Abstract
Cutaneous melanomas express a high number of potential neoepitopes, yet a substantial fraction of melanomas shift into immunologically cold phenotypes. Using cellular systems, mouse models and large datasets, we identify the tumor-secreted growth factor midkine (MDK) as a multilayered inhibitor of antigen-presenting cells. Mechanistically, MDK acts systemically in primary tumors, lymph nodes and the bone marrow, promoting a STAT3-mediated impairment of differentiation, activation and function of dendritic cells (DCs), particularly, conventional type 1 DCs (cDC1s). Furthermore, MDK rewires DCs toward a tolerogenic state, impairing CD8+ T cell activation. Downregulating MDK improves DC-targeted vaccination, CD40 agonist treatment and immune checkpoint blockade in mouse models. Moreover, we present an MDK-associated signature in DCs that defines poor prognosis and immune checkpoint blockade resistance in individuals with cancer. An inverse correlation between MDK- and cDC1-associated signatures was observed in a variety of tumor types, broadening the therapeutic implications of MDK in immune-refractory malignancies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
RNA-seq data have been uploaded to the Gene Expression Omnibus (GEO) repository under the following accession IDs: B16.R2L, GSE150401; bone marrow preparations incubated for 3 days with FLT3LG, GSE227286; BMDCs incubated with FLT3LG for 9 days, GSE227287.
Genomic data from human primary and metastatic SKCM, ACC, LUAD, BRCA, MESO and UCEC were derived from the TCGA Research Network (http://cancergenome.nih.gov/). The mutational burden status of individuals with metastatic melanoma from TCGA was retrieved from cBioPortal v6.0.20 (https://www.cbioportal.org/).
Source data are provided with this paper. All other data supporting the findings of this study are available from the corresponding author on reasonable request.
References
Kalaora, S., Nagler, A., Wargo, J. A. & Samuels, Y. Mechanisms of immune activation and regulation: lessons from melanoma. Nat. Rev. Cancer 22, 195–207 (2022).
Lawrence, M. S. et al. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature 505, 495–501 (2014).
Wolchok, J. D. et al. Final, 10-year outcomes with nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 392, 11–22 (2024).
Herrscher, H. & Robert, C. Immune checkpoint inhibitors in melanoma in the metastatic, neoadjuvant, and adjuvant setting. Curr. Opin. Oncol. 32, 106–113 (2020).
Atkins, M. B. et al. The state of melanoma: emergent challenges and opportunities. Clin. Cancer Res. 27, 2678–2697 (2021).
Shain, A. H. & Bastian, B. C. From melanocytes to melanomas. Nat. Rev. Cancer 16, 345–358 (2016).
Cerezo-Wallis, D. & Soengas, M. S. Understanding tumor–antigen presentation in the new era of cancer immunotherapy. Curr. Pharm. Des. 22, 6234–6250 (2016).
Grasso, C. S. et al. Conserved interferon-γ signaling drives clinical response to immune checkpoint blockade therapy in melanoma. Cancer Cell 39, 500–515 (2021).
Olmeda, D. et al. Whole-body imaging of lymphovascular niches identifies pre-metastatic roles of midkine. Nature 546, 676–680 (2017).
Cerezo-Wallis, D. et al. Midkine rewires the melanoma microenvironment toward a tolerogenic and immune-resistant state. Nat. Med. 26, 1865–1877 (2020).
Neumaier, E. E., Rothhammer, V. & Linnerbauer, M. The role of midkine in health and disease. Front. Immunol. 14, 1310094 (2023).
Filippou, P. S., Karagiannis, G. S. & Constantinidou, A. Midkine (MDK) growth factor: a key player in cancer progression and a promising therapeutic target. Oncogene 39, 2040–2054 (2020).
Zhao, S. et al. Midkine upregulates MICA/B expression in human gastric cancer cells and decreases natural killer cell cytotoxicity. Cancer Immunol. Immunother. 61, 1745–1753 (2012).
Tang, Y., Kwiatkowski, D. J. & Henske, E. P. Midkine expression by stem-like tumor cells drives persistence to mTOR inhibition and an immune-suppressive microenvironment. Nat. Commun. 13, 5018 (2022).
Sorrelle, N., Dominguez, A. T. A. & Brekken, R. A. From top to bottom: midkine and pleiotrophin as emerging players in immune regulation. J. Leukoc. Biol. 102, 277–286 (2017).
Heras-Murillo, I., Adan-Barrientos, I., Galan, M., Wculek, S. K. & Sancho, D. Dendritic cells as orchestrators of anticancer immunity and immunotherapy. Nat. Rev. Clin. Oncol. 21, 257–277 (2024).
Marciscano, A. E. & Anandasabapathy, N. The role of dendritic cells in cancer and anti-tumor immunity. Semin. Immunol. 52, 101481 (2021).
Maier, B. et al. A conserved dendritic-cell regulatory program limits antitumour immunity. Nature 580, 257–262 (2020).
Gerhard, G. M., Bill, R., Messemaker, M., Klein, A. M. & Pittet, M. J. Tumor-infiltrating dendritic cell states are conserved across solid human cancers. J. Exp. Med. 218, e20200264 (2021).
Nirschl, C. J. et al. IFNγ-dependent tissue-immune homeostasis is co-opted in the tumor microenvironment. Cell 170, 127–141 (2017).
Ardouin, L. et al. Broad and largely concordant molecular changes characterize tolerogenic and immunogenic dendritic cell maturation in thymus and periphery. Immunity 45, 305–318 (2016).
Bayerl, F. et al. Tumor-derived prostaglandin E2 programs cDC1 dysfunction to impair intratumoral orchestration of anti-cancer T cell responses. Immunity 56, 1341–1358 (2023).
Jin, P. et al. Molecular signatures of maturing dendritic cells: implications for testing the quality of dendritic cell therapies. J. Transl. Med. 8, 4 (2010).
Brasel, K., De Smedt, T., Smith, J. L. & Maliszewski, C. R. Generation of murine dendritic cells from FLT3-ligand-supplemented bone marrow cultures. Blood 96, 3029–3039 (2000).
Meeth, K., Wang, J. X., Micevic, G., Damsky, W. & Bosenberg, M. W. The YUMM lines: a series of congenic mouse melanoma cell lines with defined genetic alterations. Pigment Cell Melanoma Res. 29, 590–597 (2016).
Murphy, T. L. & Murphy, K. M. Dendritic cells in cancer immunology. Cell. Mol. Immunol. 19, 3–13 (2022).
Hildner, K. et al. BATF3 deficiency reveals a critical role for CD8α+ dendritic cells in cytotoxic T cell immunity. Science 322, 1097–1100 (2008).
Wculek, S. K. et al. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 20, 7–24 (2020).
Spranger, S., Dai, D., Horton, B. & Gajewski, T. F. Tumor-residing BATF3 dendritic cells are required for effector T cell trafficking and adoptive T cell therapy. Cancer Cell 31, 711–723 (2017).
Bottcher, J. P. et al. NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell 172, 1022–1037 (2018).
Broz, M. L. et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell 26, 638–652 (2014).
Barry, K. C. et al. A natural killer–dendritic cell axis defines checkpoint therapy-responsive tumor microenvironments. Nat. Med. 24, 1178–1191 (2018).
Cueto, F. J. et al. DNGR-1 limits FLT3L-mediated antitumor immunity by restraining tumor-infiltrating type I conventional dendritic cells. J. Immunother. Cancer 9, e002054 (2021).
Dong, Y. et al. PTEN functions as a melanoma tumor suppressor by promoting host immune response. Oncogene 33, 4632–4642 (2014).
Spranger, S., Bao, R. & Gajewski, T. F. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature 523, 231–235 (2015).
Liersch, R. et al. Analysis of a novel highly metastatic melanoma cell line identifies osteopontin as a new lymphangiogenic factor. Int. J. Oncol. 41, 1455–1463 (2012).
Ohl, L. et al. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity 21, 279–288 (2004).
Onai, N. & Manz, M. G. The STATs on dendritic cell development. Immunity 28, 490–492 (2008).
Su, Y. L., Banerjee, S., White, S. V. & Kortylewski, M. STAT3 in tumor-associated myeloid cells: multitasking to disrupt immunity. Int. J. Mol. Sci. 19, 1803 (2018).
Hogquist, K. A. et al. T cell receptor antagonist peptides induce positive selection. Cell 76, 17–27 (1994).
Arneth, B. M. Activation of CD4 and CD8 T cell receptors and regulatory T cells in response to human proteins. PeerJ 6, e4462 (2018).
Dudziak, D. et al. Differential antigen processing by dendritic cell subsets in vivo. Science 315, 107–111 (2007).
Vonderheide, R. H. & Glennie, M. J. Agonistic CD40 antibodies and cancer therapy. Clin. Cancer Res. 19, 1035–1043 (2013).
Gros, A. et al. PD-1 identifies the patient-specific CD8+ tumor-reactive repertoire infiltrating human tumors. J. Clin. Invest. 124, 2246–2259 (2014).
Liu, Q., Sun, Z. & Chen, L. Memory T cells: strategies for optimizing tumor immunotherapy. Protein Cell 11, 549–564 (2020).
Navarro-Barriuso, J. et al. Comparative transcriptomic profile of tolerogenic dendritic cells differentiated with vitamin D3, dexamethasone and rapamycin. Sci. Rep. 8, 14985 (2018).
Jerby-Arnon, L. et al. A cancer cell program promotes T cell exclusion and resistance to checkpoint blockade. Cell 175, 984–997 (2018).
Aran, D., Hu, Z. & Butte, A. J. xCell: digitally portraying the tissue cellular heterogeneity landscape. Genome Biol. 18, 220 (2017).
Li, H. et al. Dysfunctional CD8 T cells form a proliferative, dynamically regulated compartment within human melanoma. Cell 176, 775–789 (2019).
Thorsson, V. et al. The immune landscape of cancer. Immunity 48, 812–830 (2018).
Eddy, J. A. et al. CRI iAtlas: an interactive portal for immuno-oncology research. F1000Res 9, 1028 (2020).
Ayers, M. et al. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Invest. 127, 2930–2940 (2017).
Riaz, N. et al. Tumor and microenvironment evolution during immunotherapy with nivolumab. Cell 171, 934–949 (2017).
Gide, T. N. et al. Distinct immune cell populations define response to anti-PD-1 monotherapy and anti-PD-1/anti-CTLA-4 combined therapy. Cancer Cell 35, 238–255 (2019).
Chen, P. L. et al. Analysis of immune signatures in longitudinal tumor samples yields insight into biomarkers of response and mechanisms of resistance to immune checkpoint blockade. Cancer Discov. 6, 827–837 (2016).
Hugo, W. et al. Genomic and transcriptomic features of response to anti-PD-1 therapy in metastatic melanoma. Cell 165, 35–44 (2016).
Li, J. et al. Mature dendritic cells enriched in immunoregulatory molecules (mregDCs): a novel population in the tumour microenvironment and immunotherapy target. Clin. Transl. Med. 13, e1199 (2023).
Herradon, G., Ramos-Alvarez, M. P. & Gramage, E. Connecting metainflammation and neuroinflammation through the PTN–MK–RPTPβ/ζ axis: relevance in therapeutic development. Front. Pharmacol. 10, 377 (2019).
Weckbach, L. T. et al. Midkine drives cardiac inflammation by promoting neutrophil trafficking and NETosis in myocarditis. J. Exp. Med. 216, 350–368 (2019).
Sonobe, Y. et al. Midkine inhibits inducible regulatory T cell differentiation by suppressing the development of tolerogenic dendritic cells. J. Immunol. 188, 2602–2611 (2012).
Naik, S. H. et al. Cutting edge: generation of splenic CD8+ and CD8– dendritic cell equivalents in FMS-like tyrosine kinase 3 ligand bone marrow cultures. J. Immunol. 174, 6592–6597 (2005).
Olmeda, D. et al. Live imaging of neolymphangiogenesis identifies acute antimetastatic roles of dsRNA mimics. EMBO Mol. Med. 13, e12924 (2021).
Helft, J. et al. GM-CSF mouse bone marrow cultures comprise a heterogeneous population of CD11c+MHCII+ macrophages and dendritic cells. Immunity 42, 1197–1211 (2015).
Bindea, G. et al. ClueGO: a Cytoscape plug-in to decipher functionally grouped Gene Ontology and pathway annotation networks. Bioinformatics 25, 1091–1093 (2009).
Hanzelmann, S., Castelo, R. & Guinney, J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinformatics 14, 7 (2013).
Colaprico, A. et al. TCGAbiolinks: an R/Bioconductor package for integrative analysis of TCGA data. Nucleic Acids Res. 44, e71 (2016).
Acknowledgements
We thank our colleagues at the CNIO Melanoma Group for help and support. We also thank the CNIO Core Units, especially cores for Animal Facility (I. Blanco, G. Luque and S. Ruiz) and Histopathology (E. Jose Caleiras and P. González), Flow Cytometry (J. Garcia, A. M. E. Ilie, I. Fernández and S. Garcia), Confocal Microscopy (M. Pérez and J. Gómez) and Genomics Units (O. Domínguez). We also thank C. H. K. Lehmann (University Hospital Erlangen) for help with the preparation of anti-DEC-205-OVA and IgG-OVA. M.S.S. is funded by grants from the Ministerio de Ciencia e Innovación (project PID2020-117621RB-I00 and PID2023-147213OB-I00, funded by the Agencia Estatal de Investigación AEI/10.13039/501100011033), ‘La Caixa’ Banking Foundation under project codes HR17-00232 and HR20-00465 and the European Research Council through the advanced grant METALERT-STOP (grant agreement 884699, EU-Horizon 2020 Programme). D.O. is funded by grants from the Instituto de Salud Carlos III, AES-2021, through the project PI21/00641, which is cofunded by the European Fund FEDER. X.C., M.C.-A. and A.S. were funded by the Immutrain Marie Skłodowska-Curie ITN Grant (grant agreement 641549, EU-Horizon 2020 Programme). D.D. was funded by the German Research Foundation TRR305-TPB5 429280966 and DU548/9-1 515982377. D.S. is funded by MCIN PID2022-137712OB-I00 MCIN/AEI/10.13039/501100011033 European Union NextGeneration EU/PRTR and by ‘la Caixa’ Foundation (LCF/PR/HR23/52430012). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
M.S.S. and X.C. conceived and designed the study. X.C. developed and implemented protocols to define MDK-induced DC effects in vitro and in vivo and performed flow cytometry analyses. X.C., M.C.-A. and T.G.C. overexpressed and depleted MDK in cancer cell lines and collected conditioned media. M.C.-A., N.J.-L. and T.G.C. contributed to the mouse experiments, organ processing and flow cytometry. D.C.-W., D.O. and T.G.C. performed in vivo anti-PD-1 treatments. X.C. performed anti-CD40 treatments, and X.C. and T.G.C. performed the in vivo vaccination assays and antigen presentation experiments. T.G.C. helped with mouse colony maintenance. N.J.-L. and X.C. performed histological assessments of immune cells. X.C. and D.C.-W. performed OT-I cytotoxicity assays in vitro. C.M. helped with the in vitro experiments and performed RT–qPCR analyses. S.F. contributed to the RT–qPCR analysis. C.T. helped with antigen cross-presentation assays in vitro. L.M. helped with the acquisition of data by flow cytometry and analysis. D.M. acquired confocal and wide-field images and analyzed and quantified phagocytic activity. D.O., J.S. and S.O.-S. performed computational analyses to identify MDK- and DC-associated gene signatures, performed ssGSEA in TCGA cohorts and helped with interpretation of the bioinformatics data. D.O. performed analyses to predict responses to ICB. X.C. performed additional bioinformatic analyses. D.S., P.S. and A.S. helped in the design of MDK-induced DC effects in vitro and in vivo. D.S. provided the Batf3tm1Kmm mice, and D.D. provided and produced anti-DEC-205-OVA and IgG-OVA. The manuscript was written by M.S.S. and X.C. and was approved by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cancer thanks Niroshana Anandasabapathy, Rolf Brekken and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Transcriptomic analyses of Mdk-depleted melanoma and effect of MDK on DCs.
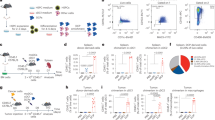
(a) Summary of the workflow followed to identify MDK-associated gene signatures to then define their impact on mouse models and patient-derived datasets. (b) Significantly dysregulated processes (GO Term) of upregulated genes (n = 362) defined by RNAseq in B16.R2L-shMdk (n = 4) vs shCtrl (n = 4) independent tumor allografts. Data were analyzed using Cytoscape (v3.10.1) and ClueGo (v2.5.10). Statistical analysis included correction with Bonferroni step down. (c) Normalized Enrichment Scores (NES) of the indicated gene ontology (GO) terms defined by GSEA in B16.R2L-shMdk (n = 4) vs shCtrl (n = 4) independent allografts: Innate Immune Responses (GO:0045087), T Differentiation (GO:0030217), Myeloid and Leukocyte Differentiation (GO:0002573) and DC activation (GO:0001773). (d) Representative GSEA plots for GO terms Antigen processing and presentation (GO:0019882), DC differentiation (GO:0097028) enriched in B16.R2L-shMdk (n = 4) vs the shCtrl (n = 4) allografts. (e) Gating strategy used to identify cDCs, cDC1s and cDC2s from FLT3LG-BMDCs cultures. (f) Pie charts from FLT3LG-BMDCs cultures treated with or without rMDK and analyzed as in panel Fig. 1d (n = 4 independent allografts). (g) Representative flow cytometry plots and the corresponding quantification (h) of cell viability of FLT3LG-BMDCs in the absence or presence of rMDK (n = 4 independent mice per condition). Data represented as mean +/- SEM. Unpaired two-tailed t-test was used to assess statistical significance. (i) RT-qPCR to assess the expression of cDC1-associated genes (Batf3, Irf8 and Nfil3) in marrow cells cultured with 50 ng/ml of FLT3LG in the absence (Control, n = 3) or in the presence of murine rMDK (10 ng/ml, n = 3) during the first three days of the differentiation. Data represented as mean +/- SEM Statistical significance was determined by two-way ANOVA corrected for Sidak’s multiple comparisons. Data correspond to BMDCs isolated from 3 independent mice. (j) Amount of MDK (ng/ml) secreted by different melanoma cell lines used in the gain-of-function analyses for B16.F10, B16.F1, YUMM1.1, YUMM2.1 and WM164. Sample size n = 3 independent biological replicates for all groups. Data represented as mean +/- SEM. Statistical significance was determined by one-way ANOVA with Brown-Forsythe test. p-value: **** = <0.0001. (k) Percentage of BMDCs obtained from FLT3LG-BMDCs cultured with conditioned media from B16.F10 (n = 4), YUMM1.1 (n = 5) and YUMM2.1 (n = 5) overexpressing MDK or isogenic control (NEG). Statistical significance was determined by an unpaired two-tailed t-test. All violin plots show median values (center dash-line), quartiles (dotted-lines) and minimum and maximum values (edges of the violin), with individual values plotted. Data correspond to BMDC cultures from independent mice. (l) Amount of MDK (ng/ml) secreted by B16.R2L cell line used in loss-of-function analyses with shRNA and siRNA. Sample size: B16.R2L-shCtrl n = 3; -shMdk n = 4; -siCtrl n = 3; -siMdk #34 n = 8; -siMdk #35 n = 8; -siMdk #36 n = 8. Data represented as mean +/- SEM of independent biological replicates. Statistical significance was determined by one-way ANOVA with Brown-Forsythe test. (m) mRNA expression of the indicated cDC1-associated genes in MutuDC1940s cultured with conditioned media from B16.R2L-shMdk (n = 3) relative to -shCtrl (dash line, n = 5). Data represented as mean +/- SEM. Statistical significance was determined by two-way ANOVA corrected for Sidak’s multiple comparisons (p-value: **** = <0.0001, *** = 0.0005). Data correspond to independent experiments. (n) mRNA expression of cDC1-associated genes in marrow cells cultured with FLT3LG (50 ng/ml) treated with conditioned media from B16.R2L-siMdk#34 and -siMdk#35 relative to -siCtrl (dash line), during the first three days of the differentiation. Data correspond to three technical replicates from two independent experiments. All values are plotted. (o) Percentage cDC1s obtained from FLT3LG-BMDCs cultured with non-tumoral conditioned media (Non-TCM, n = 3), conditioned media from B16.R2L-siCtrl (n = 3) or -siMdk#36 (n = 3). Data correspond to BMDC cultures from three independent mice per group. Statistical significance was determined by one-way ANOVA corrected for multiple comparisons (Tukey test). All plots show median values (center line) with individual values plotted.
Extended Data Fig. 2 Impact of MDK on cDC1-associated features.
(a) Growth curves of B16.R2L-shCtrl and -shMdk implanted cells, inoculated in WT or Batf3-deficient mice (Batf3-/-). The number of animals used were n = 9 (WT, shCtrl); n = 9 (WT, shMdk); n = 9 (Batf3-/-, shCtrl) and n = 8 mice (Batf3-/-, shMdk). (b) Immunostaining of intratumoral CD8+ T cells (pink) of B16.R2L (shCtrl vs shMdk) from WT or Batf3-/- mice. Shown are representative images of whole lesion sections of animals in (a), for samples sizes as follows: n = 4 (WT, shCtrl); n = 4 (WT, shMdk); n = 5 (Batf3-/-, shCtrl) and n = 5 tumors (Batf3-/-, shMdk). Scale bar = 50 μm. (c) Left: Quantification of intratumoral CD4+ T cells by mm2 in allografts of B16.R2L generated by implantation in WT mice (shCtrl, n = 5; shMdk, n = 4) or Batf3-/- mice (shCtrl, n = 5; shMdk, n = 4). Data represented as mean +/- SEM. One-way ANOVA corrected for multiple comparisons with Tukey test. Right: Representative images of B16.R2L tumors generated in WT mice as in the left panel, shCtrl, n = 5, and shMdk, n = 4). Data are also shown for similar analyses, but in Batf3-/- mice (shCtrl, n = 5; shMdk, n = 4) Scale bar = 50 μm. (d) Normalized Enrichment Scores (NES) by GSEA of’Hallmarks’ in MDK-iSlow (blue, n = 121) vs MDK-iShigh (red, n = 121) patients. (e-g) Enrichment of the DC differentiation (GO:0097028), DC activation (GO:0001773) and “LPS-activated DCs” (Ref. 23) signatures in MDK-iSlow (n = 121) and MDK-iShigh (n = 121) melanoma patients calculated by ssGSEA. Unpaired two-tailed t-test with Mann-Whitney test was applied. Box plots show median values (center lines), 25th and 75th percentiles (box edges) and minimum and maximum values (whiskers), with individual values plotted. (h) Correlation of the cDC1combo gene set enrichment score by ssGSEA and the MDK-iScore in TCGA metastatic melanoma (met-SKCM) patients (n = 364). Correlation was calculated with two-tailed Pearson correlation coefficient. (i) Distribution of patients from TCGA metastatic melanoma (met-SKCM; n = 364) that have a mutation in BRAF, NRAS, PTEN, CTNNB1 or NF1 (represented as purple triangles) overlaid in the correlation chart of the cDC1combo score vs the MDK-iScore (as in Extended Data Fig. 2h). Multiple linear regression was used to evaluate correlation between mutations and the MDK-iScore for each patient.
Extended Data Fig. 3 Flow cytometry gating strategy to identify dendritic cells.
(a-e) Gating strategy to identify DCs and progenitor dendritic cells: (a) intratumoral DCs, cDC1s and cDC2s; (b) total cDCs (resident and migratory), cDC1s, and cDC2s in draining lymph nodes; (c) circulating cDCs, DC1s, DC2s, and pre-DCs in blood; and (d) identification of pre-DCs, common myeloid progenitors (CMPs), macrophage/dendritic cell progenitors (MDPs) and common dendritic cell progenitors (CDPs), as well as (e) mature cDCs, DC1s, DC2s in the bone marrow.
Extended Data Fig. 4 Systemic analysis of the impact of MDK on DCs.
(a) Mdk expression in the isogenic melanoma cell lines used in vivo to assess depletion or overexpression of this gene in Fig. 3 (n = 3 independent depletions). Data are presented as mean values +/- SEM. Statistical significances were determined by an unpaired two-tailed t-test. (b) Draining lymph nodes from B16.R2L-shCtrl and -shMdk stained with CD11c (green), CD103 (red), CD8 (purple) and DAPI (blue), and merged. Scale bar = 200 μm. Representative images of lymph nodes from three independent mice per each group. Insets highlighted in the merged are shown in Fig. 3f. (c) Total number of resident and migratory cDC2s in draining lymph nodes of B16.R2L-shCtrl implanted mice (n = 4 per condition). Statistical significances were determined by an unpaired two-tailed t-test. (d) Total number of resident and migratory cDC1s in draining lymph nodes of B16.R2-shCtrl (n = 7 mice) and -shMdk (n = 6 mice) for loss of function studies (p-value: **** <0.0001, * = 0.0336, black asterisks represent significance between resident cDC1s, and grey asterisks represent difference between migratory cDC1s). Also shown are data for B16.F1-Neg (n = 6 mice) and -Mdk (n = 6 mice) for gain of function analyses (p-value: * = 0.0163, black asterisks represent significance between resident cDC1s). Data are represented as mean values +/- SEM. Statistical significance was determined by two-way ANOVA corrected for multiple comparisons with Sidak’s multiple comparison test. (e) Percentage of circulating cDC2s in blood of mice bearing implants of B16.R2L-shCtrl (n = 16) vs shMdk (n = 19). Unpaired two-tailed t-test. (f) Percentage of circulating cDC1s, cDC2s, and pre-DCs in blood in mice bearing B16.R2-shCtrl (n = 7) or shMdk (n = 7). Statistical significance was determined by unpaired two-tailed t-test. Plots correspond to a representative experiment from two repetitions. (g) Number of cDC2s in bone marrow (femur and tibia) from mice bearing B16.R2L transduced with shCtrl (n = 5) or shMdk (n = 5). Statistical significances were determined by an unpaired two-tailed t-test. All violin plots show median values (center dash-line), quartiles (dotted-lines) and minimum and maximum values (edges of the violin), with individual values plotted.
Extended Data Fig. 5 Transcriptomic analyses of bone marrow cells (BM) treated with rMDK.
(a) Gene set enrichment analysis (GSEA) of cDC1 and cDC2 signatures18 in bone marrow cells cultured with FLT3LG (50 ng/ml) in the presence of rMDK (10 ng/ml) or Control (n = 3 independent mice per group) for 3 days. NES, normalized enrichment score; FDR, False discovery rate. (b) STRING network analysis of Mdk-associated genes revealing STAT3 as a hub connecting cDC1-associated proteins and immunomodulators. Genes induced and repressed by MDK are shown in blue and red, respectively. Data was obtained from RNAseq of rMDK-treated bone marrow cells cultured with FLT3LG or Control (n = 3 independent mice per group) for 3 days. (c) Representative histograms and dot plots of the staining of P-STAT3Y705 from DCs untreated or treated with IL-6 (50 ng, 30 min) or rMDK (10 ng, 1 h; and 20 ng, 1 h) as defined by flow cytometry (shown are data from 3 independent experiments). (d) RT-qPCR to assess the expression of Batf3, Irf8 and Nfil3 in bone marrow cells incubated with the STAT3 inhibitor (Silibinin; 100 μM) 3 h before treatment with rMDK (10 ng/ml) for 24 h. Data correspond to BDMCs isolated from 3 independent mice. Dash-line represents expression of genes in control cells (not treated with rMDK). Statistical significance was determined by two-way ANOVA corrected for multiple comparisons with Sidak’s multiple comparison test. (e) RT-qPCR to assess the expression of Batf3 in bone marrow cells incubated with inhibitors of IKK2 (TPCA-1, 500 nM) or PI3K (GDC-0941, 100 nM) added to cells 3 h before treatment with rMDK (10 ng/ml) for 24 h. Dash-line represents expression of genes in control cells (no treated with rMDK). BDMCs were isolated from three independent mice. Data represented as mean +/- SEM. Statistical significance was determined by two-way ANOVA corrected for multiple comparisons with Sidak’s multiple comparison test. (f) STRING network analysis of MDK (red) and STAT3 (pink) interactions in the context of the receptor ALK (orange) and of transcription factors and other modulators of DC differentiation (GO:0097028; in blue). Statistics: Protein-protein interaction (PPI) enrichment p-value:< 1×10-16). Thickness of the connecting lines represents interaction edge confidence: low (0.150), medium (0.400), high (0.700) and highest (0.900).
Extended Data Fig. 6 Functional impact of MDK on DCs.
(a) Schematic summary of the methods used to assess DC functions controlled by MDK (see the Methods section for specific details). (b) Dot plot representation of OVA-AF594 endocytosis by BMDCs (CD11c+B220-), in the absence or presence of rMDK (10 mg/ml, 48 h). Left panels correspond to negative controls of BMDCs kept at 4 °C. (c) Quantification of endocytosis by cDCs without (grey) and with rMDK (red). Endocytic index= (mean fluorescence intensity (MFI) of the phagocytosing cells) x (% fluorescence-positive cells). Statistical significance was determined by unpaired two-tailed t-test. Data correspond to in vitro BMDC cultures from three mice per group, repeated independently. (d) Expression of MHC-II, MHC-I, CD40 on BMDCs control (n = 3) vs treated with rMDK (n = 3) for 48 h defined by flow cytometry. Data are plotted as integrated mean fluorescence intensity (iMFI: mean fluorescence intensity by % fluorescence-positive cells), and the statistical significance was determined by unpaired two-tailed t-test. Plots show median values (center line) with individual values plotted Data correspond to in vitro BMDCs of a representative experiment performed with three independent mice per group. This analysis was repeated twice with similar results. (e) Flow cytometry analysis of percentage of MHC-I and mean fluorescence intensity (MFI) of MHC-I on cDC1s obtained from FLT3LG-BMDCs cultured with non-tumoral conditioned media (non-TCM, n = 3), conditioned media from B16.R2L-siCtrl (n = 3) or -siMdk (n = 3). Data correspond to in vitro BMDCs from three independent mice per group. Statistical significance was determined by one-way ANOVA corrected for multiple comparisons (Tukey test). Plots show median values (center line) with individual values plotted. (f) Flow cytometry analysis of H-2Kb-SIINFEKL expression in BMDCs preincubated with rMDK (10 ng/ml, 24 h) and then exposed to OVA and LPS (100 ng/ml) for the indicated time points. Sample size: Control n = 2 BDMCs; rMKD n = 3 BDMCs per condition; OVA n = 3 independent BMDC preparations per condition. Each of these BMDCs were treated in duplicate. Data are represented as MFI and statistical difference was determined by one-way ANOVA corrected for multiple comparisons (Tukey test). (g) Expression of MHC-I and CD40 in MutuDC1940s incubated with vehicle control (DMSO) or the Alk inhibitor (NVP-TAE-684; 500 nM) 3 h before treatment with rMDK (10 ng/ml, 24 h). Sample size: Control + DMSO n = 4, Control + TAE n = 5, rMDK + DMSO n = 5, rMDK + TAE n = 6. Data correspond to independent replicates generated by flow cytometry and plotted as mean fluorescence intensity (MFI). Statistical difference was determined by one-way ANOVA corrected for multiple comparisons (Tukey test). Violin and box plots show median values (center lines), 25th and 75th percentiles (box edges) and minimum and maximum values (whiskers), with individual values plotted.
Extended Data Fig. 7 Comparison between rMDK-treated BMDCs vs mregDC signatures.
(a) Heatmaps of genes that define a mregDC signature18 (including genes involved in DCs maturation, migration, Th2 responses, regulatory DCs, and TLR responses) in BMDCs treated with rMDK or Control (n = 3 independent mice per group). Data derived from transcriptomic analysis of Fig. 6a. Scale bar corresponds to reads per kilobase million (RPKM, log2 scale) normalized per gene (0 = min, 1 = max). (b) Summary drawing of the killing inhibition experiment: Naïve OT-1 CD8+ T cells were primed in vitro by the addition of SIINFKEL (40pM) and IL-2 (5 ng/ml). Then, these in vitro-primed OT-1 CD8+ T cells were co-cultured with BMDCs and B16.F10 melanoma cells expressing OVA + /- MDK. Next day, killing was measured by the staining of DAPI and Annexin V. (c) OT-1 T cell killing of YUMM1.1-OVA-NEG (blue) and -MDK cells (red) in the presence or absence of BMDCs preincubated or not with tumor conditioned media from YUMM1.1-OVA-MDK or with rMDK (10 ng/ml). Sample size: YUMM1.1-OVA-NEG + OT-I, n = 5; YUMM1.1-OVA-MDK + OT-I, n = 6; YUMM1.1-OVA-NEG + OT-I + BMDCs, n = 6; YUMM1.1-OVA-MDK + OT-I + BMDCs, n = 6; YUMM1.1-OVA-NEG + MDK CM + OT-I + BMDCs, n = 6; YUMM1.1-OVA-NEG + rMDK + OT-I + BMDCs, n = 6. Data represented as mean +/- SEM. Significance assessed by unpaired two-tailed t-test.
Extended Data Fig. 8 Impact of MDK on immune-based therapies.
(a) Growth curves of B16.F10-OVA inoculated in mice vaccinated with IgG-OVA (black), vaccinated with αDEC-205-OVA (green), or vaccinated with αDEC-205-OVA + rMDK (purple). Samples sizes: IgG-OVA control, n = 3; αDEC-205-OVA vaccination, n = 7; αDEC-205-OVA vaccination + rMDK, n = 7. Representative experiment from two repetitions. (b) Immune staining intratumoral CD8+ cells (pink) in B16.F10-OVA inoculated in mice, vaccinated with αDEC-205-OVA or vaccinated with αDEC-205-OVA + rMDK. Scale bars = 50 μm. Shown are representative images from 6 lesions per condition. (c) Individual fold change (Log2 scale) in tumor volume after treatment (day 24 post-treatment vs day of initiation of the treatment) with CD40 agonist or IgG2a (5 mg kg−1) in mice bearing B16.R2L-shCtrl or shMdk. Samples sizes are: shCtrl + αCD40, n = 7; shCtrl + IgG2a, n = 6; shMdk + αCD40, n = 9; and shMdk + IgG2a, n = 6. (d) Percentage of PD-1+ and CD44+ CD8+ T cells in blood from mice inoculated with B16.R2L-shCtrl or shMdk cells and treated with the CD40 agonist antibody or IgGa (5 mg kg−1). Statistical significance was defined by unpaired two-tailed t-test. Sample size: shCtrl + CD40, n = 5; shCtrl + IgG2a, n = 5; shMdk + CD40, n = 6; and shMdk + IgG2a, n = 5. Violin plots show median values (center dash-line), quartiles (dotted-lines) and minimum and maximum values (edges of the violin), with individual values plotted. (e) Individual Log2 fold change in tumor volume after treatment with IgG2a isotype control (5 mg kg−1) or with αPD-1 antibody (clone RMP1-14; 5 mg kg−1) in mice bearing B16.R2L-shCtrl or -shMdk. Data at day 20. Sample size was: shCtrl + IgG2a (n = 5); shCtrl + αPD-1 (n = 6); shMdk + IgG2a (n = 6); and (shMdk + αPD-1; n = 6). (f) Quantification by flow cytometry of circulating cDC1s (CD3-CD19-F4/80-CD11c+MHC-II+CD24+Sirp1a-) in blood in wild-type (WT) and Batf3-/- mice implanted with B16.R2L-shCtrl or B16R2L-shMdk treated with IgG2a control (5 mg kg−1) or with αPD-1 antibody (clone RMP1-14; 5 mg kg−1). Samples sizes are WT + shCtrl + IgG2a, n = 4; WT + shMdk + IgG2a, n = 5; Batf3-/- + shCtrl + IgG2a, n = 3; Batf3-/- + shMdk + IgG2a, n = 6; WT + shCtrl + αPD-1, n = 6; WT + shMdk + αPD-1, n = 6; Batf3-/- + shCtrl + αPD-1, n = 5; and Batf3-/- + shMdk + aPD-1, n = 6. (g) Survival curves and (h) individual tumor growth curves of wild-type (WT) and Batf3-/- mice implanted with B16.R2L-shCtrl or B16R2L-shMdk treated with IgG2a control (5 mg kg−1) or with αPD-1 antibody (clone RMP1-14; 5 mg kg−1). Log-rank (Mantel-Cox) test was performed to compare survival among groups. Samples sizes are WT + shCtrl + IgG2a, n = 11; WT + shMdk + IgG2a, n = 16; Batf3-/- + shCtrl + IgG2a, n = 13; Batf3-/- + shMdk + IgG2a, n = 10; WT + shCtrl + αPD-1, n = 8; WT + shMdk + αPD-1, n = 7; Batf3-/- + shCtrl + αPD-1, n = 5; and Batf3-/- + shMdk + aPD-1, n = 6. Treatments were performed every three days. Data summarizes results from two independent experiments.
Extended Data Fig. 9 Tolerogenic features of DCs linked to MDK as defined in melanoma patients.
(a) Stratification of TCGA Melanoma patients (n = 473) according to the expression of the MDK-educated tolerogenic DC signature. Patients within the 20th highest and lowest percentile of this MDK-tDC score (brown and black, respectively) were considered for subsequent analyses. (b) Overall survival by Kaplan-Meier curves of TCGA Melanoma patients M-tDClow (n = 92) and M-tDChigh (n = 92). Patients in the upper and lower 33rd percentiles of this scoring are labeled in black and brown, respectively. Log-rank (Mantel-Cox) test was performed to compare survival among groups. (c) MSigDB Hallmark gene set collection identified by GSEA to be differentially regulated in M-tDClow (n = 92) and M-tDChigh (n = 92) TCGA melanoma patients. NES, Normalized enrichment score. (d) Enrichment scores defined by ssGSEA of different signatures related to DC infiltration and function (see Supplementary Table 3 and 4 for the indicated references and gene lists) in TCGA melanoma patients M-tDClow (n = 92) and M-tDChigh (n = 92). Statistical significance was determined by two-way ANOVA corrected for multiple comparisons with Sidak’s multiple comparison test. Box plots show median values (center lines), 25th and 75th percentiles (box edges) and minimum and maximum values (whiskers), with individual values plotted. (e) NES by GSEA of the indicated signatures associated with good response and resistance to ICBs for M-tDClow (n = 92) and M-tDChigh (n = 92) TCGA melanoma patients: Responders to combination of αCTLA-4 and αPD-1 (Ref. 55), responders to nivolumab (Ref. 53) and resistant to αPD-1 (IPRES, lower panel)56. FDR, False discovery rate.
Supplementary information
Supplementary Information
Supplementary Tables 1–9.
Supplementary Video 1
Representative video of the immunofluorescence images of BMDCs treated with or without rMDK (10 ng ml–1) phagocytosing pHRodo E. coli BioParticles (green) at the indicated times in BMDCs stained with DiD′ (red); scale bar, 100 μm.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Catena, X., Contreras-Alcalde, M., Juan-Larrea, N. et al. Systemic rewiring of dendritic cells by melanoma-secreted midkine impairs immune surveillance and response to immune checkpoint blockade. Nat Cancer 6, 682–701 (2025). https://doi.org/10.1038/s43018-025-00929-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43018-025-00929-y
This article is cited by
-
Transdermal delivery of CRISPR/Cas9-mediated melanoma gene therapy via polyamines-modified thermosensitive hydrogels
Journal of Nanobiotechnology (2025)