Abstract
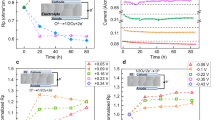
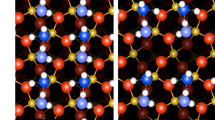
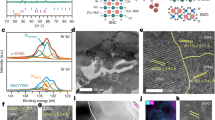
Atom trapping of scarce precious metals onto a suitable support at high temperatures has emerged as an effective approach to build thermally stable single-atom catalysts. Here, following a similar mechanism based on atom trapping through support effects, we demonstrate a reverse atom-trapping strategy to controllably extract strontium atoms from a rigid lanthanum strontium cobalt ferrite ((La0.6Sr0.4)0.95Co0.2Fe0.8O3−δ, LSCF) surface with ease. The lattice oxygen redox activity of LSCF is accordingly fine-tuned, leading to enhanced cathode performance in a solid-oxide fuel cell. An over 30−70% increases in maximum power density of the single cells at intermediate temperatures is achieved by LSCF with surface strontium vacancies compared to the pristine surface. In addition, the strontium-deficient surface excludes strontium segregation and formation of electrochemically inert SrO islands, thus improving the longevity of the cathode. This development can be broadly applicable for modifying structurally stable oxide surfaces, and opens more possibilities of scalable single-atom extraction strategies.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Atomic coordinates of the optimized computational models are provided as Supplementary Data 1 with this paper. Other data that support the findings of this study can be found in the article and the Supplementary Information; this information is also available from the corresponding author upon request.
Change history
05 August 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41929-024-01214-4
References
Fu, Q., Saltsburg, H. & Flytzani-Stephanopoulos, M. Active nonmetallic Au and Pt species on ceria-based water-gas shift catalysts. Science 301, 935–938 (2003).
Kyriakou, G. et al. Isolated metal atom geometries as a strategy for selective heterogeneous hydrogenations. Science 335, 1209–1212 (2012).
Qiao, B. et al. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 3, 634–641 (2011).
Jones, J. et al. Thermally stable single-atom platinum-on-ceria catalysts via atom trapping. Science 353, 150–154 (2016).
Xiong, H. et al. Thermally stable and regenerable platinum–tin clusters for propane dehydrogenation prepared by atom trapping on ceria. Angew. Chem. Int. Ed. 56, 8986–8991 (2017).
Peterson, E. et al. Low-temperature carbon monoxide oxidation catalysed by regenerable atomically dispersed palladium on alumina. Nat. Commun. 5, 4885 (2014).
Moliner, M. et al. Reversible transformation of Pt nanoparticles into single atoms inside high-silica chabazite zeolite. J. Am. Chem. Soc. 138, 15743–15750 (2016).
Ganzler, A. M. et al. Tuning the structure of platinum particles on ceria in situ for enhancing the catalytic performance of exhaust gas catalysts. Angew. Chem. Int. Ed. 56, 13078–13082 (2017).
Zhou, P. et al. Thermolysis of noble metal nanoparticles into electron-rich phosphorus-coordinated noble metal single atoms at low temperature. Angew. Chem. Int. Ed. 58, 14184–14188 (2019).
Wei, S. et al. Direct observation of noble metal nanoparticles transforming to thermally stable single atoms. Nat. Nanotechnol. 13, 856–861 (2018).
Qu, Y. et al. Direct transformation of bulk copper into copper single sites via emitting and trapping of atoms. Nat. Catal. 1, 781–786 (2018).
Hwang, J. et al. Perovskites in catalysis and electrocatalysis. Science 358, 751–756 (2017).
Jin, S. et al. Thousandfold change in resistivity in magnetoresistive La–Ca–Mn–O films. Science 264, 413–415 (1994).
Bednorz, J. G. & Müller, K. A. Possible high TC superconductivity in the Ba–La–Cu–O system. Z. Phys. B 64, 189–193 (1986).
Shao, Z. & Haile, S. A high-performance cathode for the next generation of solid-oxide fuel cells. Nature 431, 170–173 (2004).
Salamon, M. B. & Jaime, M. The physics of manganites: structure and transport. Rev. Mod. Phys. 73, 583 (1988).
Adler, S. B. Factors governing oxygen reduction in solid oxide fuel cell cathodes. Chem. Rev. 104, 4791–4844 (2004).
Wachsman, E. D. & Lee, K. T. Lowering the temperature of solid oxide fuel cells. Science 334, 935–939 (2011).
Lee, Y.-L., Kleis, J., Rossmeisl, J. & Morgan, D. Ab initio energetics of LaBO3(001) (B = Mn, Fe, Co, and Ni) for solid oxide fuel cell cathodes. Phys. Rev. B 80, 224101 (2009).
Lee, Y.-L., Kleis, J., Rossmeisl, J., Shao-Horn, Y. & Morgan, D. Prediction of solid oxide fuel cell cathode activity with first-principles descriptors. Energy Environ. Sci. 4, 3966–3970 (2011).
Mueller, D. N., Machala, M. L., Bluhm, H. & Chueh, W. C. Redox activity of surface oxygen anions in oxygen-deficient perovskite oxides during electrochemical reactions. Nat. Commun. 6, 6097 (2015).
Suntivich, J. et al. Estimating hybridization of transition metal and oxygen states in perovskites from O K-edge X-ray absorption spectroscopy. J. Phys. Chem. C. 118, 1856–1863 (2014).
Suntivich, J. et al. Design principles for oxygen-reduction activity on perovskite oxide catalysts for fuel cells and metal–air batteries. Nat. Chem. 3, 546–550 (2011).
Hong, W. T. et al. Probing LaMO3 metal and oxygen partial density of states using X-ray emission, absorption, and photoelectron spectroscopy. J. Phys. Chem. C. 119, 2063–2072 (2015).
Peña, M. A. & Fierro, J. L. G. Chemical structures and performance of perovskite oxides. Chem. Rev. 101, 1981–2018 (2001).
Goldschmidt, V. M. Die gesetze der krystallochemie. Naturwissenschaften 14, 477–485 (1926).
Lee, W., Han, J. W., Chen, Y., Cai, Z. & Yildiz, B. Cation size mismatch and charge interactions drive dopant segregation at the surfaces of manganite perovskites. J. Am. Chem. Soc. 135, 7909–7925 (2013).
Irvine, J. et al. Evolution of the electrochemical interface in high-temperature fuel cells and electrolysers. Nat. Energy 1, 15014 (2016).
Boldrin, P. & Brandon, N. P. Progress and outlook for solid oxide fuel cells for transportation applications. Nat. Catal. 2, 571–577 (2019).
Ding, D., Lai, S. Y., Gerdes, K. & Liu, M. Enhancing SOFC cathode performance by surface modification through infiltration. Energy Environ. Sci. 7, 552–575 (2014).
Smith, D. W. An acidity scale for binary oxides. J. Chem. Educ. 64, 480–481 (1987).
Wang, A., Li, J. & Zhang, T. Heterogeneous single-atom catalysis. Nat. Rev. Chem. 2, 65–81 (2018).
Lang, R. et al. Non defect-stabilized thermally stable single-atom catalyst. Nat. Commun. 10, 234 (2019).
Nicollet, C. et al. Acidity of surface-infiltrated binary oxides as a sensitive descriptor of oxygen exchange kinetics in mixed conducting oxides. Nat. Catal. 3, 913–920 (2020).
Flood, H. & Förland, T. The acidic and basic properties of oxides. Acta Chem. Scand. 1, 592–604 (1947).
Kostogloudis, G. C. & Ftikos, C. Properties of A-site-deficient La0.6Sr0.4Co0.2Fe0.8O3−δ-based perovskite oxides. Solid State Ion. 126, 143–151 (1999).
Doshi, R., Richard, V. L., Carter, J. D., Wang, X. & Krumpelt, M. Development of solid-oxide fuel cells that operate at 500 °C. J. Electrochem. Soc. 146, 1273–1278 (1999).
Mineshige, A. et al. Introduction of A-site deficiency into La0.6Sr0.4Co0.2Fe0.8O3−δ and its effect on structure and conductivity. Solid State Ion. 176, 1145–1149 (2005).
Konysheva, E. Y., Xu, X. & Irvine, J. T. S. On the existence of A-site deficiency in perovskites and its relation to the electrochemical performance. Adv. Mater. 24, 528–532 (2012).
de Castro, I. A. et al. Molybdenum oxides—from fundamentals to functionality. Adv. Mater. 29, 1701619 (2017).
Zhukovskii, V. M., Yanushkevich, T. M. & Tel’nykh, T. F. Fazovaya diagramma sistemy MoO3–SrO. Zh. Neorg. Khim. 17, 2827–2830 (1972).
Zhang, Y. et al. Thermal-expansion offset for high-performance fuel cell cathodes. Nature 591, 246–251 (2021).
Opitz, A. K. et al. The chemical evolution of the La0.6Sr0.4CoO3−δ surface under SOFC operating conditions and its implications for electrochemical oxygen exchange activity. Top. Catal. 61, 2129–2141 (2018).
Wu, T. et al. Iron-facilitated dynamic active-site generation on spinel CoAl2O4 with self-termination of surface reconstruction for water oxidation. Nat. Catal. 2, 763–772 (2019).
Lee, K. T. & Manthiram, A. Characterization of Nd0.6Sr0.4Co1−yFeyO3−δ (0 ≤ y ≤ 0.5) cathode materials for intermediate temperature solid oxide fuel cells. Solid State Ion. 176, 1521–1527 (2005).
Chen, J.-M. et al. A complete high-to-low spin state transition of trivalent cobalt ion in octahedral symmetry in SrCo0.5Ru0.5O3−δ. J. Am. Chem. Soc. 136, 1514–1519 (2014).
Thole, B. T. & van der Laan, G. Branching ratio in X-ray absorption spectroscopy. Phys. Rev. B 38, 3158 (1988).
Nam, D. N. H., Jonason, K., Nordblad, P., Khiem, N. V. & Phuc, N. X. Coexistence of ferromagnetic and glassy behavior in the La0.5Sr0.5CoO3 perovskite compound. Phys. Rev. B 59, 4189 (1999).
Khiem, N. V., Bau, L. V., An, N. M., Phuc, N. X. & Nam, D. N. H. Effects of Fe doping on the magnetic and transport properties of La0.5Sr0.5(Co1−xFex)O3 (0 ≤ x ≤ 0.6). Phys. Rev. B 327, 187–189 (2003).
Marrero-López, D., Peña-Martínez, J., RuizMorales, J. C., Gabás, M., Núñez, P., Aranda, M. A. G. & Ramos-Barrado, J. R. Redox behaviour, chemical compatibility and electrochemical performance of Sr2MgMoO6−δ as SOFC anode. Solid State Ion. 180, 1672–1682 (2010).
Ciucci, F. Modeling electrochemical impedance spectroscopy. Curr. Opin. Electrochem 13, 132–139 (2019).
Wan, T. H., Saccoccio, M., Chen, C. & Ciucci, F. Influence of the discretization methods on the distribution of relaxation times deconvolution: implementing radial basis functions with DRT tools. Electrochim. Acta 184, 483–499 (2015).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169 (1996).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865 (1996).
Dudarev, S. L., Botton, G. A., Savrasov, S. Y., Humphreys, C. J. & Sutton, A. P. Electron-energy-loss spectra and the structural stability of nickel oxide: an LSDA+U study. Phys. Rev. B 57, 1505–1509 (1998).
Ritzmann, A. M., Dieterich, J. M. & Carter, E. A. Density functional theory + U analysis of the electronic structure and defect chemistry of LSCF (La0.5Sr0.5Co0.25Fe0.75O3−δ). Phys. Chem. Chem. Phys. 18, 12260–12269 (2016).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188 (1976).
Henkelman, G., Uberuaga, B. P. & Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901–9904 (2000).
Allred, A. L. Electronegativity values from thermochemical data. J. Inorg. Nucl. Chem. 17, 215–221 (1961).
Acknowledgements
This work was supported by the National Key R&D Program of China (2018YFA0702003 to Y.D.L.), the National Natural Science Foundation of China (21890383 to Y.D.L., 21871159 and 22171157 to D.W., and 52002249 to Y.H.L.), the Beijing Natural Science Foundation (2214061 to Z.Z.), the Science and Technology Key Project of Guangdong Province of China (2020B010188002 to D.W.), the Guangdong Basic and Applied Basic Research Foundation (2019A1515110025 to Y.H.L.), the Fundamental Research Funds for the Central Universities (WUT: 2019III012GX and 2020III002GX to J.S.W.), and the China Postdoctoral Science Foundation (2019M660607 to Z.Z.). The S/TEM work was performed at the Nanostructure Research Center (NRC), which is supported by the State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, and the State Key Laboratory of Silicate Materials for Architectures (all of the laboratories are at Wuhan University of Technology). We thank the 1W1B and 4B9B beamlines of Beijing Synchrotron Radiation Facility (BSRF) and the BL14W1 beamline of Shanghai Synchrotron Radiation Facility (SSRF) for providing beam time to support this project. We also thank L. Zheng of Institute of High Energy Physics, Chinese Academy of Sciences and K. Cao of ShanghaiTech University for providing insightful discussions. Z.Z. acknowledges support from the Shuimu Tsinghua Scholar Program.
Author information
Authors and Affiliations
Contributions
D.W. and Y.D.L. supervised the research. Z.Z. conceived the idea, designed the experiments and analysed the data. Z.Z. and J.H. prepared the catalysts and Z.Z., Y.H.L., Z.L., J.Z. and J.H. performed the catalyst characterization. Z.Z., Y.H.L. and L.F. conducted the electrochemical tests. R.Y. performed the STEM studies and Z.Z., R.Y. and J.S.W. analysed the data. Z.Z. and Y.Z. developed the theoretical framework and Z.Z., J.Y. and L.X. carried out the DFT computations. Z.Z., J.Y., J.O.W. and Y.W. collected and analysed the X-ray absorption spectroscopy data. Z.Z. wrote the manuscript and all authors contributed to the discussions and revisions of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks John Buckeridge and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1-32 and Tables 1–7.
Supplementary Data 1
Atomic coordinates of optimized computational models.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhuang, Z., Li, Y., Yu, R. et al. Reversely trapping atoms from a perovskite surface for high-performance and durable fuel cell cathodes. Nat Catal 5, 300–310 (2022). https://doi.org/10.1038/s41929-022-00764-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-022-00764-9
This article is cited by
-
Optimizing surface active sites via burying single atom into subsurface lattice for boosted methanol electrooxidation
Nature Communications (2025)
-
Boosting extraordinary energy-storage in BaTiO3-based ferroelectric ceramics via surface reconstruction cation-defects engineering
Journal of Materials Science: Materials in Electronics (2025)
-
Single-atom alloys for sustainability-related electrocatalytic applications
Frontiers of Chemical Science and Engineering (2025)
-
Efficient power generation in a solid oxide fuel cell using N2O as the oxidant
Rare Metals (2025)
-
Enhanced stability of perovskite cathode via entropy engineering for CO2 electrolysis
Rare Metals (2025)