Abstract
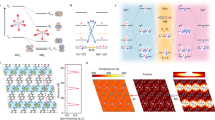
Co–Mn spinel oxide is a promising next-generation electrocatalyst that has previously shown oxygen reduction reaction activity that rivals that of Pt in alkaline fuel cells. Although the performance is encouraging, understanding the catalytic mechanisms in the oxygen reduction reaction is critical to advancing and enabling low-cost alkaline fuel cell technology. Here we use multimodal in situ synchrotron X-ray diffraction and resonant elastic X-ray scattering to investigate the interplay between the structure and oxidation state of a Co–Mn spinel oxide electrocatalyst. We show that the Co–Mn spinel oxide electrocatalyst exhibits a kinetically limited cubic-to-tetragonal phase transition, which is correlated to a reduction in both the Co and Mn valence states. Additionally, the electrocatalyst exhibits a reversible and rapid increase in tensile strain at low potentials during cyclic voltammetry, and joint density-functional theory is used to provide insight into how reactive adsorbates induce strain in spinel oxide nanoparticles.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this work are available in the Article and its Supplementary Information or from the authors upon request.
References
Debe, M. K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 486, 43–51 (2012).
Gasteiger, H. A., Kocha, S. S., Sompalli, B. & Wagner, F. T. Activity benchmarks and requirements for Pt, Pt-Alloy and non-Pt oxygen reduction catalysts for PEMFCs. Appl. Catal. B 56, 9–35 (2005).
Xiong, Y., Yang, Y., Disalvo, F. J. & Abruña, H. D. Pt-decorated composition-tunable Pd-Fe@Pd/C core-shell nanoparticles with enhanced electrocatalytic activity toward the oxygen reduction reaction. J. Am. Chem. Soc. 140, 7248–7255 (2018).
Wang, G. et al. Pt skin on AuCu intermetallic substrate: a strategy to maximize Pt utilization for fuel cells. J. Am. Chem. Soc. 136, 9643–9649 (2014).
Wang, D. et al. Structurally ordered intermetallic platinum–cobalt core–shell nanoparticles with enhanced activity and stability as oxygen reduction electrocatalysts. Nat. Mater. 12, 81–87 (2012).
Shao, M., Chang, Q., Dodelet, J. P. & Chenitz, R. Recent advances in electrocatalysts for oxygen reduction reaction. Chem. Rev. 116, 3594–3657 (2016).
Ge, X. et al. Oxygen reduction in alkaline media: from mechanisms to recent advances of catalysts. ACS Catal. 5, 4643–4667 (2015).
Yang, Y. et al. Octahedral spinel electrocatalysts for alkaline fuel cells. Proc. Natl Acad. Sci. USA 116, 24425–24432 (2019).
Wang, Y. et al. Synergistic Mn-Co catalyst outperforms Pt on high-rate oxygen reduction for alkaline polymer electrolyte fuel cells. Nat. Commun. 10, 1506 (2019).
Yang, Y. et al. In situ X-ray absorption spectroscopy of a synergistic Co-Mn oxide catalyst for the oxygen reduction reaction. J. Am. Chem. Soc. 141, 1463–1466 (2019).
Martens, I. et al. Probing the dynamics of platinum surface oxides in fuel cell catalyst layers using in situ X-ray diffraction. ACS Appl. Energy Mater. 2, 7772–7780 (2019).
Chattot, R. et al. Disclosing Pt-bimetallic alloy nanoparticle surface lattice distortion with electrochemical probes. ACS Energy Lett. 5, 162–169 (2020).
Chattot, R. et al. Electrochemical strain dynamics in noble metal nanocatalysts. J. Am. Chem. Soc. 143, 17068–17078 (2021).
Stragier, H. et al. Diffraction anomalous fine structure: a new X-ray structural technique. Phys. Rev. Lett. 69, 3064–3067 (1992).
Kawaguchi, T. et al. Roles of transition metals interchanging with lithium in electrode materials. Phys. Chem. Chem. Phys. 17, 14064–14070 (2015).
Komatsu, H. et al. Site-selective analysis of nickel-substituted Li-rich layered material: migration and role of transition metal at charging and discharging. J. Phys. Chem. C 122, 20099–20107 (2018).
Weinstock, D. et al. Structure-selective operando X-ray spectroscopy. ACS Energy Lett. 7, 261–266 (2022).
Yu, Y. et al. A review on performance degradation of proton exchange membrane fuel cells during startup and shutdown processes: causes, consequences and mitigation strategies. J. Power Sources 205, 10–23 (2012).
Warren, B. E. X-Ray Diffraction (Dover Publications, 1990).
Bunǎu, O. & Joly, Y. Self-consistent aspects of X-ray absorption calculations. J. Phys. Condens. Matter 21, 345501 (2009).
Guda, S. A. et al. Optimized finite difference method for the full-potential XANES simulations: application to molecular adsorption geometries in MOFs and metal-ligand intersystem crossing transients. J. Chem. Theory Comput. 11, 4512–4521 (2015).
Bourke, J. D., Chantler, C. T. & Joly, Y. FDMX: extended X-ray absorption fine structure calculations using the finite difference method. J. Synchrotron Radiat. 23, 551–559 (2016).
Bundschu, C. R. et al. Oxygen reduction pathway for spinel metal oxides in alkaline media: an experimentally supported ab initio study. J. Am. Chem. Soc. 146, 4680–4686 (2024).
Hancock, D. Y. et al. Jetstream2: accelerating cloud computing via Jetstream. In Proc. ACM International Conference Proceeding Series PEARC 2021: Evolution Across All Dimensions (eds Paris, J. et al.) 1–8 (ACM, 2021).
Boerner, T. J., Deems, S., Furlani, T. R., Knuth, S. L. & Towns, J. ACCESS: advancing innovation: NSF’s advanced cyberinfrastructure coordination ecosystem: services and support. In Proc. PEARC 2023: Computing for the Common Good (eds Sinkovits, R. et al.) 173–176 (ACM, 2023).
Newnham, R. E. Properties of Materials (Oxford Univ. Press, 2005).
Zhang, Z., Koppensteiner, J., Schranz, W. & Carpenter, M. A. Variations in elastic and anelastic properties of Co3O4 due to magnetic and spin-state transitions. Am. Mineral. 97, 399–406 (2012).
Meena, P. L., Kumar, R. & Sreenivas, K. Structural, elastic and magnetic properties of spinel Co3O4. J. Pure Appl. Phys. 56, 890–895 (2018).
Beer, F. P., Johnston, E. R., DeWolf, J. T. & Mazurek, D. F. Mechanics of Materials 6th edn (McGraw-Hill, 2012).
Yang, E. et al. Origin of unusual spinel-to-layered phase transformation by crystal water. Chem. Sci. 9, 433–438 (2018).
Wojtowicz, P. J. Theoretical model for tetragonal-to-cubic phase transformations in transition metal spinels. Phys. Rev. 116, 32 (1959).
Kieffer, J., Valls, V., Blanc, N. & Hennig, C. New tools for calibrating diffraction setups. J. Synchrotron Radiat. 27, 558–566 (2020).
Vugrin, K. W., Swiler, L. P., Roberts, R. M., Stucky-Mack, N. J. & Sullivan, S. P. Confidence region estimation techniques for nonlinear regression in groundwater flow: three case studies. Water Resour. Res. 43, 3423 (2007).
Virtanen, P. et al. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat. Methods 17, 261–272 (2020).
Solé, V. A., Papillon, E., Cotte, M., Walter, P. & Susini, J. A multiplatform code for the analysis of energy-dispersive X-ray fluorescence spectra. Spectrochim. Acta B Spectrosc. 62, 63–68 (2006).
Acknowledgements
The research was primarily supported as part of the Center for Alkaline Based Energy Solutions (CABES), an Energy Frontier Research Center funded by the US Department of Energy (DOE), Office of Science, Basic Energy Sciences (BES), under award no. DE-SC0019445. This work is based on research conducted at the Center for High-Energy X-ray Sciences (CHEXS), which is supported by the National Science Foundation (BIO, ENG and MPS Directorates) under award no. DMR-1829070. Research conducted at the Center for High-Energy X-ray Science (CHEXS) was supported by the National Science Foundation (BIO, ENG and MPS Directorates) under awards DMR-1829070 and DMR-2342336. This work used CPU and Large Memory nodes at Indiana Jetstream2 through allocation CHE240012 from the Advanced Cyberinfrastructure Coordination Ecosystem: Services & Support (ACCESS) program, which is supported by National Science Foundation grants #2138259, #2138286, #2138307, #2137603 and #2138296.
Author information
Authors and Affiliations
Contributions
A.S., H.D.A., Y.Y. and D.W. conceived the idea. A.S., J.J.H., D.W., Y.Y., S.S. and J.P.C.R. designed and conducted the X-ray experiments. Y.Y., Q.L. and J.J.H. prepared and ran the in situ electrochemical cell. Y.Y. synthesized Co–Mn spinel oxide catalysts. J.J.H., D.W. and A.S. processed and analysed the X-ray and electrochemical data. C.R.B. and T.A.A. carried out the JDFT calculations. J.J.H. conducted the strain modelling and REXS simulations. J.J.H., Y.Y., C.R.B. and A.S. wrote the manuscript. All authors, with the exception of D.W., reviewed the manuscript. D.W. passed away prior to the writing of the manuscript, but is credited due to his substantial contributions to this work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Yvonne Grunder, Zhaolin Liu, Sheng Sun and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–11 and Notes 1–4.
Supplementary Data 1
JDFT atomic position data for Co3O4.
Supplementary Data 2
JDFT atomic position data for Co2MnO4.
Supplementary Data 3
JDFT atomic position data for Co2xMnO4.
Supplementary Data 4
JDFT atomic position data for CoMnCoO4.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, J.J., Yang, Y., Weinstock, D. et al. Multimodal in situ X-ray mechanistic studies of a bimetallic oxide electrocatalyst in alkaline media. Nat Catal 8, 116–125 (2025). https://doi.org/10.1038/s41929-025-01289-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-025-01289-7