Abstract
Beta (β)-cell senescence contributes to type 2 diabetes mellitus (T2DM). While exercise is vital for T2DM management and significantly affects cellular ageing markers, its effect on β-cell senescence remains unexplored. Here, we show that short-term endurance exercise training (treadmill running, 1 h per day for 10 days) in two male and female mouse models of insulin resistance decreases β-cell senescence. In vivo and in vitro experiments revealed that this effect is mediated, at least in part, by training-induced increases in serum glucagon, leading to activation of 5′-AMP-activated protein kinase (AMPK) signalling in β-cells. AMPK activation resulted in the nuclear translocation of NRF2 and decreased expression of senescence markers and effectors. Remarkably, human islets from male and female donors with T2DM treated with serum collected after a 10-week endurance exercise training programme showed a significant decrease in the levels of senescence markers. These findings indicate that exercise training decreases senescence in pancreatic islets, offering promising therapeutic implications for T2DM.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Bulk RNAseq data are deposited to the Gene Expression Omnibus with accession no. GSE227516. Source data are provided with this paper.
References
Khan, M. A. B. et al. Epidemiology of type 2 diabetes—global burden of disease and forecasted trends. J. Epidemiol. Glob. Health 10, 107–111 (2020).
Sun, H. et al. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 183, 109119 (2022).
Dunning, T., Sinclair, A. & Colagiuri, S. New IDF Guideline for managing type 2 diabetes in older people. Diabetes Res. Clin. Pract. 103, 538–540 (2014).
Hooten, N. N., Pacheco, N. L., Smith, J. T. & Evans, M. K. The accelerated aging phenotype: the role of race and social determinants of health on aging. Ageing Res. Rev. 73, 101536 (2022).
Salomon, J. A. et al. Healthy life expectancy for 187 countries, 1990–2010: a systematic analysis for the Global Burden Disease Study 2010. Lancet 380, 2144–2162 (2012).
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013).
Bahour, N. et al. Diabetes mellitus correlates with increased biological age as indicated by clinical biomarkers. Geroscience 44, 415–427 (2022).
Aguayo-Mazzucato, C. et al. Acceleration of β cell aging determines diabetes and senolysis improves disease outcomes. Cell Metab. 30, 129–142 (2019).
Carapeto, P. V. & Aguayo-Mazzucato, C. Effects of exercise on cellular and tissue aging. Aging (Albany NY) 13, 14522–14543 (2021).
Radák, Z. et al. Exercise training decreases DNA damage and increases DNA repair and resistance against oxidative stress of proteins in aged rat skeletal muscle. Pflugers Arch. 445, 273–278 (2002).
Werner, C. et al. Physical exercise prevents cellular senescence in circulating leukocytes and in the vessel wall. Circulation 120, 2438–2447 (2009).
Kaliman, P. et al. Neurophysiological and epigenetic effects of physical exercise on the aging process. Ageing Res. Rev. 10, 475–486 (2011).
Ntanasis-Stathopoulos, J., Tzanninis, J. G., Philippou, A. & Koutsilieris, M. Epigenetic regulation on gene expression induced by physical exercise. J. Musculoskelet. Neuronal Interact. 13, 133–146 (2013).
Wohlgemuth, S. E. et al. An exploratory analysis of the effects of a weight loss plus exercise program on cellular quality control mechanisms in older overweight women. Rejuvenation Res. 14, 315–324 (2011).
Barclay, R. D., Burd, N. A., Tyler, C., Tillin, N. A. & Mackenzie, R. W. The role of the IGF-1 signaling cascade in muscle protein synthesis and anabolic resistance in aging skeletal muscle. Front. Nutr. 6, 146 (2019).
Lanza, I. R. et al. Endurance exercise as a countermeasure for aging. Diabetes 57, 2933–2942 (2008).
Englund, D. A. et al. Exercise reduces circulating biomarkers of cellular senescence in humans. Aging Cell 20, e13415 (2021).
Verdijk, L. B. et al. Skeletal muscle hypertrophy following resistance training is accompanied by a fiber type-specific increase in satellite cell content in elderly men. J. Gerontol. A Biol. Sci. Med. Sci. 64, 332–339 (2009).
Keller, C., Keller, P., Giralt, M., Hidalgo, J. & Pedersen, B. K. Exercise normalises overexpression of TNF-α in knockout mice. Biochem. Biophys. Res. Commun. 321, 179–182 (2004).
de Brachène, A. C. et al. Exercise as a non-pharmacological intervention to protect pancreatic beta cells in individuals with type 1 and type 2 diabetes. Diabetologia 66, 450–460 (2023).
Suryadevara, V. et al. SenNet recommendations for detecting senescent cells in different tissues. Nat. Rev. Mol. Cell Biol. https://doi.org/10.1038/s41580-024-00738-8 (2024).
Midha, A. et al. Unique human and mouse β-cell senescence-associated secretory phenotype (SASP) reveal conserved signaling pathways and heterogeneous factors. Diabetes 70, 1098–1116 (2021).
De Cecco, M. et al. L1 drives IFN in senescent cells and promotes age-associated inflammation. Nature 566, 73–78 (2019).
Saul, D. et al. A new gene set identifies senescent cells and predicts senescence-associated pathways across tissues. Nat. Commun. 13, 4827 (2022).
Baker, D. J. et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479, 232–236 (2011).
Hayashi, T. et al. Metabolic stress and altered glucose transport: activation of AMP-activated protein kinase as a unifying coupling mechanism. Diabetes 49, 527–531 (2000).
Hayashi, T., Hirshman, M. F., Kurth, E. J., Winder, W. W. & Goodyear, L. J. Evidence for 5′ AMP-activated protein kinase mediation of the effect of muscle contraction on glucose transport. Diabetes 47, 1369–1373 (1998).
Musi, N. et al. AMP-activated protein kinase (AMPK) is activated in muscle of subjects with type 2 diabetes during exercise. Diabetes 50, 921–927 (2001).
Calegari, V. C. et al. Endurance training activates AMP-activated protein kinase, increases expression of uncoupling protein 2 and reduces insulin secretion from rat pancreatic islets. J. Endocrinol. 208, 257–264 (2011).
Kari, S., Vasko, V. V., Priya, S. & Kirschner, L. S. PKA activates AMPK through LKB1 signaling in follicular thyroid cancer. Front. Endocrinol. (Lausanne) 10, 769 (2019).
Witters, L. A., Kemp, B. E. & Means, A. R. Chutes and ladders: the search for protein kinases that act on AMPK. Trends Biochem. Sci 31, 13–16 (2006).
Ducommun, S. et al. Motif affinity and mass spectrometry proteomic approach for the discovery of cellular AMPK targets: identification of mitochondrial fission factor as a new AMPK substrate. Cell. Signal. 27, 978–988 (2015).
Urhausen, A., Gabriel, H. & Kindermann, W. Blood hormones as markers of training stress and overtraining. Sports Med. 20, 251–276 (1995).
Chen, Z. et al. Dipeptidyl peptidase-4 inhibition improves endothelial senescence by activating AMPK/SIRT1/Nrf2 signaling pathway. Biochem. Pharmacol. 177, 113951 (2020).
Wang, Z. et al. Cordycepin prevents radiation ulcer by inhibiting cell senescence via NRF2 and AMPK in rodents. Nat. Commun. 10, 2538 (2019).
Childs, B. G., Durik, M., Baker, D. J. & van Deursen, J. M. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat. Med. 21, 1424–1435 (2015).
Garatachea, N. et al. Exercise attenuates the major hallmarks of aging. Rejuvenation Res. 18, 57–89 (2015).
Woods, J. A., Wilund, K. R., Martin, S. A. & Kistler, B. M. Exercise, inflammation and aging. Aging Dis. 3, 130–140 (2012).
Stein, G. H., Drullinger, L. F., Soulard, A. & Dulić, V. Differential roles for cyclin-dependent kinase inhibitors p21 and p16 in the mechanisms of senescence and differentiation in human fibroblasts. Mol. Cell. Biol. 19, 2109–2117 (1999).
Lee, B. Y. et al. Senescence-associated β-galactosidase is lysosomal β-galactosidase. Aging Cell 5, 187–195 (2006).
Apfeld, J., O’Connor, G., McDonagh, T., DiStefano, P. S. & Curtis, R. The AMP-activated protein kinase AAK-2 links energy levels and insulin-like signals to lifespan in C. elegans. Genes Dev. 18, 3004–3009 (2004).
van Vliet, T. et al. Physiological hypoxia restrains the senescence-associated secretory phenotype via AMPK-mediated mTOR suppression. Mol. Cell 81, 2041–2052 (2021).
Nguyen-Tu, M.-S. et al. Opposing effects on regulated insulin secretion of acute vs chronic stimulation of AMP-activated protein kinase. Diabetologia 65, 997–1011 (2022).
Zhang, W. et al. Network pharmacology for systematic understanding of Schisandrin B reduces the epithelial cells injury of colitis through regulating pyroptosis by AMPK/Nrf2/NLRP3 inflammasome. Aging (Albany NY) 13, 23193–23209 (2021).
Pisania, A. et al. Quantitative analysis of cell composition and purity of human pancreatic islet preparations. Lab. Invest. 90, 1661–1675 (2010).
De Miguel, Z. et al. Exercise plasma boosts memory and dampens brain inflammation via clusterin. Nature 600, 494–499 (2021).
Hall, L. G., Thyfault, J. P. & Johnson, J. D. Exercise and inactivity as modifiers of β cell function and type 2 diabetes risk. J. Appl. Physiol. (1985) 134, 823–839 (2023).
Langlois, A., Forterre, A., Pinget, M. & Bouzakri, K. Impact of moderate exercise on fatty acid oxidation in pancreatic β-cells and skeletal muscle. J. Endocrinol. Invest. 44, 1815–1825 (2021).
Aguayo-Mazzucato, C. et al. β cell aging markers have heterogeneous distribution and are induced by insulin resistance. Cell Metab. 25, 898–910 (2017).
Acknowledgements
This study was supported by institutional start-up funds to C.A.-M., National Institutes of Health (NIH) grants 1R01DK132535 to C.A.-M. and P30 DK036836 to Joslin Diabetes Center (Cores), Thomas J. Beatson Jr. Foundation grant 2020-010 and the Richard and Susan Smith Family Foundation Award to C.A.-M. Support was also provided by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES)-Finance Code 001 (to P.C.), the 2021 STEP-UP (Short-term Research Experience for Underrepresented Persons) programme supported by award number 1R25DK113652 from the NIH-National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (to J.K.), NIDDK grant K23-DK114550 and BADERC P&F grant (to R.J.W.M.), NIH grant R01DK099511 and R01 DK112283 (to L.J.G.) and American Diabetes Association grant #1-17-PMF-009 (to A.B.A.-W.). G.A.R. was supported by a Wellcome Trust Investigator Award (212625/Z/18/Z), MRC Programme grant (MR/R022259/1), Diabetes UK Project grant (BDA16/0005485), CRCHUM start-up funds, an Innovation Canada John R. Evans Leader Award, JDRF, CIHR, NIH-NIDDK multi-PI project grant (R01DK135268) and CIHR-JDRF Team grant (CIHR-IRSC:0682002550; JDRF 4-SRA-2023-1182-S-N). Human pancreatic islets and/or other resources were provided by the NIDDK-funded IIDP (RRID:SCR _014387) at City of Hope, NIH grant #2UC4DK098085.
Author information
Authors and Affiliations
Contributions
P.C., K.I., F.H., J.K., A.B.A.-W. and R.J.W.M. conducted the experiments. P.C., M.F.H., L.J.G., G.A.R. and C.A.-M. designed the study. P.C. and C.A.-M. wrote the manuscript. All authors reviewed and edited the paper.
Corresponding author
Ethics declarations
Competing interests
G.A.R. has received grant funding from and is a consultant for Sun Pharmaceutical Industries Ltd. C.A.-M. has served as a consultant for eGenesis and Novo Nordisk. The other authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Sandra Galic and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Ashley Castellanos-Jankiewicz, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
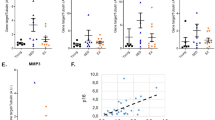
Extended Data Fig. 1 Distance run per day.
Extended Data Figure 1 refers to Fig. 1. Distance (kilometers) run per day for young 6-weeks-old C57BL/6 females (a, n=5-11/group) and for retired breeders, male 6–9 months-old (b, n=5-10/group) in the wheel-running group (trained). Each line represents an individual mouse.
Extended Data Fig. 2 Body composition parameters.
Extended Data Figure 2 refers to Figs. 1 and 3. (a) Body weight in grams of female trained mice (6 weeks of age, n=5-11/group) from control and trained (wheel) groups. Relates to Fig. 1a and b. (b) Body weight in grams of sedentary and trained (treadmill) at the end of the protocol for 12 -weeks-old (n=10 animals/group, males), relates to Fig. 1d–h; (c) Representative pictures from DEXA Fat and lean mass were measured by dual-energy X-ray absorptiometry (DEXA) (d-g). (h) Body weight in grams of sedentary and trained (treadmill) animals sedentary and control with insulin resistance induced by S961 (males, n=5/group, 6–9 months-old), relates to Fig. 1i-l; (i) Body weight in grams of sedentary and trained (treadmill) animals sedentary and control (males, n=5/group, 6–9 months-old); relates to Fig. 3a–c.N= 5 animals/group, males, 6–9 months old. Two sided-test and Welch’s correction. Mean ± SD. *P<0.05.
Extended Data Fig. 3 Glucose and insulin tolerance tests.
Extended Data Figure 3 refer to Fig. 1d–h. AUC for ITT (a) in 12-weeks-old C57BL/6 males after treadmill exercise protocol; n=10/group (a), serum levels from S961 treated mice, n=4-5/group (b), AUC and blood glucose levels for IPGTT (c, e) and ITT (d, f) for trained and sedentary 6–9 months-old animals (n=5/group) from reversibility prevention (c and d) and reversibility groups (e and f). N=4-10/group. Two sided-test and Welch’s correction. Mean ± SD. ***P<0.001.
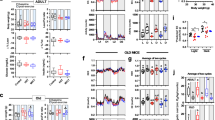
Extended Data Fig. 4 Glucose, IL6 and insulin response.
Extended Data Figure 4 refers to Figs. 4 and 5. Glucose levels (a) and concentration of IL-6 (b) after exercise-protocol (6–9-month-old C57BL/6 male mice; n=10/group in A and n=5/group in B). (c) insulin from glucose stimulated insulin secretion performed in islets from male mice (C57BL6, 3–6 months-old, cultured in presence of glucagon and trained serum. N=5 animals/group. Two sided-test and Welch’s correction. Mean ± SD. *P<0.05, **P<0.01, ****P<0.0001.
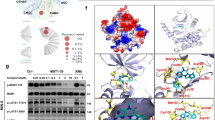
Extended Data Fig. 5 AMPK activation and P21CIP1 levels in islets treated with human from exercise participants without T2D.
Extended Data Figure 5 refers to Fig. 8. Experiment conducted in individuals without T2D. The results showed increased AMPK phosphorylation (A and B) but without changes in senescence marker P21CIP1 (A and C). Samples from individuals ranging from 22 to 57 years old, male, n=6/group. Two sided-test and Welch’s correction. Mean ± SD. *P<0.05.
Supplementary information
Supplementary Information
Supplementary Fig. 1.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Fig. 8
Statistical source data.
Source Data Fig. 3
Western blots for Fig. 3d,l.
Source Data Fig. 4
Western blots for Fig. 4g,l.
Source Data Fig. 5
Western blots for Fig. 5e,l.
Source Data Fig. 6
Western blots for Fig. 6a.
Source Data Fig. 7
Western blots for Fig. 7a.
Source Data Fig. 8
Western blots for Fig. 8c.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 5
Western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Carapeto, P., Iwasaki, K., Hela, F. et al. Exercise activates AMPK in mouse and human pancreatic islets to decrease senescence. Nat Metab 6, 1976–1990 (2024). https://doi.org/10.1038/s42255-024-01130-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-024-01130-8
This article is cited by
-
Exercise-induced meteorin-like protein protects human pancreatic beta cells from cytokine-induced apoptosis
Diabetologia (2025)
-
Exercise inhibits cellular senescence in pancreatic islets
Nature Metabolism (2024)