Abstract
Biological nitrogen fixation is an important source of new nitrogen, influencing ocean fertility and carbon uptake. While recently documented in Arctic waters, its role in the Southern Ocean remains uncertain. We measured nitrogen fixation along the Western Antarctic Peninsula and at Palmer Station over two austral summer months. Rates from 15N2 assay were below conservative detection limits but detectable under less stringent detection thresholds. Continuous acetylene reduction assay provided further support. nifH gene sequencing identified Gammaproteobacteria as the dominating identified diazotrophs, while Epsilonproteobacteria contributed disproportionally to nifH expression when putative nitrogen fixation was highest. Combined with environmental observations, we hypothesize that vertical water mixing resuspended sediments into the water column and contributed to the limited nitrogen fixation. Given the sporadic and low rates, further research is needed to determine whether nitrogen fixation plays a minor role or represents an overlooked process with biogeochemical significance in the Southern Ocean.
Similar content being viewed by others
Introduction
Nitrogen is a fundamental element of life, present in biomolecules such as proteins and nucleic acids. In many regions of the world oceans such as oligotrophic regions, nitrogen availability limits photosynthesis and the biological uptake and sequestration of carbon dioxide (CO2)1. Biological dinitrogen (N2) fixation to ammonium is a major source of new nitrogen in the ocean2 and can support up to half of new production in oligotrophic waters1. However, estimates of global N2 fixation harbor substantial uncertainties, ranging from less than 100 to over 200 Tg N year−12,3,4. By comparison, global ocean denitrification estimates are ~400 Tg N year−15. Other sources of nitrogen, such as riverine input and atmospheric deposition, are not large enough to balance the nitrogen budget in the ocean2. The implication is that either the oceanic nitrogen inventory is decreasing (with implications for ocean fertility), or that nitrogen sources (sinks) are under- (over-) estimated. In order to address a potential missing source of nitrogen, researchers are actively looking for other biomes where N2 fixation may be important. Until recently, N2 fixation was believed to be conducted predominantly by cyanobacteria such as Trichodesmium living in warm tropical and subtropical waters6. However, recent studies have not only expanded the range of habitats over which N2 fixation may be important, including coastal7 and polar oceans8, but also the microbial taxa who may be responsible for it9,10,11,12. In polar waters, most observations of N2 fixation have been collected in the Arctic with a dominance of diazotrophs more eurythermal than Trichodesmium such as UCYN-A and cluster III nifH phylotypes, and with rates ranging from below detection limit to 17.2 nmol N L−1 d−18,13,14. In contrast, few observations are available in Antarctic waters. Traditional views would dictate that the cold and nitrogen rich waters of the Southern Ocean should not be conducive to N2 fixation. However, low N2 fixation rates have been reported in the high-latitude waters of the South Pacific and South Indian Oceans15,16. Recently, Shiozaki et al.17 published evidence of N2 fixation in eastern Antarctic waters with a particularly high rate (44.4 nmol N L−1 d−1) at the ice-edge17. This high rate has recently been questioned by White et al. as it was calculated from an outlier and the observed UCYN-A abundance was not sufficient to support such a high rate18. More recently, N2 fixation was reported during late summers in the coastal waters of Chile Bay at the Western Antarctic Peninsula (WAP)19.
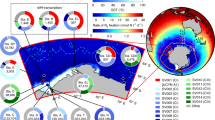
In this study, we provide evidence of potential but limited N2 fixation rates in Antarctic waters. We focus our study on the Western Antarctic Peninsula (Fig. 1), a region which has experienced warming, sea ice retreat, and changes in phytoplankton community structure over the last decades20,21,22. We conducted a survey of N2 fixation along the WAP shelf from January to February in 2018, and time-series observations at Palmer Station during the growing season from January to March in 2019. The rates measured by 15N2 incubation method were below the most stringent detection limit at all offshore stations in 2018 and from February to March at Palmer Station in 2019. However, some measured N2 fixation rates were above less stringent detection limits suggesting potential N2 fixation. This was corroborated by estimates with our high-frequency instrument FARACAS, a flow-through method that measures N2 fixation continuously based on acetylene reduction23. While Shiozaki et al.17 found a dominance of UCYN-A in nifH gene expression17, our nifH gene amplicon sequencing results showed that Proteobacteria were likely the dominant diazotroph in our study region. Combined with the analysis of environmental properties, we hypothesize that the diazotrophs were associated with the sediments. In our study, the potential N2 fixation fluxes were sporadic and low. Additional work is needed to determine if N2 fixation in Antarctic waters is an interesting biological process with little biogeochemical implications, or if our limited observations encountered an important new biome of N2 fixation.
Results and Discussion
Measuring low N2 fixation rates
Due to relatively low fluxes compared to the available pool, measuring N2 fixation in the ocean using 15N2 assays has long been a methodological challenge and a matter of discussion, with methodological bias toward potential over-estimation24 or under-estimation25,26,27. More recently, Gradoville et al.28 pointed out the need to qualify systematically the detection limits when using 15N2 assays in particular when reporting low rates to avoid interpreting false positives28. They proposed two ways to qualify the detection threshold. The first one is to propagate all the experimental uncertainties associated with each parameter used for the determination of the rate, defined as to the minimal quantifiable rate (MQR)29. The second one is to define a minimum 15N enrichment between the start and end of the incubations of 4‰ (0.00146 atom%) according to Montoya et al.30, referred here as the limit of detection (LODMontoya). In addition, we defined an alternative limit of detection (LODsd) using three times the standard deviation of the initial 15N abundance in our samples as a threshold. In our experiments, while the isotopic composition of the particulate organic N (PON) was measured in triplicate after the incubations, the initial value was measured in monoplicate only. In order to define the LODsd, we modeled the standard deviation of the initial 15N abundance in PON using the observed trend over time at Palmer station (see the “Method” section for more details).
N2 fixation was detected at seven, one, and none of the time points out of 18 at Palmer Station depending on whether we use the MQR, LODMontoya, and LODsd as thresholds, respectively (Fig. 2). Interestingly, the only sample above the LODMontoya is not above the MQR. This discrepancy is due to the fact that only the 15N enrichment difference between the beginning and the end of the incubation in the particulate matter is used to calculate LOD while all the parameters used in the calculation of the N2 fixation rate are included to calculate MQR. Nevertheless, since the N2 fixation rates presented here were always close to the different detection limits used (Supplementary Data 1 and 2), we interpret them as potential N2 fixation rates.
Nitrogen fixation rates (nmol N L−1 d−1) measured by 15N2 incubation (blue bars) from January to March and by FARACAS (blue line) from Jan. 27th to 28th in 2019. The error bars show standard deviation of the triplicate incubations (except for duplicates on Feb. 11th). Rates above the MQR, LODMontoya, or LODsd are depicted with green marks.
Time series of nitrogen fixation measurements and other environmental factors
15N2 fixation rates were above the MQR between Jan. 24th and Feb. 22nd (with the exception of Feb. 17th) with rates ranging 0.21–6.59 nmol L−1 d−1 when detected and then remained below the MQR for the rest of the studied period. N2 fixation rates were only above the LODMontoya on Mar. 5th at a rate of 1.53 nmol L−1 d−1. These measured N2 fixation rates were comparable to most of the maximum N2 fixation rates reported by Shiozaki et al.17, except for one of their extremely high value (44.4 nmol N L−1 d−1) at Station E near the sea ice edge17, although our study was conducted during austral summer when there was no sea ice formation in the northern WAP. This rate was also comparable to the rates in surface waters under light conditions between 2013 and 2017 reported by Alcamán-Arias et al.19 at Chile Bay, but considerably lower than those in 2018 and 201919. On a more global scale, between Jan. 24th and Feb 14th N2 fixation rates were higher than the 7th decile of the compiled rates reported in Shao et al.31. However, when normalized to the high biomass observed in the study region (N2 fixation rate/ particulate organic N), our rates (<0.001 d−1) are much lower than what is measured in subtropical waters (typically ranging 0.01–0.1 d−1)31, suggesting a limited impact of N2 fixation in this Antarctic ecosystem. To capture temporal patterns of N2 fixation at higher resolutions than 15N2 incubations, measurements by FARACAS were carried out, which also showed detectable N2 fixation from Jan. 27th to 28th (Fig. 2), ranging from 1.2 to 5.8 nmol N L−1 d−1. These rates were comparable to 15N2 incubation measurements. The stir bar in FARACAS incubator was found to be stuck before 9 pm on Jan. 28th and could have caused overestimation of N2 fixation rates between 4 am and 9 pm because of ethylene buildup in the incubator. The discrepancy between the N2 fixation rates measured by two different methods could also be caused by uncertainties in the conversion ratio between acetylene reduction and N2 fixation and release of newly fixed N into dissolved phase not captured by the 15N2 incubation. The increasing N2 fixation trends at night as observed after 9 pm on 28th is consistent with the higher rates under dark conditions at the WAP19, potentially contributed by the heterotrophic diazotrophs (as described in the next section).
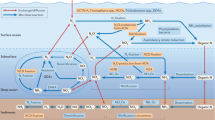
The days before Feb. 2nd and 14th, when N2 fixation rates were at their highest, were characterized by a drastic change in environmental conditions. Strong winds on Jan 22nd (>15 m/s) caused considerable water mixing with a substantial drop in biological oxygen saturation (as estimated with O2/Ar) at the ocean surface from around +10% to below −10% (Fig. 3). The water temperature did not change substantially, but salinity increased from 33.4 to 33.8, the highest value observed over the study period (Fig. 3) and was consistent with upwelling of bottom waters or Upper Circumpolar Deep Water (salinity 34.62–34.68)32. Vertical profiles of water density at station B 1 km from Palmer Station also showed elevated values in the surface layer during this time (Supplementary Fig. 1), indicating the enhanced vertical water mixing associated with the strong winds. Nutrients (N and P, Supplementary Fig. 1) also increased during this water mixing event, and triggered a diatom bloom in early February, as shown by the increase in chlorophyll, O2/Ar ratios (Fig. 3), and particulate organic N (Supplementary Data 1). The color of the filtered biomass, when N2 fixation was measurable, was also visually distinct from other samples (Supplementary Fig. 2), indicating potentially different plankton communities or particle sources. Based on these environmental conditions, and our nifH analyses showing likely sediment-associated diazotrophs (see “Discussion” below), we hypothesize that the strong winds resuspended sediments in the shallow water column (bottom depth within 10 m).
1-h averaged chlorophyll, water temperature, salinity, wind speed and O2/Ar ratio are shown by blue lines in each panel. Each red dot represents a 10-min average at the sampling time point for discrete DNA/RNA samples. The shaded area indicates the time periods when 15N2 incubation experiments were performed and the signals were below (gray shaded) or above the MQR or the LODMontoya (green shaded).
The importance of sedimentary N2 fixation is increasingly evident33,34, especially in coastal oceans35,36,37,38. Mixing could inoculate the water column with sedimentary diazotrophs39 and/or alleviate water column diazotrophic iron limitation through resuspension40,41. Limited organic matter availability (with lower chlorophyll and negative O2/Ar ratios), or higher N/P ratios (Supplementary Fig. 1) may explain the undetectable N2 fixation on other windy days later during the growing season. Further studies are needed to ascertain the factors controlling diazotrophy in the region.
To expand the spatial coverage of N2 fixation observations along the WAP, we conducted 15N2 incubations at 5 different stations along the WAP during the 2018 PAL-LTER cruise from Jan 22nd to Feb 3rd (Fig. 1). All N2 fixation rates were below the detection limits (LODmontoya, LODsd, and MQR) except for station 150.00 where the measured N2 fixation rate of 1.9 nmol L−1 d−1 which was associated with a high standard deviation (±8.3 nmol L−1 d−1) exceeded the LODmontoya of only 0.3 nmol L−1 d−1. As opposed to the time-series sampling site at Palmer Station, where bottom depth is 10–20 m, these 5 regional cruise stations were farther offshore, with the water depth ranging from 250 to 460 m. The absence of detectable N2 fixation rates in surface waters at these offshore deeper stations also aligns with our hypothesis that sedimentary diazotrophs are the main contributors to measurable N2 fixation rates in the study region.
Diazotroph community composition in Antarctica
DNA and RNA were extracted from a total of 55 filters collected from Jan. 13th to Mar. 24th in 2019. nifH genes were amplified from all 55 DNA samples. The sequences show the presence of putative diazotrophs in the WAP coastal waters (Fig. 4). A maximum likelihood phylogenetic tree was built for the top 15 nifH ASVs among DNA and RNA samples from this study (named as “nifH ASV 1”, etc.) together with the top 15 ASVs from eastern Antarctica (named as “ASV001” to “ASV015” in Shiozaki et al.)17, 18 OTUs from Chile Bay19 and other representative nifH sequences from environmental samples or diazotroph isolates (Fig. 5).
The analysis includes a total of 206 nifH sequences, color coded by source: blue and purple for the top 15 nifH amplicon sequencing variants (ASVs) in DNA and RNA samples in this study, respectively, with overlap in nifH ASV 3; red labels denote top ASVs identified in eastern Antarctica waters by Shiozaki et al.17; pink labels for sequences identified from Chile Bay by Alcamán-Arias et al.19. Additional nifH sequences from other environmental samples are represented in various colors. Bootstrap values were determined from 1000 iterations (blue circles).
While there are some similarities in diazotrophic communities between different Antarctic waters (e.g., clustering of nifH ASV 1 and ASV013, nifH ASV 14 and Ued OTU03, ASV004 and Ued OTU12), most of the top nifH ASVs from our study are distinct from those in eastern Antarctic or Chile Bay waters (Fig. 5). The top sequences from our study are also different from Arctic samples based on the phylogenetic tree (Fig. 5). For example, UCYN-A was described as a major contributor to N2 fixation in eastern Antarctica17 and the Arctic waters14,42. In contrast, no nifH ASVs from our study sites at the WAP are close to UCYN-A (ASV001) on the phylogenetic tree (Fig. 5). The absence of UCYN-A was also reported in the Chile Bay waters19, indicating distinct diazotroph communities between eastern and western Antarctic waters. In our study, Proteobacteria were the most well-represented diazotrophic phylum, with Gammaproteobacteria as the dominant class (Fig. 4). 22 out of the 55 RNA samples showed positive nifH RT-PCR amplifications. Shown on Fig. 4 are the 13 RNA samples with more than 1000 reads per library after removal of the non-nifH gene sequences. Epsilonproteobacteria and Bacteroidia dominated the RNA samples. The discrepancy between DNA and RNA nifH compositions indicates that some rare diazotrophs may contribute disproportionally to N2 fixation rates.
Potential for sedimentary diazotrophy
Some Epsilonproteobacteria can fix N2 and have been shown to be potentially important in high-latitude coastal sediments43. We hypothesize that Epsilonproteobacteria were likely key diazotrophs at the WAP. When measurable N2 fixation rates and substantial nifH RNA reads were detected (Fig. 4), the most abundant RNA reads were identified as Epsilonproteobacteria, with nifH ASV 35 being the most abundant ASV on Jan. 28 and Feb. 2 and nifH ASV 108 and nifH ASV 152 accounting for >70% of the reads on Feb. 23. Strains under genus Arcobacter were among the top blast hits for both nifH ASV 108 and nifH ASV 152. Arcobacter has been reported in diverse habitats including estuarine sediments44,45. The top blast hit was Candidatus Sulfurimonas under genus Sulfurimonas (score = 468, e-value = 2e−127) for nifH ASV 35. Genus Sulfurimonas is under the order Campylobacterales, which has been reported in high-latitude ocean sediments46,47. Similarly, species of genus Sulfurimonas can be dominant in pelagic and sedimentary redoxclines48, again agreed with the hypothesis that nifH ASV 35 resulted from resuspended sediments. Furthermore, nifH ASV 35, nifH ASV 108 and nifH ASV 152, which were the dominantly expressed RNA ASVs during measurable N2 fixation in our study, clustered together with sequences isolated from sedimentary samples (Fig. 5). nifH ASV 1 and nifH ASV 20 cluster with ASV013 on the phylogenetic tree, together with four other sedimentary samples, further supporting a sedimentary origin. Notably, Chile Bay diazotrophs also clustered together with sedimentary samples but differed from the top nifH ASVs in our RNA samples on the phylogenetic tree (Fig. 5).
A bloom of the genus Arcobactor and Sulfurimonas was reported after oxygenation of sediments collected from the coastal Baltic Sea43, a similar eutrophic high latitude coastal environment to the WAP, which was traditionally believed unfavorable for N2 fixation. A recent study also showed that N2 fixation occurred under simulated sediment resuspensions with coastal Baltic Sea sediments, dominated by sulfur-reducing bacteria49. Reported to be prominent in diazotrophic communities from temperate estuary sediments39, sulfur-reducing Deltaproteobacteria were also present at our study site. Sediment resuspension at the WAP likely led to a re-oxygenation similar to one observed in the Baltic Sea study43. The authors hypothesized that re-oxygenation may have led to favorable microaerophilic conditions for these genera, and high heterogeneity in sediments created micro niches which permitted anaerobic N2 fixation43. N2 fixation may also have been stimulated by subdued inhibition by ammonium when resuspended and diluted into the water column50.
Considering that a large portion of the nifH sequences were not identified based on the currently available reference database (Fig. 4), an alternative to the sediment source is that we may have encountered new heterotrophic N2 fixers. Recent studies have shown that heterotrophic N2 fixers may be more important in the ocean than previously thought9,10,11,12. Our study demonstrates that they may also be important in Antarctic waters. For example, many of our nifH ASVs also showed substantial levels of sequence identity (i.e., over 85%) to nifH genes found in genomes of prevalent heterotrophic bacterial diazotrophs recently reconstructed from surface ocean metagenomes10.
In our study, characterization of the community was also performed on days when N2 fixation was below the detection limit. On some of these days, the nifH gene was successfully amplified (RT-PCR) from RNA samples (Fig. 4) (e.g., Mar. 24th). This could result from low absolute abundance of active diazotrophs or below detection limit N2 fixation, or a change in diazotroph community between the start of the incubations and molecular sampling. For example, an RNA sample collected in the afternoon of Jan. 27th, right before the second 15N2 incubation, displayed notably different active taxa based on nifH gene expression, dominated by nifH ASV 8 under class Bacteroidia (93.7%). The top blast hit of nifH ASV 8 is an uncultured bacterium from the OMZ water column (score = 348, e-value = 2e−91). This substantial change in nifH gene expression within 10 h could be caused by diel variability or changes in water masses, perhaps associated with tidal activity.
With the results indicating that resuspended sediments may have contributed to measurable N2 fixation in coastal Antarctic waters, our study challenges the traditional view that polar, coastal, and nutrient-rich oceans are inhospitable to diazotrophs and adds to recent studies expanding their potential niches7,17,19,51,52,53,54,55. However, our observations showed N2 fixation to be low (or absent when using more stringent thresholds) and sporadic. The major diazotroph communities were distinct from other studies in Antarctic waters17,19, showing the Southern Ocean to be a potentially diverse N2 fixer habitat. This time-series dataset also complements our understanding of temporal variabilities of N2 fixation in Antarctica waters. It remains to be determined how widespread diazotrophy is in Antarctic waters and how it is influenced by sediment resuspension. Furthermore, given that vertical mixing is common in this region, sediment resuspension may also influence elemental cycling within the water column56. In light of the disproportionate influence of climate change in polar regions (e.g., warming, stratification, and community changes), further studies are needed to examine the importance of N2 fixation in this newly discovered biome.
Methods
High-resolution continuous measurement of N2 fixation rates (FARACAS)
High frequency measurements of aquatic N2 fixation were conducted at Palmer Station, Antarctica, from January to March in 2019 using the FARACAS system23. Due to technical issues, the FARACAS data were only usable at the start of the deployment during January 27–28th. Unfiltered seawater was continuously pumped into the laboratory from 6 m depth in Arthur Harbor, west of Palmer Station. C2H2 gas was prepared in Tedlar bag by reacting high-purity calcium carbide (CaC2, Alfa Aesar) with ultrapure water, and then dissolved in 0.2 µm filtered seawater to a saturation of 70% (v/v%). The 70% C2H2 tracer water was then mixed with unfiltered seawater at a ratio of 1:6, reaching a final saturation of 10%. This water mixture was pumped into a modified 9 L glass incubator, where flow-through incubation was performed. Ambient temperature was maintained by a water-jacket outside of incubator and light was simulated using an LED-wrap based on the solar altitude. The incubator was thoroughly acid washed every two days. C2H4 production rates were measured during the incubation using a Picarro CRDS (cavity ring-down laser absorption spectroscopy) C2H4 analyzer (model G1106, Santa Clara, CA), and converted to N2 fixation rates using a factor of 4:1.
15N2 incubation
Concurrently, discrete 15N2 addition incubations were conducted from late January to March 2019 at Palmer Station, following the protocols outlined in Mohr et al.25. Briefly, 15N2 enriched water was made by dissolving 10 mL 15N2 tracer (Cambridge Isotope Laboratories, 98%+) into 1 L 0.2 µm filtered seawater after degassing under vacuum for more than 1 h. Surface seawater was collected into 4 L transparent polycarbonate bottles directly from the shore adjacent to the inlet of unfiltered water source for the FARACAS system at Palmer Station or into 2.5 L transparent polycarbonate bottles from Niskin bottles during the 2018 LTER cruise. In case of unfavorable weather conditions at Palmer Station, water was collected from the tap in the Palmer Station pump house. 15N2 enriched water was injected to the bottom of each triplicate bottles (5% v:v) at Palmer Station or directly injected as 15N2 during the 2018 LTER cruise, after which the triplicate bottles were incubated for 24 h in an outdoor transparent tank, with continuously circulating surface seawater controlling the temperature. Dissolved 15N2 samples were collected in gas tight Exetainer vials (Labco) from initial 15N2 enriched water as well as from each bottle after incubation. The samples were poisoned with HgCl2 and stored until analysis using Membrane Inlet Mass Spectrometer (Bay Instruments). Particulate matter was recovered by filtering the water through pre-combusted 25 mm GF/F filters (Whatman) at the end of each incubation. Non-incubated particulate matter was also sampled at each time point (in monoplicate) to reflect the natural 15N/14N ratio in the environment. Filters were stored at −80 °C and dried before N isotope analysis using an elemental analyzer coupled with a mass spectrometer. N2 fixation rates were calculated according to Montoya et al.30. At Palmer Station, the natural (i.e., non-incubated) 15N abundance of particulate matter measured at each time point in monoplicate showed a scattered pattern but a linear increasing trend over the time course of the survey (Sieve Bootstrap Based Test, “notrend_test” function in “funtimes” R package, p-value < 0.0001). We assume that this trend reflects an ecological reality and the scattered nature of the samples is mostly due to analytical uncertainty. In order to account for this, we modeled the linear increasing trend (r = 0.75, p-value = 0.0003, n = 18, Supplementary Fig. 3) and used the fitted values to define the initial 15N abundance in particulate matter for each time point. During the 2018 LTER cruise, no pattern in the 15N abundance of non-incubated was observed and the average of all values was used for all stations. To determine the LODsd, the standard deviation of the initial 15N abundance in the PON was calculated using the rolling standard deviation method with a width of 3, to account for the trend, leading to a value of 0.00063 atom%. LODMontoya and MQR were calculated according to Gradoville et al.28.
DNA and RNA sample collection
DNA and RNA samples were collected twice a day around mid-day and mid-night from January 13th to March 24th in 2019. In order to avoid potential contamination within the pipes between Palmer Station pump house and the wet lab, unfiltered seawater was collected into acid-washed LDPE Cubitainers (Thermo Scientific) from the tap in the pump house. A peristaltic pump was used to filter seawater onto 0.22 µm polyethersulfone membrane filters (Millipore) or 0.22 µm Sterivex filters (Millipore). The filtration was limited to no more than 30 min to minimize RNA degradation, achieving a filtration volume ranging between 600–4000 mL. The filters were flash frozen immediately using liquid nitrogen and then stored at −80 °C until further analysis.
DNA and RNA extraction, nested PCR and nifH gene sequencing
DNA and RNA were extracted from 0.22 µm membrane or 0.22 µm Sterivex filters using Allprep DNA/RNA mini kit (Qiagen) following the manufacture’s instruction with an additional 2-min bead-beating step at 30 Hz using 0.2 g of 0.1 mm Zr beads. DNA and RNA were eluted using 50 uL EB buffer or RNase-free water respectively. RNA samples were further cleaned using RNase-Free DNase set (Qiagen) and the RNA Clean & Concentrator kit (Zymo) to remove DNA, and finally eluted in 15 µL RNase free water. DNA and RNA concentrations were measured on a Qubit fluorometer using Qubit DNA/RNA HS Assay Kit. In order to synthesize cDNA from RNA samples for nifH gene amplification, reverse transcription was conducted using QuantiTect Reverse Transcription Kit (Qiagen) following the manufacturer’s guidelines. nifH genes were then amplified using a nested PCR protocol (nifH3 and nifH4 primers during the 1st round, nifH1 and nifH2 primers with Illumina adapter during the 2nd round) as described by Turk et al.57. The thermal cyle of nested PCR was 95 °C for 5 min followed by 35 cycle of 1 min at 95 °C, 55 °C, 72 °C, respectively, and then 5 min at 72 °C. Finally, customized dual indexed barcodes (6 bp) with heterogenous spacers58 were added through a ligation process using KAPA HiFi ReadyMix59. The thermal cycle of ligation PCR was 95 °C for 3 min followed by 8 cycle of 30 s at 95 °C, 55 °C, 72 °C, respectively, and then 5 min at 72 °C. Samples were pooled in equal molar concentrations before sending for Illumina sequencing at Duke Center for Genomic and Computational Biology in one MiSeq 300PE run.
Sequence data processing
Raw sequencing data was demultiplexed using QIIME 1 (version 1.9.1). The reads were then merged, and barcodes, primers and adapters were trimmed using BBDuk (version 1.1.2). ASVs were inferred using DADA2 pipeline after quality filtering and dereplication60. Chimeras were then removed, and taxonomy was assigned using the function “assignTaxonomy” with reference nifH ARB database61. All ASVs were blasted against Refseq database using blastx in DIAMOND62 and the ASVs that do not encode nitrogenase were filtered out as non-nifH sequences. Some of the featured ASVs were further blasted on National Center for Biotechnology Information (NCBI) database for more information. In total, 25403 ASVs were identified from 55 DNA samples and 22 RNA samples. The 55 DNA samples had an average of 101846 reads and the 13 RNA samples (>1000 reads) had an average of 41147 reads. All nifH gene sequencing data has been deposited in NCBI.
Phylogenetic analyses were conducted in MEGA7 (version 7.0.26)63. 206 sequences were first aligned using ClustalW and then constructed the phylogenetic tree by Maximum Likelihood method based on the Tamura-Nei model64. Bootstrap values were calculated based on 1000 times of replications.
Environmental parameters
Temperature, salinity, chlorophyll, and wind speed data were downloaded from the Antarctic Meteorological Research Center (AMRC) FTP site (ftp://amrc.ssec.wisc.edu/pub/palmer/) and averaged over a 10-min time span at the sampling time or an 1-hour time span during the time series. Temperature was measured at the Palmer Station pump house using a Seabird SBE 38 thermometer. Conductivity was measured using Seabird SBE45 thermosalinograph in the Palmer Station aquarium lab. On the days when these temperature and conductivity data were not available, data were interpolated based on the Palmer Station tide gauge sensor data and salinity was calculated65 in order to obtain a wider time coverage. Chlorophyll was measured using a WetLab fluorometer (FLRT 3759) in the aquarium lab. Wind speed was measured at the weather station in the backyard of Palmer Station. O2/Ar ratios were measured in the wet lab using an equilibrator inlet mass spectrometer (EIMS)66 and averaged to the same 10-min or 1-h resolution as other environmental parameters.
Data availability
Source data used to create all the figures are available on Figshare (https://doi.org/10.6084/m9.figshare.28386431.v2). The sequence datasets generated for this study have been deposited at DDBJ/ENA/GenBank under the accession KIWL00000000. The version described in this paper is the first version, KIWL01000000.
Code availability
No custom code or mathematical algorithm used to be deemed central to the conclusions.
References
Karl, D. et al. The role of nitrogen fixation in biogeochemical cycling in the subtropical North Pacific Ocean. Nature 388, 533 (1997).
Gruber, N. The marine nitrogen cycle: overview and challenges. Nitrogen Mar. Environ. 2, 1–50 (2008).
Luo, Y. -W., Lima, I. D., Karl, D. M., Deutsch, C. A. & Doney, S. C. Data-based assessment of environmental controls on global marine nitrogen fixation. Biogeosciences 11, 691–708 (2014).
Tang, W., Li, Z., & Cassar, N. Machine learning estimates of global marine nitrogen fixation. J. Geophys. Res. Biogeosciences124, 717–730 (2019).
Codispoti, L. A. An oceanic fixed nitrogen sink exceeding 400 Tg N a−1 vs the concept of homeostasis in the fixed-nitrogen inventory. Biogeosciences 4, 233–253 (2007).
Capone, D. G. et al. Nitrogen fixation by Trichodesmium spp.: an important source of new nitrogen to the tropical and subtropical North Atlantic Ocean. Glob. Biogeochem. Cycles 19, 2 (2005).
Tang, W. et al. Revisiting the distribution of oceanic N 2 fixation and estimating diazotrophic contribution to marine production. Nat. Commun. 10, 831 (2019).
Sipler, R. E. et al. Preliminary estimates of the contribution of Arctic nitrogen fixation to the global nitrogen budget. Limnol. Oceanogr. Lett. 2, 159–166 (2017).
Delmont, T. O. et al. Nitrogen-fixing populations of Planctomycetes and Proteobacteria are abundant in surface ocean metagenomes. Nat. Microbiol. 3, 804–813 (2018).
Delmont, T. O. et al. Heterotrophic bacterial diazotrophs are more abundant than their cyanobacterial counterparts in metagenomes covering most of the sunlit ocean. ISME J. 16, 927–936 (2022).
Farnelid, H. et al. Nitrogenase gene amplicons from global marine surface waters are dominated by genes of non-cyanobacteria. PloS ONE 6, e19223 (2011).
Bombar, D., Paerl, R. W. & Riemann, L. Marine non-cyanobacterial diazotrophs: moving beyond molecular detection. Trends Microbiol. 24, 916–927 (2016).
Blais, M. et al. Nitrogen fixation and identification of potential diazotrophs in the Canadian Arctic. Glob. Biogeochem. Cycles 26, 3 (2012).
Shiozaki, T. et al. Diazotroph community structure and the role of nitrogen fixation in the nitrogen cycle in the Chukchi Sea (western Arctic Ocean). Limnol. Oceanogr. 63, 2191–2205 (2018).
Hörstmann, C. et al. Hydrographic fronts shape productivity, nitrogen fixation, and microbial community composition in the southern Indian Ocean and the Southern Ocean. Biogeosciences 18, 3733–3749 (2021).
Raes, E. J. et al. N2 Fixation and new insights into nitrification from the ice-edge to the equator in the South Pacific Ocean. Front. Mar. Sci. 7, 389 (2020).
Shiozaki, T. et al. Biological nitrogen fixation detected under Antarctic sea ice. Nat. Geosci. 13, 729–732 (2020).
White, A. E., Granger, J. & Turk-Kubo, K. Questioning high nitrogen fixation rate measurements in the Southern Ocean. Nat. Geosci. 15, 29–30 (2022).
Alcamán-Arias, M. E. et al. Dark Diazotrophy during the Late Summer in Surface Waters of Chile Bay, West Antarctic Peninsula. Microorganisms 10, 1140 (2022).
Lin, Y. et al. Decline in plankton diversity and carbon flux with reduced sea ice extent along the Western Antarctic Peninsula. Nat. Commun. 12, 1–9 (2021).
Stammerjohn, S., Massom, R., Rind D. & Martinson D. Regions of rapid sea ice change: an inter-hemispheric seasonal comparison. Geophys. Res. Lett. 39, 6 (2012).
Montes-Hugo, M. et al. Recent changes in phytoplankton communities associated with rapid regional climate change along the western Antarctic Peninsula. Science 323, 1470–1473 (2009).
Cassar, N., Tang, W. Y., Gabathuler, H. & Huang, K. Method for high frequency underway N-2 fixation measurements: flow-through incubation acetylene reduction assays by cavity ring down laser absorption spectroscopy (FARACAS). Anal. Chem. 90, 2839–2851 (2018).
Dabundo, R. et al. The contamination of commercial 15N2 gas stocks with 15N–labeled nitrate and ammonium and consequences for nitrogen fixation measurements. PloS ONE9, e110335 (2014).
Mohr, W., Wallace, G. & LaRoche, D. W. J. Methodological underestimation of oceanic nitrogen fixation rates. PloS ONE 5, e12583 (2010).
Großkopf, T. et al. Doubling of marine dinitrogen-fixation rates based on direct measurements. Nature 488, 361 (2012).
Wannicke, N. et al. New Perspectives on Nitrogen Fixation Measurements Using 15N2 Gas. Front. Mar. Sci. 5, 120 (2018).
Gradoville, M. R. et al. Diversity and activity of nitrogen-fixing communities across ocean basins. Limnol. Oceanogr. 62, 1895–1909 (2017).
White, A. E. et al. A critical review of the 15N2 tracer method to measure diazotrophic production in pelagic ecosystems. Limnol. Oceanogr. Methods 18, 129–147 (2020).
Montoya, J. P., Voss, M., Kahler, P. & Capone, D. G. A simple, high-precision, high-sensitivity tracer assay for N (inf2) fixation. Appl. Environ. Microbiol. 62, 986–993 (1996).
Shao, Z. et al. Global oceanic diazotroph database version 2 and elevated estimate of global N2 fixation. Earth Syst. Sci. Data 15, 3673–3709 (2023).
Moffat, C., Owens, B. & Beardsley, R. On the characteristics of circumpolar deep water intrusions to the west Antarctic Peninsula continental shelf. J.Geophys. Res. Oceans 114, C5 (2009).
Newell, S. E., McCarthy, M. J., Gardner, W. S. & Fulweiler, R. W. Sediment nitrogen fixation: a call for re-evaluating coastal N budgets. Estuaries Coasts 39, 1626–1638 (2016).
Fulweiler, R., Nixon, S., Buckley, B. & Granger, S. Reversal of the net dinitrogen gas flux in coastal marine sediments. Nature 448, 180 (2007).
Gardner, W. S. et al. Nitrogen fixation and dissimilatory nitrate reduction to ammonium (DNRA) support nitrogen dynamics in Texas estuaries. Limnol. Oceanogr. 51, 558–568 (2006).
McCarthy, M. J., Newell, S. E., Carini, S. A. & Gardner, W. S. Denitrification dominates sediment nitrogen removal and is enhanced by bottom-water hypoxia in the Northern Gulf of Mexico. Estuaries Coasts 38, 2279–2294 (2015).
Wang, R. et al. Nitrogen fixation in surface sediments of the East China Sea: occurrence and environmental implications. Mar. Pollut. Bull. 137, 542–548 (2018).
Yao, X. et al. Nitrogen fixation occurring in sediments: contribution to the nitrogen budget of lake Taihu, China. J. Geophys. Res. Biogeosciences 123, 2661–2674 (2018).
Hallstrøm, S. et al. Pelagic N2 fixation dominated by sediment diazotrophic communities in a shallow temperate estuary. Limnol. Oceanogr. 67, 364–378 (2021).
Burdige, D. J. & Christensen, J. P. Iron biogeochemistry in sediments on the western continental shelf of the Antarctic Peninsula. Geochim. et Cosmochim. Acta 326, 288–312 (2022).
Sherrell, R. M., Annett, A. L., Fitzsimmons, J. N., Roccanova, V. J. & Meredith, M. P. A ‘shallow bathtub ring’ of local sedimentary iron input maintains the Palmer Deep biological hotspot on the West Antarctic Peninsula shelf. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 376, 20170171 (2018).
Cheung, S. et al. High biomass turnover rates of endosymbiotic nitrogen-fixing cyanobacteria in the western Bering Sea. Limnol. Oceanogr. Lett. 7, 501–509 (2022).
Broman, E., Sachpazidou, V., Pinhassi, J. & Dopson, M. Oxygenation of hypoxic coastal Baltic Sea sediments impacts on chemistry, microbial community composition, and metabolism. Front. Microbiol. 8, 2453 (2017).
Collado, L. & Figueras, M. J. Taxonomy, epidemiology, and clinical relevance of the genus Arcobacter. Clin. Microbiol. Rev. 24, 174–192 (2011).
Sasi Jyothsna, T., Rahul, K., Ramaprasad, E., Sasikala, C. & Ramana, C. V. Arcobacter anaerophilus sp. nov., isolated from an estuarine sediment and emended description of the genus Arcobacter. Int. J. Syst. Evolut. Microbiol. 63, 4619–4625 (2013).
Rasigraf, O., Schmitt, J., Jetten, M. S. & Lüke, C. Metagenomic potential for and diversity of N-cycle driving microorganisms in the Bothnian Sea sediment. MicrobiologyOpen 6, e00475 (2017).
Jorgensen, S. L. et al. Correlating microbial community profiles with geochemical data in highly stratified sediments from the Arctic Mid-Ocean Ridge. Proc. Natl. Acad. Sci. USA 109, E2846–E2855 (2012).
Henkel, J. V. et al. Candidatus Sulfurimonas marisnigri sp. nov. and Candidatus Sulfurimonas baltica sp. nov., thiotrophic manganese oxide reducing chemolithoautotrophs of the class Campylobacteria isolated from the pelagic redoxclines of the Black Sea and the Baltic Sea. Syst. Appl. Microbiol. 44, 126155 (2021).
Liesirova, T. et al. Nitrogen-fixing sulfate reducing bacteria in shallow coastal sediments under simulated resuspension. Estuar. Coast. Shelf Sci. 280, 108165 (2022).
Darnajoux, R., Reji, L., Zhang, X. R., Luxem, K. E. & Zhang, X. Ammonium sensitivity of biological nitrogen fixation by anaerobic diazotrophs in cultures and benthic marine sediments. J. Geophys. Res. Biogeosciences127, e2021JG006596 (2022).
Conley, D. J. et al. Controlling eutrophication: nitrogen and phosphorus. Science 323, 5917 (2009).
Zehr, J. P. & Paerl, H. W. Molecular ecological aspects of nitrogen fixation in the marine environment. In Microbial Ecology of the Oceans (ed. Kirchman, D. L.) Ch. 13 481–525 (Wiley, 2008).
Voss, M., Croot, P., Lochte, K., Mills, M. & Peeken, I. Patterns of nitrogen fixation along 10 N in the tropical Atlantic. Geophys. Res. Lett. 31, 23 (2004).
Sohm, J. A. et al. Nitrogen fixation in the South Atlantic Gyre and the Benguela upwelling system. Geophys. Res. Lett. 38, 16 (2011).
Mulholland, M. R. et al. High rates of N2 fixation in temperate, western North Atlantic coastal waters expand the realm of marine diazotrophy. Glob. Biogeochem. Cycles 33, 826–840 (2019).
Pedersen, J. N., Bombar, D., Paerl, R. W. & Riemann, L. Diazotrophs and N2-fixation associated with particles in coastal estuarine waters. Front. Microbiol. 9, 2759 (2018).
Turk, K. A. et al. Nitrogen fixation and nitrogenase (nifH) expression in tropical waters of the eastern North Atlantic. ISME J. 5, 1201 (2011).
Fadrosh, D. W. et al. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome 2, 1–7 (2014).
Kozich, J. J., Westcott, S. L., Baxter, N. T., Highlander, S. K. & Schloss, P. D. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 79, 5112–5120 (2013).
Callahan, B. J. et al. DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583 (2016).
Moynihan, M. A. nifHdada2 GitHub repository. Zenodo. https://doi.org/10.5281/zenodo.3958370 (2020).
Buchfink, B., Reuter, K. & Drost, H. -G. Sensitive protein alignments at tree-of-life scale using DIAMOND. Nat. Methods 18, 366–368 (2021).
Kumar, S., Stecher, G. & Tamura, K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874 (2016).
Tamura, K. & Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 10, 512–526 (1993).
Hill, K., Dauphinee, T. & Woods, D. The extension of the practical salinity scale 1978 to low salinities. IEEE J. Ocean. Eng. 11, 109–112 (1986).
Cassar, N. et al. Continuous high-frequency dissolved O2/Ar measurements by equilibrator inlet mass spectrometry. Anal. Chem. 81, 1855–1864 (2009).
Acknowledgements
We thank the scientists, scientific support personnel, the crew on R/V Laurence M. Gould, and the PAL-LTER team for their assistance in sample collection. We are grateful to Hans Gabathuler for instrument support. This work is supported by NSF OPP-1643534 to N.C. and NSF OPP-1440435 to H.W.D.
Author information
Authors and Affiliations
Contributions
N.C. designed the study. S.G. performed measurements and collected samples at Palmer Station, with contributions from W.T. and H.W.D. H.B. conducted measurements at offshore stations. S.G. and H.B. analyzed nitrogen fixation data. S.G. performed molecular sequencing with contributions from J.R. and Y.L. S.G. analyzed environmental data. S.G., Y.L., and A.M.E. conducted sequencing data analyses. S.G. and N.C. wrote the manuscript with contributions from all co-authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Earth & Environment thanks Douglas Capone and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Clare Davis and Alice Drinkwater. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gu, S., Berthelot, H., Lin, Y. et al. Evidence of limited N2 fixation in the Southern Ocean. Commun Earth Environ 6, 264 (2025). https://doi.org/10.1038/s43247-025-02225-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43247-025-02225-0