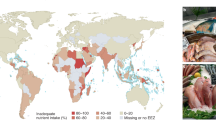
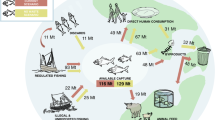
Abstract
Wild fish harvests from freshwaters and oceans per person on Earth have been stagnating for decades due to increased food demand from a burgeoning global human population, raising the stakes for maximizing the nutritional benefits from limited fish stocks. Here we adopt an allocation optimization approach using biogeographic and nutrient data for the world’s fishes to identify ideal portfolios of species for consumption in every country. We find that, across nations, biodiversity increases opportunities to fulfil multiple nutritional requirements with less fish biomass. This advantage emerges through complementarity among species; portfolios of complementary species provide >60% more nutrients than the same biomass of the most nutrient-rich species. Moreover, biodiverse fisheries enable harvest allocation towards species with traits enhancing fishery resilience (for example, small size, low trophic position) and offer greater redundancy, whereby a wider range of comparably nutritious species is available. Our analysis underscores that conserving fish biodiversity can improve nutrition and fishery resilience while reducing harvest pressure on already-stressed aquatic ecosystems.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Data for replicating the analysis and results are available via Figshare at https://doi.org/10.6084/m9.figshare.24615465 (ref. 49). The Aquatic Resource Trade in Species (ARTIS) database is accessible at https://artisdata.weebly.com/ (ref. 27).
Code availability
Code supporting the results is available via Figshare at https://doi.org/10.6084/m9.figshare.24615465 (ref. 49).
References
Foley, J. A. et al. Solutions for a cultivated planet. Nature 478, 337–342 (2011).
Godfray, H. C. J. et al. Food security: the challenge of feeding 9 billion people. Science 327, 812–818 (2010).
van der Plas, F. et al. Jack-of-all-trades effects drive biodiversity–ecosystem multifunctionality relationships in European forests. Nat. Commun. 7, 11109 (2016).
Fanin, N. et al. Consistent effects of biodiversity loss on multifunctionality across contrasting ecosystems. Nat. Ecol. Evol. 2, 269–278 (2018).
Hanley-Cook, G. T. et al. Food biodiversity: quantifying the unquantifiable in human diets. Crit. Rev. Food Sci. Nutr. 63, 7837–7851 (2022).
Golden, C. D. et al. Aquatic foods to nourish nations. Nature 598, 315–320 (2021).
Hicks, C. C. et al. Harnessing global fisheries to tackle micronutrient deficiencies. Nature 574, 95–98 (2019).
Hilborn, R. et al. Effective fisheries management instrumental in improving fish stock status. Proc. Natl Acad. Sci. USA 117, 2218–2224 (2020).
Bernhardt, J. R. & O’Connor, M. I. Aquatic biodiversity enhances multiple nutritional benefits to humans. Proc. Natl Acad. Sci. USA 118, e1917487118 (2021).
Heilpern, S. A. et al. Species trait diversity sustains multiple dietary nutrients supplied by freshwater fisheries. Ecol. Lett. 26, 1887–1897 (2023).
Walker, B. et al. Response diversity as a sustainability strategy. Nat. Sustain. https://doi.org/10.1038/s41893-022-01048-7 (2023).
Heilpern, S. A. et al. Substitution of inland fisheries with aquaculture and chicken undermines human nutrition in the Peruvian Amazon. Nat. Food 2, 192–197 (2021).
Belton, B. & Thilsted, S. H. Fisheries in transition: food and nutrition security implications for the global South. Glob. Food Sec. 3, 59–66 (2014).
van Dooren, C. A review of the use of linear programming to optimize diets, nutritiously, economically and environmentally. Front. Nutr. 5, 48 (2018).
Fats and Fatty Acids in Human Nutrition: Report of an Expert Consultation (FAO, 2010).
Vitamin and Mineral Requirements in Human Nutrition (WHO and FAO, 2004).
Yachi, S. & Loreau, M. Biodiversity and ecosystem productivity in a fluctuating environment: the insurance hypothesis. Proc. Natl Acad. Sci. USA 96, 1463–1468 (1999).
Heilpern, S. A. et al. Declining diversity of wild-caught species puts dietary nutrient supplies at risk. Sci. Adv. 7, eabf9967 (2021).
Mouillot, D., Graham, N. A. J., Villéger, S., Mason, N. W. H. & Bellwood, D. R. A functional approach reveals community responses to disturbances. Trends Ecol. Evol. 28, 167–177 (2013).
Olden, J. D., Hogan, Z. S. & Zanden, M. J. V. Small fish, big fish, red fish, blue fish: size-biased extinction risk of the world’s freshwater and marine fishes. Glob. Ecol. Biogeogr. 16, 694–701 (2007).
Comte, L. & Olden, J. D. Climatic vulnerability of the world’s freshwater and marine fishes. Nat. Clim. Change 7, 718–722 (2017).
Robinson, J. P. W. et al. Small pelagic fish supply abundant and affordable micronutrients to low- and middle-income countries. Nat. Food 3, 1075–1084 (2022).
Hutchings, J. A., Myers, R. A., García, V. B., Lucifora, L. O. & Kuparinen, A. Life-history correlates of extinction risk and recovery potential. Ecol. Appl. 22, 1061–1067 (2012).
Garcia, S. M. et al. Reconsidering the consequences of selective fisheries. Science 335, 1045–1047 (2012).
Duffy, J. E., Lefcheck, J. S., Stuart-Smith, R. D., Navarrete, S. A. & Edgar, G. J. Biodiversity enhances reef fish biomass and resistance to climate change. Proc. Natl Acad. Sci. USA 113, 6230–6235 (2016).
Ross, S. R. P.-J., Petchey, O. L., Sasaki, T. & Armitage, D. W. How to measure response diversity. Methods Ecol. Evol. 14, 1150–1167 (2023).
Gephart, J. A. et al. Globalization of wild capture and farmed aquatic foods. Nat. Commun. 15, 8026 (2024).
Fiorella, K. J., Bageant, E. R., Thilsted, S. H. & Heilpern, S. A. Commercially traded fish portfolios mask household utilization of biodiversity in wild food systems. Proc. Natl Acad. Sci. USA 121, e2403691121 (2024).
Cisneros-Montemayor, A. M., Pauly, D., Weatherdon, L. V. & Ota, Y. A global estimate of seafood consumption by coastal indigenous peoples. PLoS ONE 11, e0166681 (2016).
Heilpern, S. A. et al. Nutritional challenges of substituting farmed animals for wild fish in human diets. Environ. Res. Lett. 18, 114030 (2023).
Ambikapathi, R. et al. Global food systems transitions have enabled affordable diets but had less favourable outcomes for nutrition, environmental health, inclusion and equity. Nat. Food 3, 764–779 (2022).
Loken, B., Dhar, M. & Rapando, N. P. Healthy and sustainable diets must be culturally acceptable too. Nat. Food 5, 723–724 (2024).
Robinson, J. P. W. et al. Managing fisheries for maximum nutrient yield. Fish Fish. 23, 800–811 (2022).
Lynch, A. J. et al. Inland fish and fisheries integral to achieving the Sustainable Development Goals. Nat. Sustain. https://doi.org/10.1038/s41893-020-0517-6 (2020).
Halpern, B. S. et al. Opinion: putting all foods on the same table: achieving sustainable food systems requires full accounting. Proc. Natl Acad. Sci. USA 116, 18152–18156 (2019).
Carmona, C. P. et al. Erosion of global functional diversity across the tree of life. Sci. Adv. 7, eabf2675 (2021).
Díaz, S. et al. The global spectrum of plant form and function. Nature 529, 167–171 (2016).
Vaitla, B. et al. Predicting nutrient content of ray-finned fishes using phylogenetic information. Nat. Commun. 9, 3742 (2018).
Schiettekatte, N. M. D. et al. Biological trade-offs underpin coral reef ecosystem functioning. Nat. Ecol. Evol. 6, 701–708 (2022).
Vanni, M. J. & McIntyre, P. B. Predicting nutrient excretion of aquatic animals with metabolic ecology and ecological stoichiometry: a global synthesis. Ecology 97, 3460–3471 (2016).
McIntyre, P. B., Liermann, C. A. R. & Revenga, C. Linking freshwater fishery management to global food security and biodiversity conservation. Proc. Natl Acad. Sci. USA 113, 12880–12885 (2016).
Miranda, R. et al. Monitoring extinction risk and threats of the world’s fishes based on the Sampled Red List Index. Rev. Fish Biol. Fish. 32, 975–991 (2022).
Froese, R. & Pauly, D. FishBase version 02/2019 (2019); www.fishbase.org
Csárdi, G. & Berkelaar, M. lpSolve: Interface to ‘Lp_solve’ v. 5.5 to Solve Linear/Integer Programs (2024).
Laliberté, E. & Legendre, P. A distance-based framework for measuring functional diversity from multiple traits. Ecology 91, 299–305 (2010).
Dinno, A. paran: Horn’s Test of Principal Components/Factors (2018).
Baselga, A. & Orme, C. D. L. betapart: an R package for the study of beta diversity. Methods Ecol. Evol. 3, 808–812 (2012).
R Core Team R: A language and environment for statistical computing (R Foundation for Statistical Computing, 2020).
Heilpern, S. et al. Data and code for ‘Leveraging biodiversity to maximize nutrition and resilience of global fisheries’. Figshare https://doi.org/10.6084/m9.figshare.24615465 (2025).
Acknowledgements
We thank C. Bowser, E. Duvall, M. Miranda, F. Pacheco, M. Valdverde and L. Zarri for providing comments that improved the manuscript. We also thank the many researchers that analysed fish nutrient content and developed predictive models for unmeasured species. This project is partially supported by Schmidt Sciences programmes, through an Eric & Wendy Schmidt AI in Science Postdoctoral Fellowship (S.A.H.) and an AI2050 Senior Fellowship (C.G.); a Cornell Presidential Postdoctoral Fellowship (S.A.H.); the National Science Foundation and the National Institute of Food and Agriculture (NSF and USDA/NIFA) no. 2023-67021-39829 (C.G.); the Air Force Office of Scientific Research no. FA9550-23-1-0322 (C.G.) and no. FA9550-23-1-0569 (DURIP; CG); and a David and Lucile Packard Fellowship for Science and Engineering (P.B.M.).
Author information
Authors and Affiliations
Contributions
S.A.H. conceived the study with substantial input from F.W.S. and P.B.M; all authors contributed to refining the study design; S.A.H. and F.W.S. developed the methodology and analysis; S.A.H. wrote the first draft with support from P.B.M.; all authors contributed to reviewing and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Sustainability thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Variation in nutrient content across all food fish species (grey), and for specific countries (green; first column: Iceland; second column: Kiribati; third column: Kenya), as depicted by the first three principal components using all nutrient content values (for example, protein, iron, zinc, calcium, vitamin A and omega-3s [EPA+DHA]).
Complementary species (orange) are the species included within optimal portfolios, while the best species (purple) is the single species that minimizes the biomass needed to support all RDAs. When a single best species is also included in an optimal portfolio, it is orange and outlined in black. More biodiverse countries are typically represented by a wider variation in nutritional trait space. Species included in the optimal portfolio are drawn from the edges of nutritional space, but do not overlap.
Extended Data Fig. 2 The species richness of an optimal portfolio (SOpt) increases as more RDAs are considered but never exceeds four species.
Each point represents a country (n=291) colored by it’s biodiversity endowment (S). Increasing the number of RDAs considered also leads to an increase in the minimum portion size needed (Pmin), but more speciose portfolios are associated with lower Pmin. Box plots display the median (center line), interquartile range (box) and whiskers (minimum and maximum values within 1.5 × IQR).
Extended Data Fig. 3 The wider aggregate nutrient trait content variation in more biodiverse countries increased the potential to assemble optimal species portfolios or select single best species with nutritionally extreme values.
(A) Species from countries with higher biodiversity exhibit more variation in nutrient content, measured as the convex hull volume considering six key nutrients derived from fish. (B) The mean nutrient trait distance of all species within a country tends to be lower than the mean nutrient trait distance of species in optimal portfolios (the black line represents the 1:1 line, with points above representing countries where the mean nutrient trait distance of optimal species is larger than of all species within that respective country). (C) The mean nutrient trait distance of all species within a country tends to be lower than the mean nutrient trait distance of single best species (the black line represents the 1:1 line, with points above representing countries where the mean nutrient trait distance of the single best species is larger than of all species within that respective country). Each point corresponds to a country (n=290).
Extended Data Fig. 4 Randomly assembled portfolios from countries with high biodiversity required less biomass to meet RDAs, but differences with portfolios based on complementarity and selection were exceptionally large (n=290).
Random portfolios were assembled by drawing a random sample of the same number of species represented in the optimal portfolio from each country (see methods). For full statistical results see Table S1. Panel B is a close-up of panel A.
Extended Data Fig. 5 Countries well-endowed with biodiversity sustain nutritionally complete diets with less biomass regardless of (A) %RDA thresholds or (B) number of RDAs considered.
The biodiversity effect (that is, estimated effect of S on on Pmin) does not vary based on (C) thresholds but (D) becomes stronger when more RDAs are considered. The biodiversity effect was obtained by using a generalized linear model and extracting the estimates and 95% confidence intervals.
Extended Data Fig. 6 The species composition of optimal portfolios depends on the number and type of nutrient RDAs are considered.
Each point represents a country (n=290) colored by the number of species within an optimal portfolio as indicated in the legend in (A), with lines connecting the same country. Points are jittered for interpretability. Dissimilarity, turnover and nestedness are measured as the pairwise differences between each successive number of RDAs for each country, with 0 indicating that there are no differences in optimal species composition and 1 indicating complete compositional turnover when comparing successive number of RDAs. For example, for the vast majority of countries the species composition of optimal portfolios that meet 2 RDAs is completely different than the species that are optimal to meet one RDA. When increasing from one to three RDAs, compositional changes are primarily driven (B) turnover, which represents changes in species composition driven by replacement of some species by others that were not previously included. When increasing from four to six RDAs, compositional changes are represented by a mixture of turnover and (C) nestedness, the latter representing changes in species composition driven by gains or losses of species.
Extended Data Fig. 7 Biodiversity gradient with random species draws from a global pool retains the same relationship between species richness and the minimum amount of fish biomass to meet RDAs based on complementarity and selection.
To build this random biodiversity gradient we sampled the global pool of species without replacement from 1 to 756 species, which is the species richness range for the number of food fishes available across nations. For every level of species richness, we performed this randomization 30 times for all 290 countries for a total of 8,700 random assemblages.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Heilpern, S.A., Simon, F.W., Sethi, S.A. et al. Leveraging biodiversity to maximize nutrition and resilience of global fisheries. Nat Sustain (2025). https://doi.org/10.1038/s41893-025-01577-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41893-025-01577-x