. (A) UMAP plot showing the clustering of immune cells identified from scRNA-seq data of peripheral blood mononuclear cells (PBMCs) from 2 HCs, 3 survivor of sepsis patients and 2 die of sepsis patients (GSE167363). The cells were grouped into 9 distinct clusters based on their gene expression profiles, each represented by a different color. (B) The 9 distinct clusters were annotated as B cells, T cells, NK cells, myeloid cells, platelets, and erythroid cells with the help of SingleR. The clusters were annotated with their respective cell type labels, providing a clear visualization of the distribution and separation of different cell types. (C) Heatmap showing the expression levels of subpopulation specific marker genes across these 6 different immune cell types. The cell types were color-coded at the top of the heatmap. The heatmap was divided into six clusters C1 to C6, each representing a group of genes with similar expression patterns. The color gradient from blue to red indicated low to high expression levels, respectively. The genes were listed on the right side, with some genes highlighted in red for emphasis. (D) Bar plot depicted the relative proportions of these 6 different immune cell types in different disease status. Each condition was represented by a stacked bar, with the cell types color-coded as in previous panels. The plot allowed for a comparison of the relative abundance of each cell type in the different conditions, highlighted any significant differences in cell composition. (E) Violin plot showing the expression of CHIT1 across these 6 different immune cell types. This scatter plot showed the expression levels of the CHT1 gene across various cell types. The x-axis represented different cell types, and the y-axis represented the expression level of CHT1. Each point represented a single cell, and the color of the points corresponds to the cell type, as indicated by the legend. The plot indicated that CHIT1 expression was particularly elevated in myeloid cells compared to other immune cell populations.
Myeloid cells subpopulation analysis found CHIT1 mainly expressed in neutrophils and CHIT1 + neutrophils highly accumulated in sepsis non-survivors
Following the discovery of CHIT1 enrichment in myeloid cells within the peripheral blood of sepsis patients, further analysis was conducted to determine the specific myeloid cell subpopulations where CHIT1 was predominantly expressed. A subset comprising 20,958 myeloid cells was selected for subpopulation analysis, which led to the identification of four distinct clusters (Fig. 6A). These clusters were categorized as CD14 + + monocytes/macrophages, neutrophils, DCs, and CD14 + monocytes/macrophages, based on SingleR classification and gene expression profiles of the cell subgroups (Fig. 6B). A heatmap displayed the unique gene clusters expressed by each myeloid subpopulation (Fig. 6C). Notably, CHIT1 was found to be predominantly expressed in neutrophils compared to other myeloid cell types (Fig. 6D). Additionally, CHIT1 + neutrophils were observed to accumulate significantly more in sepsis non-survivors than in HCs and sepsis survivors (Fig. 6E), indicating that CHIT1 might play a role in the progression and mortality of sepsis by modulating neutrophil activity.
Myeloid cells subpopulation analysis found CHIT1 mainly expressed in neutrophils and CHIT1 + neutrophils highly accumulated in sepsis non-survivors. (A) UMAP plot showed myeloid cells could be grouped into 4 distinct clusters based on their gene expression profiles, each represented by a different color. (B) These 4 distinct clusters were annotated as D14 + + monocytes/macrophages, neutrophils, dendritic cells (DC), and CD14 + monocytes/macrophages with the help of SingleR. The clusters were annotated with their respective cell type labels, providing a clear visualization of the distribution and separation of different cell types. (C) Heatmap showing the expression levels of subpopulation specific marker genes across these 4 different immune cell types. The cell types were color-coded at the top of the heatmap. The heatmap was divided into six clusters C1 to C4, each representing a group of genes with similar expression patterns. The color gradient from blue to red indicated low to high expression levels, respectively. The genes were listed on the right side, with some genes highlighted in red for emphasis. (D) Violin plot displaying the expression levels of the CHIT1 gene across these 4 different immune cell types. This scatter plot showed the expression levels of the CHT1 gene across various cell types. The x-axis represented different cell types, and the y-axis represented the expression level of CHT1. Each point represented a single cell, and the color of the points corresponds to the cell type, as indicated by the legend. (E) Bar plot depicted the relative proportions of these 4 different immune cell types in different disease status. This comparison highlighted CHIT1 + neutrophils was highly accumulated in sepsis related death patients.
Higher expression of pro-inflammation related genes were found in CHIT1 + neutrophils and sepsis non-survivors
Following the identification of CHIT1 expression predominantly in neutrophils and the observation that CHIT1 + neutrophils were significantly more abundant in sepsis non-survivors compared to HCs and sepsis survivors, further investigation was conducted to understand the mechanisms underlying sepsis progression and 28-day mortality. Hyperinflammation, which was found to be strongly associated with sepsis, could be driven by neutrophils through various mechanisms. These included the release of proteases and reactive oxygen species, as well as the formation of neutrophil extracellular traps (NETs), which could exacerbate organ damage, increase sepsis severity, and impact inflammation, coagulation, and tissue damage22. Consistent with these findings, CHIT1 + neutrophils exhibited elevated expression levels of pro-inflammatory genes such as pro-inflammation related genes(including S100A8, S100A9, S100A11, S100A120), pro-inflammation related receptors (including IL1R2 and IFNGR2, TLR2) and chemokines (CXCL8) compared to other myeloid cell subpopulations. Furthermore, these pro-inflammatory genes were expressed at higher levels in CHIT1 + neutrophils from sepsis non-survivors compared to those from HCs and sepsis survivors (Fig. 7A and H). This suggested that CHIT1 + neutrophils may play a critical role in sepsis progression and 28-day mortality by intensifying inflammation.
High expression of pro-inflammation related genes were found in CHIT1 + neutrophils and sepsis non-survivors. Violin Plots of illustrating the expression of pro-inflammation related genes of S100A8 (A), S100A9 (B), S100A11 (C), S100A12 (D), IL1R2 (E), IFNGR2 (F), TLR2 (G), and CXCL8 (H) in the 4 different myeloid cells subpopulation and in patients with different disease status. The x-axis represented the identity of the cell types, while the y-axis represented the expression level of pro-inflammation related genes. The plots were color-coded to indicate the different conditions. This comparison highlighted pro-inflammation related genes were highly expressed in CHIT1 + neutrophils and sepsis non-survivors.
Reduced expression of HLA related genes were found in CHIT1 + neutrophils and sepsis non-survivors
It had been established in previous research that HLA-DR expression offered important insights into predicting mortality and the risk of secondary infections. HLA-DR levels were found to be inversely related to the severity of sepsis and the extent of immune dysfunction23. In line with these findings, reduced expression of HLA-related genes, including HLA-DMA, HLA-DPA1, HLA-DPB1, HLA-DRA, HLA-DRB1, and HLA-DRB5, was observed in CHIT1 + neutrophils compared to other myeloid cell subpopulations. Additionally, these HLA-related genes were expressed at lower levels in CHIT1 + neutrophils from sepsis non-survivors compared to those from HCs and sepsis survivors (Fig. 8A and F). This indicated that CHIT1 + neutrophils may contribute to sepsis progression and 28-day mortality by downregulating HLA-related genes.
Reduced expression of HLA related genes were found in CHIT1 + neutrophils and sepsis non-survivors. Violin Plots of illustrating the expression of HLA related genes of HLA-DMA (A), HLA-DPA1 (B), HLA-DPB1 (C), HLA-DRA (D), HLA-DRB1 (E), and HLA-DRB5 (F) in the 4 different myeloid cells subpopulation and in patients with different disease status. The x-axis represented the identity of the cell types, while the y-axis represented the expression level of pro-inflammation related genes. The plots were color-coded to indicate the different conditions. This comparison highlighted HLA related genes were significantly reduced in CHIT1 + neutrophils and sepsis non-survivors.
CHIT1 + neutrophils was the terminally differentiated myeloid cells subpopulations and could interact other immune cells via ICAM1-(ITGAM + ITGB2) pathway
Cell trajectory analysis of myeloid cells was conducted using Monocle 2. It was found that CHIT1 + neutrophils were a terminally differentiated myeloid cell subpopulation (Fig. 9A and B). Furthermore, it was observed that in the context of disease, cells from HCs differentiated along separate paths towards sepsis non-survivors and sepsis survivors (Fig. 9C), suggesting that the differentiation of myeloid cells played a role in sepsis progression. Additionally, CellChat analysis revealed that CHIT1 + neutrophils could interact with various immune cell types, including NK cells, erythroid cells, CD14 + + monocytes/macrophages, DCs, and CD14 + monocytes/macrophages, via the ICAM1-(ITGAM + ITGB2) pathway (Fig. 9D and F).
CHIT1 + neutrophils was the terminally differentiated myeloid cells subpopulation and could interact with other immune cells via ICAM1-(ITGAM + ITGB2) pathway. (A) Pseudotime analysis of myeloid cells trajectory. This panel showed a pseudotime analysis of cell trajectories using a 2D plot with two principal components component 1 and component 2. The color gradient represented pseudotime, ranging from early blue to later edstages of cell development or differentiation. The plot illustrates the progression of cells along the trajectory, with branches indicating potential bifurcation points. (B) Cell type distribution along pseudotime. This panel displayed the distribution of different cell types along the pseudotime trajectory. The plot used a 2D representation with component 1 and component 2. Each color represented a different cell type. The plot showed how the proportion of each cell type changes over pseudotime, indicating potential transitions between cell states. (C) Condition-specific pseudotime trajectory. This panel illustrated the pseudotime trajectory for different conditions. The plot used a 2D representation with component 1 and component 2. The color coding helped visualize how cells from different conditions followed similar or distinct trajectories over pseudotime. (D) Cell-chat analysis showed CHIT1 + neutrophils could interact with other immune cell types, including B cells, T cells, NK cells, platelets, erythroid cells, D14 + + monocytes/macrophages, dendritic cells (DC) and CD14 + monocytes/macrophages. (E) Dot plot of ICAM1-(ITGAM + ITGB2) pathway activity in interaction of CHIT1 + neutrophils with other immune cells. F: Violin plots showing the expression of ICAM1, ITGAM, and ITGAX genes across different immune cell types.
Discussion
Sepsis, characterized by the body’s abnormal response to infection, is a severe and complex condition that can lead to widespread inflammation and organ failure. The diagnosis and treatment of sepsis are challenging, resulting in high rates of morbidity and mortality globally24,25. A comprehensive understanding of their underlying mechanisms and clinical manifestations is crucial for effective management and treatment. In this context, the use of mRNA-seq and scRNA-seq had been employed to investigate the molecular mechanisms of sepsis, facilitating detailed analyses of gene expression and cellular heterogeneity. In the present study, it was observed that CHIT1 and CHIT1 + neutrophils played significant roles in the progression of sepsis.
CHIT1 is located on human chromosome 1 at the 1p36.13 locus and encodes an enzyme responsible for hydrolyzing chitin, a polymer presented in insect exoskeletons, crustaceans, and fungal cell walls. This gene is situated in a chromosomal region associated with various genetic disorders and has been studied for its role in disease susceptibility and genetic functions. The enzyme chitinase 1, encoded by CHIT1, is essential for several biological processes, being predominantly expressed in immune cells such as macrophages and neutrophils. This enzyme plays a crucial role in the immune response against pathogens, especially chitin-rich organisms like fungi and arthropods. Additionally, chitinase 1 assists in tissue remodeling and repair by degrading chitin, which modulates the extracellular matrix necessary for tissue regeneration and wound healing26. The involvement of CHIT1 in various diseases and conditions underscores its significance in both normal physiology and pathology. Elevated levels of CHIT1 had been linked to several inflammatory conditions, suggesting its role in disease mechanisms. For example, increased CHIT1levels in Gaucher disease, a lysosomal storage disorder, serve as a biomarker for monitoring disease progression and treatment efficacy27. High CHIT1 levels werealso observed in chronic obstructive pulmonary disease (COPD) and asthma, where it may contribute to inflammatory processes28. Furthermore, elevated CHIT1 levels had been detected in various cancers, including breast and colorectal cancer, indicating a potential role in tumor progression and metastasis29. Additionally, CHIT1 levels had been studied in autoimmune diseases such as rheumatoid arthritis and systemic lupus erythematosus, where they may reflect disease activity and severity30. Up to now, only two publications had studied the relationship between CHIT1 and sepsis. Study by Zhang.et al. investigated the role of PALLD, PRKCH, AKAP12, PDK4, and CHIT1 proteins in diagnosis and prognosis of neonatal sepsis (NS). Their results showed that these proteins were all elevated in the serum of NS and had diagnostic value for identifying NS. Among these proteins, CHIT1 was highlighted as a potential non-invasive biomarker for the diagnosis and prognosis of NS, contributing to the development of new diagnostic tools for NS31. Another study by Klostergaard. et at. investigated the associations between polymorphisms of CHIT1 and mannose-binding lectin 2 (MBL2) with sepsis risk in adult patients with acute myeloid leukemia (AML) undergoing high-dose chemotherapy. Despite the important role of CHIT1 and MBL2 played in the innate immune system, no significant associations were found between CHIT1 polymorphism and sepsis (P = 0.85) or sepsis related death (P = 0.14). The study suggested that severe neutropenia and mucositis following chemotherapy may override the protective effects of the MBL system, indicating that recombinant MBL replacement therapy was unlikely to reduce sepsis risk in AML patients32. The present study provided novel insights into the role of CHIT1 and CHIT1 + neutrophils in sepsis progression. Specifically, it was observed that higher CHIT1 expression levels in sepsis patients were associated with increased 28-day mortality rates. Through MR analysis, CHIT1 was identified as a high-risk susceptibility gene and a potential druggable target for sepsis. Additionally, scRNA-seq analysis revealed that CHIT1 was predominantly expressed in neutrophils, particularly in non-survivors of sepsis. The study further suggested that CHIT1 + neutrophils contributed to sepsis progression through mechanisms such as overexpression of pro-inflammatory genes, reduced HLA-DR levels, and recruitment of other immune cells via the ICAM1-ITGAM + ITGB2 pathway. Unlike the study by Zhang et al., which focused on neonatal sepsis and highlighted CHIT1 as a potential non-invasive biomarker for diagnosis and prognosis in neonates, the current study investigated the role of CHIT1 in adult sepsis and identified its association with mortality. Furthermore, while Klostergaard Anja et al. examined the genetic polymorphisms of CHIT1 in adult AML patients undergoing high-dose chemotherapy and found no significant associations with sepsis risk or sepsis-related death, this study demonstrated a direct link between CHIT1 expression levels and sepsis progression in a broader patient population. The current study innovated by providing a comprehensive analysis of CHIT1 expression in sepsis using scRNA-seq data, revealing its predominant expression in neutrophils and its differential levels between survivors and non-survivors. This detailed cellular-level analysis allowed for a deeper understanding of the role of CHIT1 + neutrophils in sepsis progression. Moreover, the identification of CHIT1 as a potential druggable target based on MR analysis represented a significant advancement, offering new directions for therapeutic interventions in sepsis management. Despite these contributions, the study had some limitations. The sample size, especially for the scRNA-seq analysis, may be relatively small, which could affect the robustness and generalizability of the findings. Additionally, while the study suggested potential mechanisms by which CHIT1 + neutrophils contributed to sepsis progression, further experimental validation was needed to confirm these pathways. Finally, the clinical applicability of CHIT1 as a biomarker or therapeutic target required additional validation through larger-scale clinical trials and in diverse patient populations.
Sepsis is recognized as a state of unbalanced hyperinflammation and immune suppression, where immune mechanisms initially activated for protection can become harmful due to excessive inflammation and immune suppression. Various cell types and mediator systems contribute to the severe inflammation seen in sepsis, including white blood cells (such as neutrophils, macrophages, and NK cells), endothelial cells, cytokines, complement factors, and the coagulation cascade. Neutrophils, also known as polymorphonuclear cells, are crucial components of the innate immune response and play a significant role in regulating adaptive immunity. Equipped with various enzymes and inflammatory agents, neutrophils can exacerbate inflammation in sepsis by releasing proteases and reactive oxygen species. They form NETs, which are structures composed of chromatin fibers and antimicrobial agents such as myeloperoxidase, elastase, and cathepsin G33. While NETs help combat bacterial infections by trapping and killing bacteria34,35, excessive NETs formation can lead to complications such as intravascular clotting and organ failure. NETs are rich in histones, which can activate and damage blood vessels, contributing to vascular injury36. Additionally, free histones have been associated with severe outcomes in sepsis models induced by high doses of LPS or TNF37,38. In this study, CHIT1 was mainly expressed in neutrophils, with CHIT1 + neutrophils being more prevalent in sepsis non-survivors compared to survivors and HCs. Furthermore, Cellchat analysis demonstrated that CHIT1 + neutrophils could recruit other immune cell types, including NK cells, erythroid cells, D14 + + monocytes/macrophages, DC, and CD14 + monocytes/macrophages through the ICAM1-(ITGAM + ITGB2) pathway, suggesting a critical role for CHIT1 + neutrophils in sepsis.
Early research introduces the concept of a “cytokine storm” describing the intense systemic release of inflammatory cytokines in animal models exposed to bacterial infections or their products. These studies indicate that targeting pro-inflammatory cytokines such as TNF, IL-1β, IL-12, and IL-18 could significantly reduce organ damage and improve survival outcomes39. Similarly, higher expression of pro-inflammatory genes, including inflammatory genes (S100A8, S100A9, S100A11, S100A120), receptors (IL1R2, IFNGR2, TLR2), and chemokines (CXCL8), were found in CHIT1 + neutrophils compared to other myeloid cell subpopulations. Moreover, the expression of these pro-inflammatory genes was also higher in CHIT1 + neutrophils from sepsis non-survivors compared to HCs and sepsis survivors. S100A8/A9 has emerged as a valuable biomarker for sepsis and related organ damage. Targeting S100A8/A9 has shown promise in reducing inflammation and improving outcomes, highlighting its potential as both a diagnostic biomarker and a therapeutic target for sepsis40. In sepsis, persistent immune activation is driven not only by external pathogens but also by endogenous “damage-associated molecular patterns” (DAMPs) released from damaged cells. These DAMPs activate pattern recognition receptors (PRRs), which can also detect pathogen-associated molecular patterns (PAMPs), leading to a harmful cycle of continuous immune stimulation and dysfunction5. DAMPs are recognized by TLR2 on immune cells attracted to the damaged tissue, exacerbating the inflammatory response and contributing to tissue damage when blood flow is restored41,42. Inhibiting TLR2 could potentially offer cellular protection by reducing complement activation, lowering C3 opsonization, and decreasing membrane attack complex (MAC) deposition, thereby alleviating damage and mitigating inflammation43. CXCL8, secreted by various cell types, including monocytes, macrophages, fibroblasts, hepatocytes, epithelial, and endothelial cells, facilitates the movement of neutrophils to areas of inflammation by interacting with CXCR1 and CXCR2 receptors. Experimental data highlighted the crucial involvement of the CXCL8-CXCR1/2 pathway in neutrophils within inflammatory and autoimmune conditions44. Similarly, higher CHIT1 + neutrophils were observed in sepsis non-survivors in this study. Combining our findings with other studies, it was strongly indicated that CHIT1 + neutrophils may contribute to sepsis progression by releasing pro-inflammatory agents that trigger or exacerbate the inflammatory response.
Sepsis is a complex clinical condition characterized by a systemic inflammatory reaction to tissue damage or infection, which leads to a reduced immune response. This condition impacts both the innate and adaptive immune systems. Immunoparalysis, a state of immune suppression, is associated with poorer outcomes, including multiple organ dysfunction, secondary infections, and higher mortality rates. Various immune markers have been proposed for detecting immunoparalysis in sepsis, with some showing potential clinical relevance. Key features of sepsis-induced immunoparalysis include decreased lymphocyte counts and the downregulation of HLA on monocytes or neutrophils. These HLA proteins are crucial for presenting antigens to T lymphocytes. The measurement of mHLA-DR, a class II protein on monocytes, via flow cytometry is used to assess monocyte function45. In sepsis-induced immunoparalysis, well-documented characteristics included decreased levels of HLA-DR and reduced monocyte activity46. Low HLA-DR levels were linked to poor clinical outcomes, such as increased susceptibility to hospital-acquired infections, organ failure, prolonged ICU stays, and elevated mortality rates47,48. Various experimental immunotherapies had been tested to counteract sepsis-induced immunoparalysis. Notably, sargramostim, a recombinant form of human granulocyte-macrophage colony-stimulating factor (rhu GM-CSF), had demonstrated clinical benefits by shortening hospital stays and reducing the likelihood of secondary infections. This reduction in infection risk was associated with increased levels of mHLA-DR expression on peripheral blood monocytes in treated patients49. In the present study, reduced expression of HLA-related genes—HLA-DMA, HLA-DPA1, HLA-DPB1, HLA-DRA, HLA-DRB1, and HLA-DRB5—was found in CHIT1 + neutrophils compared to other myeloid cell subpopulations. Additionally, the expression of these pro-inflammatory genes was lower in CHIT1 + neutrophils from sepsis non-survivors compared to those in HCs and sepsis survivors, suggesting that CHIT1 + neutrophils may contribute to the progression and 28-day mortality of sepsis by promoting sepsis-induced immunoparalysis through the reduction of HLA-related genes.
ICAM1 was a key adhesion molecule expressed on the surface of endothelial cells and immune cells, facilitating leukocyte recruitment and activation. ICAM1 was upregulated on endothelial cells and immune cells (e.g., neutrophils) during inflammation. It bound to the integrin heterodimer ITGAM and ITGB2 on immune cells, facilitating the adhesion and migration of neutrophils and monocytes to the sites of inflammation. This interaction was critical for the immune response in sepsis. ICAM1 was found to be upregulated in sepsis and was associated with septic shock, overall mortality and sepsis-related mortality. In sepsis, the process of impeding the interaction between ICAM1 and their respective protein receptors could disrupt the adherence of immune and endothelial cells. This disruption subsequently resulted in a diminished inflammatory response50. In the present study, CellChat analysis revealed that CHIT1 + neutrophils could interact with various immune cell types, including NK cells, CD14 + + monocytes/macrophages, DCs, and CD14 + monocytes/macrophages, via the ICAM1-(ITGAM + ITGB2) pathway, which may suggest that CHIT1 + neutrophils could also modulate other immune cell and influence the severity and outcome of sepsis by ICAM1-ITGAM + ITGB2 pathway. For example, macrophages emerged as the predominant immune cell type of sepsis-induced cardiomyopathy (SIC). Notably, ITGAM was identified as a key regulatory molecule that modulated macrophage function, driving the pathogenesis of SIC through its influence on the recruitment and functional reprogramming of these cells. In vitro experiments revealed that lipopolysaccharide (LPS) stimulation triggered an upregulation of ITGAM in macrophages and played critical roles in macrophages mobilization and intercellular communication. The strategic administration of ITGAM-neutralizing antibodies to SIC mice resulted in a marked decrease in macrophage infiltration within the cardiac tissue, which was initially associated with an improvement in cardiac function51. The ICAM1-ITGAM + ITGB2 pathway was a critical component in the interaction between CHIT1 + neutrophils and other immune cells during sepsis. This pathway modulated immune cell adhesion, activation, and communication, influencing the progression and outcome of sepsis.
While MR is a powerful tool for assessing potential causal relationships, it is not immune to limitations, and its conclusions must be interpreted with caution. First, the robustness of MR hinged on the genetic variants serving as IVs. While we rigorously selected SNPs associated with CHIT1 (p < 5 × 10⁻⁸) and excluded pleiotropic variants via PhenoScanner, residual horizontal pleiotropy or linkage disequilibrium with confounding factors could not be entirely ruled out. Second, MR estimates reflected lifelong genetic effects rather than acute changes in CHIT1 levels during sepsis. This may limit direct translation to therapeutic targeting of CHIT1 in critical illness. Finally, our analysis relied on summary statistics from European-ancestry cohorts. Shared participants between exposure and outcome datasets, as well as genetic diversity across populations, could influence generalizability.CHIT1 had been identified as a potential biomarker in various lipid storage disorders, including Gaucher, Niemann-Pick, and Fabry diseases. In the context of Gaucher disease, plasma CHIT1 levels had been utilized in clinical settings for more than a decade to assess disease severity and evaluated the efficacy of therapeutic interventions52. Measuring CHIT1 levels in a clinical setting could be achieved through enzyme-linked immunosorbent assays (ELISA), which have been used to quantify CHIT1 in various biological samples, including serum and cerebrospinal fluid. For instance, the CircuLex Human Chitotriosidase ELISA Kit had been employed to measure CHIT1 levels in multiple sclerosis patients, demonstrating its applicability in clinical research53. This method could similarly be applied to sepsis patients to assess CHIT1 levels as part of diagnostic or prognostic evaluations. Using CHIT1 as a biomarker for sepsis diagnosis and prognosis could offer several benefits. Elevated CHIT1 levels had been associated with disease severity and progression in other inflammatory conditions, suggesting that it might serve as an early indicator of sepsis and help predict patient outcomes. However, there were potential limitations to consider. Variability in CHIT1 expression levels among different patient populations, due to factors such as genetic polymorphisms, underlying health conditions, could affect its reliability as a biomarker. For example, the 24-bp duplication polymorphism in the CHIT1 gene could significantly influence its expression levels, which may vary across different ethnic groups. This genetic variability could complicate the interpretation of CHIT1 levels in diverse patient populations54. Moreover, targeting CHIT1 in sepsis patients presented potential challenges and risks. Given CHIT1s role in immune responses and inflammation, interventions aimed at modulating its activity could have unintended effects on the immune system. Additionally, the precise mechanisms through which CHIT1 contributed to sepsis pathogenesis were not fully understood, which could complicate the development of targeted therapies. Further research was needed to elucidate these mechanisms and to determine the safety and efficacy of CHIT1-targeted treatments in sepsis patients.
Conclusion
In summary, our combined analysis of multi-omics and MR studies demonstrated that CHIT1 + neutrophils play a significant role in sepsis-related mortality by exacerbating both hyperinflammation and immune suppression. These results suggest potential opportunities for developing targeted immunotherapy approaches that focued on CHIT1 + neutrophils to improve outcomes in sepsis.
Data availability
All datasets used and/or analyzed during the current study were from the public database, including GSE65682 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi? acc=GSE65682), GSE167363 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi? acc=GSE167363https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi), GSE28750 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi? acc=GSE28750), GSE57065 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi? acc=GSE57065), GSE95233 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi? acc=GSE95233), eQTLGen Consortium (https://www.eqtlgen.org/cis-eqtls.html) and OpenGWAS project (https://gwas.mrcieu.ac.uk/).
Abbreviations
- SIRS:
-
Systemic inflammatory response syndrome
- scRNA-seq:
-
Single-cell RNA sequencing
- MR:
-
Mendelian randomization
- RCTs:
-
Randomized controlled trials
- GEO:
-
Gene expression omnibus
- CHIT1:
-
Chitinase 1
- HCs:
-
Healthy controls
- DEGs:
-
Differentially expressed genes
- FDR:
-
False discovery rate
- HR:
-
Hazard ratios
- CI:
-
Confidence interval
- IEU:
-
Integrative epidemiology unit
- IVs:
-
Instrumental variables
- LD:
-
Linkage disequilibrium
- GWAS:
-
Genome-wide association study
- UMIs:
-
Unique molecular identifiers
- PBMCs:
-
Peripheral blood mononuclear cells
- qRT-PCR:
-
Quantitative real-time PCR experiment
- GO:
-
Gene ontology
- KEGG:
-
Kyoto encyclopedia of genes and genomes
- BP:
-
Biological process
- ROC:
-
Receiver operating characteristic
- AUC:
-
Area under the curve
- ICU:
-
Intensive care unit
- NETs:
-
Neutrophil extracellular traps
- COPD:
-
Chronic obstructive pulmonary disease
- NS:
-
Neonatal sepsis
- MBL2:
-
Mannose-binding lectin 2
- AML:
-
Acute myeloid leukemia
- DAMPs:
-
Damage-associated molecular patterns
- PRRs:
-
pattern recognition receptors
- PAMPs:
-
Pathogen-associated molecular patterns
- MAC:
-
Membrane attack complex
- GM-CSF:
-
Granulocyte-macrophage colony-stimulating factor
- SIC:
-
Sepsis-induced cardiomyopathy
- LPS:
-
Lipopolysaccharide
- ELISA:
-
Enzyme-linked immunosorbent assays
References
Wang, H. E., Jones, A. R. & Donnelly, J. P. Revised National estimates of emergency department visits for Sepsis in the united States. Crit. Care Med. 45(9):1443–1449 .
Marshall, J. C. Why have clinical trials in sepsis failed? Trends Mol. Med. 20 (4), 195–203 (2014).
Evans, L. et al. Surviving Sepsis campaign: international guidelines for management of Sepsis and septic shock 2021. Crit. Care Med. 49 (11), e1063–e1143 (2021).
n der Poll, T., Shankar-Hari, M., Wiersinga, W. J. & va, The immunology of sepsis. Immunity 54 (11), 2450–2464 (2021).
n Der Poll, T., Scicluna, B. P., Netea, M. G. & Va, The immunopathology of sepsis and potential therapeutic targets. Nat. Rev. Immunol. 17 (7), 407–420 (2017).
Shankar-Hari, M. & Rubenfeld, G. D. Population enrichment for critical care trials: phenotypes and differential outcomes. Curr. Opin. Crit. Care. 25 (5), 489–497 (2019).
Stanski, N. L. & Wong, H. R. Prognostic and predictive enrichment in sepsis. Nat. Rev. Nephrol. 16 (1), 20–31 (2020).
Darden, D. B. et al. Single-Cell RNA-seq of human Myeloid-Derived suppressor cells in late Sepsis reveals multiple subsets with unique transcriptional responses: A pilot study. Shock 55 (5), 587–595 (2021).
Yao, R. Q. et al. Single-cell transcriptome profiling of the immune space-time landscape reveals dendritic cell regulatory program in polymicrobial sepsis. Theranostics 12 (10), 4606–4628 (2022).
Morrow, J. D. et al. Human lung DNA methylation quantitative trait loci colocalize with chronic obstructive pulmonary disease genome-Wide association loci. Am. J. Respir Crit. Care Med. 197 (10), 1275–1284 (2018).
Huang, W., Xiao, J., Ji, J. & Chen, L. Association of lipid-lowering drugs with COVID-19 outcomes from a Mendelian randomization study. Elife 10, e73873 (2021).
Scicluna, B. P. et al. A molecular biomarker to diagnose community-acquired pneumonia on intensive care unit admission. Am. J. Respir Crit. Care Med. 192 (7), 826–835 (2015).
Qiu, X. et al. Dynamic changes in human single-cell transcriptional signatures during fatal sepsis. J. Leukoc. Biol. 110 (6), 1253–1268 (2021).
Kanehisa, M., Furumichi, M., Sato, Y., Kawashima, M. & Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 51 (D1), D587–D592 (2023).
Kanehisa, M. Toward Understanding the origin and evolution of cellular organisms. Protein Sci. Vol. 28 (11), 1947–1951 (2019).
Kanehisa, M. & Goto, S. K. E. G. G. Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. Vol. 28 (1), 27–30 (2000).
Võsa, U. et al. Large-scale cis- and trans-eQTL analyses identify thousands of genetic loci and polygenic scores that regulate blood gene expression. Nat. Genet. 53 (9), 1300–1310 (2021).
Hemani, G. et al. The MR-base platform supports systematic causal inference across the human phenome. Elife 7, e34408 (2018).
Burgess, S., Butterworth, A. & Thompson, S. G. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 37 (7), 658–665 (2013).
Qiu, X. et al. Reversed graph embedding resolves complex single-cell trajectories. Nat. Methods. 14 (10), 979–982 (2017).
Jin, S. et al. Inference and analysis of cell-cell communication using cellchat. Nat. Commun. 12 (1), 1088 (2021).
Boufenzer, A. et al. Potentiation of NETs release is novel characteristic of TREM-1 activation and the Pharmacological Inhibition of TREM-1 could prevent from the deleterious consequences of NETs release in sepsis. Cell. Mol. Immunol. 18 (2), 452–460 (2021).
Zhuang, Y., Peng, H., Chen, Y., Zhou, S. & Chen, Y. Dynamic monitoring of monocyte HLA-DR expression for the diagnosis, prognosis, and prediction of sepsis. Front. Bioscience - Landmark. 22 (8), 1344–1354 (2017).
Salomão, R. et al. Sepsis: evolving concepts and challenges. Braz. J. Med. Biol. Res. 52 (4), e8595 (2019).
Li, A. T. et al. Biomarkers for the early diagnosis of Sepsis in Burns: systematic review and Meta-analysis. Ann. Surg. 275 (4), 654–662 (2022).
Kanneganti, M., Kamba, A. & Mizoguchi, E. Role of Chitotriosidase (Chitinase 1) under normal and disease conditions. J. Epithel. Biol. Pharmacol. 5, 1–9 (2012).
Camerino, D. et al. Low-perceived work ability, ageing and intention to leave nursing: A comparison among 10 European countries. J. Adv. Nurs. 56 (5), 542–552 (2006).
Przysucha, N., Górska, K. & Krenke, R. Chitinases and chitinase-like proteins in obstructive lung diseases – current concepts and potential applications. Int. J. COPD. 15, 885–899 (2020).
Libreros, S., Garcia-Areas, R. & Iragavarapu-Charyulu, V. CHI3L1 plays a role in cancer through enhanced production of pro-inflammatory/pro-tumorigenic and angiogenic factors. Immunol. Res. 57 (1-3), 99–105 (2013).
Yu, R. et al. Serum CHI3L1 as a biomarker of interstitial lung disease in rheumatoid arthritis. Front. Immunol. 14, 1211790 (2023).
Zhang, Y., Li, J. Y., Qi, H. L. & Kong, X. Y. Mechanisms of PALLD, PRKCH, AKAP12, PDK4, and CHIT1 proteins in serum diagnosis of neonatal Sepsis. Clin. Lab. 68(11) (2022).
Klostergaard, A. et al. Sepsis in acute myeloid leukaemia patients receiving high-dose chemotherapy: no impact of Chitotriosidase and mannose-binding lectin polymorphisms. Eur. J. Haematol. 85 (1), 58–64 (2010).
Brinkmann, V. et al. Neutrophil Extracell. Traps Kill Bacteria Sci. ; 303(5663):1532–1535. (2004).
Li, P. et al. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J. Exp. Med. 207 (9), 1853–1862 (2010).
Czaikoski, P. G. et al. Neutrophil extracellular traps induce organ damage during experimental and clinical sepsis. PLoS One. 11 (2), e0148142 (2016).
McDonald, B. et al. Platelets and neutrophil extracellular traps collaborate to promote intravascular coagulation during sepsis in mice. Blood 129 (10), 1357–1367 (2017).
Clark, S. R. et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 13 (4), 463–469 (2007).
Xu, J. et al. Extracellular histones are major mediators of death in sepsis. Nat. Med. 5 (11), 1318–1321 (2009).
Wiersinga, W. J., Leopold, S. J. & van der Cranendonk, D. R. Host innate immune responses to sepsis. Virulence 5 (1), 36–44 (2014).
Wang, Q. et al. S100A8/A9: an emerging player in sepsis and sepsis-induced organ injury. Biomed. Pharmacotherapy. 168, 115674 (2023).
Arslan, F. et al. Myocardial ischemia/reperfusion injury is mediated by leukocytic toll-like receptor-2 and reduced by systemic administration of a novel anti-toll-like receptor-2 antibody. Circulation 121 (1), 80–90 (2010).
Selejan, S. et al. Ischaemia-induced up-regulation of Toll-like receptor 2 in Circulating monocytes in cardiogenic shock. Eur. Heart J. 33 (9), 1085–1094 (2012).
Mulfaul, K. et al. Toll-like receptor 2 facilitates oxidative Damage-Induced retinal degeneration. Cell. Rep. 30 (7), 2209–2224e5 (2020).
Sitaru, S., Budke, A., Bertini, R. & Sperandio, M. Therapeutic Inhibition of CXCR1/2: where do we stand? Intern. Emerg. Med. 18 (6), 1647–1664 (2023).
Padovani, C. M. & Yin, K. Immunosuppression in sepsis: biomarkers and specialized Pro-Resolving mediators. Biomedicines 12 (1), 175 (2024).
Davies, R., O’Dea, K. & Gordon, A. Immune therapy in sepsis: are we ready to try again? J. Intensive Care Soc. 9 (4), 326–344 (2018).
Leijte, G. P. et al. Monocytic HLA-DR expression kinetics in septic shock patients with different pathogens, sites of infection and adverse outcomes. Crit. Care. 24 (1), 110 (2020).
Cour-Andlauer, F. et al. Decreased human leukocyte antigen DR on Circulating monocytes expression after severe pediatric trauma: an exploratory report. Pediatr. Crit. Care Med. 22 (5), e314–e323 (2021).
Joshi, I., Carney, W. P. & Rock, E. P. Utility of monocyte HLA-DR and rationale for therapeutic GM-CSF in sepsis immunoparalysis. Front. Immunol. 14, 1130214 (2023).
Laudes, I. J. et al. Disturbed homeostasis of lung intercellular adhesion Molecule-1 and vascular cell adhesion Molecule-1 during Sepsis. Am. J. Pathol. 164. (2004).
Huang, H., Wang, Q., Ma, L. & Wu, Y. ITGAM: A pivotal regulator in macrophage dynamics and cardiac function during Sepsis-Induced cardiomyopathy. Cureus 164 (4), 1435–1445 (2024).
van Dussen, L. et al. Value of plasma Chitotriosidase to assess non-neuronopathic gaucher disease severity and progression in the era of enzyme replacement therapy. J. Inherit. Metab. Dis. 37 (6), 991–1001 (2014).
Beliën, J. et al. CHIT1 at diagnosis predicts faster disability progression and reflects early microglial activation in multiple sclerosis. Nat. Commun. 15 (1), 5013 (2024).
Boot, R. G. et al. The human Chitotriosidase gene - Nature of inherited enzyme deficiency. J. Biol. Chem. 273 (40), 25680–25685 (1998).
Author information
Authors and Affiliations
Contributions
Guorui Li, Yunlong Mao and Jiaxiang Liao were involved in data acquisition, analysis, and interpretation. Guorui Li and Yunlong Mao were responsible for drafting the manuscript. Yuquan Zhou supervised the study and oversaw the manuscript’s revisions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical statement
The ethical approval for our study was obtained from the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University (Protocol license number: 2025-E0219), and adhered to the principles of the Declaration of Helsinki. We acquired informed consent from patients or their relatives. All procedures performed in this study were in accordance with the ethical standards of the institutional and with the 1964 Helsinki Declaration and this study aimed to identify new key biomarkers that could predict outcomes in sepsis patients and explore its underlying molecular mechanisms later amendments or comparable ethical standards.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, G., Mao, Y., Liao, J. et al. Integrated multiomics and Mendelian randomization identify CHIT1 as a novel sepsis biomarker and therapeutic target. Sci Rep 15, 15715 (2025). https://doi.org/10.1038/s41598-025-99619-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-99619-z