Abstract
Background
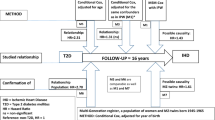
This study aimed to evaluate the association of lactation duration with incident ischemic heart disease (IHD) and to determine the potential health gains from scaling up breastfeeding practice.
Methods
130,147 parous postmenopausal females without IHD were included at baseline (2004–2008) from the China Kadoorie Biobank study. Lactation duration was self-reported and measured as lifetime, per child, and first child, respectively. Incident IHD was identified during follow-up (2004–2015). The dose-response associations between lactation duration and IHD were examined using Cox models with restricted cubic splines. Stratification analyses were conducted by socioeconomic status (SES) and residence. The number of preventable IHD cases was estimated using the population attributable fraction and potential impact fraction in various scenarios.
Results
The study shows that parous postmenopausal females who ever lactated have significantly lower risks of IHD, with adjusted hazard ratios (aHRs) varying from 0.71 (95%CI: 0.63–0.80) to 0.85 (95%CI: 0.75–0.96) for a lifetime, from 0.70 (0.63–0.78) to 0.82 (0.72–0.93) for per-child, and from 0.80 (0.74–0.87) to 0.92 (0.85–0.99) for the first-child, appearing as U-shaped associations. Similar associations are found in females with low SES and urban residence. The scaling up of breastfeeding to near-universal levels could have prevented up to 115,000 new IHD cases among Chinese females aged over 40 years in 2019.
Conclusions
Lactation demonstrates potential benefits in reducing IHD risk, appearing as U-shaped associations among Chinese parous postmenopausal females, especially for those with low SES in urban areas. Scaling up breastfeeding practices serves as a promising strategy for reducing the IHD burden in China.
Plain language summary
We investigated whether people who breastfeed their babies are less likely to develop heart disease as a consequence of reduced blood flow to the heart (IHD) later in life. We studied postmenopausal females in China and found that people who had breastfed their infants had a lower risk of developing IHD than those who had never breastfed. The risk reduction increased if people breastfed for up to 10 months per child or had low socioeconomic status and lived in urban areas. This suggests that encouraging and supporting breastfeeding, particularly for urban women with low socioeconomic status, may help reduce the IHD risk.
Similar content being viewed by others
Introduction
As the most common cardiovascular disease (CVD), ischemic heart disease (IHD) continues to pose a severe threat to public health1,2. Globally, despite a decline in IHD mortality over the past decade, IHD remains a leading cause of death, responsible for approximately 9.14 million deaths, or 16.1% of total deaths in 20191,3. The burden of IHD is also substantial in China, where the number of IHD-related deaths increased by 30.2% from 2010 to 2019, reaching 1.87 million deaths in 20193,4,5. The upward trend of age-standardized IHD mortality recently has become a cause of concern5. In response, China has set a goal of reducing the mortality of CVDs by 25% before 2025, as outlined in its Medium and Long-term Plan for Prevention and Treatment of Chronic Diseases (2017–2025)6. As the incidence of IHD continues to rise due to the ageing trends, the economic burden on families and society is expected to grow7,8. Therefore, primary prevention strategies, accurate diagnoses, and efficient treatments for IHD are urgently needed. The Lancet Women and Cardiovascular Disease Commission has called for sex‐specific research on CVDs to reduce the global burden of CVDs in women by 20309. Obvious sex-based differences have been revealed in risk factors, pathophysiology, clinical presentation, and epidemiology of IHD10,11. Compared to males, post-menopausal females are more likely to develop IHD and have a higher prevalence and mortality of IHD12,13. These differences may partly be attributed to variations in reproductive events and the consequent hormonal fluctuations affecting females’ cardiovascular system11.
Lactation is the process by which the mother secretes milk from her mammary glands to feed her newborn following childbirth14. Previous studies have revealed that lactation is associated with a reduced risk of coronary artery heart disease (CHD), coronary artery and aortic calcification, stroke, metabolic syndrome, hypertension, and diabetes15,16. A prospective study based on 267,400 female textile workers in Shanghai, China, suggested that females who did not breastfeed after childbirth had a slightly increased risk of IHD mortality17. Nonetheless, the dose-response relationship between lactation duration and the risk of IHD is poorly understood18,19,20. Socioeconomic status (SES) is a well-established determinant of health outcomes, with profound implications on CVDs21. However, the extent to which the associations between lactation and IHD are influenced by SES remains poorly understood.
The population attributable fraction (PAF) and potential impact fraction (PIF) are instrumental in estimating the proportion of disease burden that could be prevented in a population through the elimination of a specific risk factor. These metrics have been widely used to estimate the population impact of preventive interventions under different scenarios on various diseases, including CVDs, and dementia, providing a theoretical basis for policymaking22,23. The World Health Organization and the infant feeding guideline in China have recommended exclusive breastfeeding for infants until the age of 6 months, followed by continued breastfeeding until the age of 2 years or beyond, as the optimal breastfeeding strategy24,25. However, the current rate of lactation for 6 months in China is less than 30%, which falls short of the goal of 50% by 2020 set by the National Nutrition Plan (2017–2030), and the global average level of 43%26,27. By far, how scaling up breastfeeding practice could be beneficial for IHD prevention in China remains unknown.
To fill the gap of knowledge, we investigated the dose-response associations of lactation duration, measuring as lifetime lactation duration, per-child lactation duration, first-child lactation duration, with the risk of IHD among parous postmenopausal females in the China Kadoorie Biobank (CKB) study. We also examined these associations across different SES and residential subgroups to elucidate potential disparities. Furthermore, we employed scenario-based PAF and PIF to estimate the potential impact of universal breastfeeding on preventing IHD among Chinese parous postmenopausal females.
Parous postmenopausal females who breastfed have a lower risk of IHD compared to those who never lactated, especially for those with low socioeconomic status in urban areas. A U-shaped association suggests that a lactation duration of up to 10 months per child is linked to greater IHD risk reduction. Scaling up breastfeeding practices serves as a promising strategy for reducing the IHD burden in China, highlighting the potential population-level benefits of promoting extended breastfeeding.
Methods
Study design and participants
The CKB study is a large-scale prospective cohort study in the Chinese population28,29,30. The baseline survey took place between June 25, 2004, and July 15, 2008, in ten geographically defined regions (five urban, five rural) across China. 512,726 residents aged 30–79 years (born in 1930–1970) were recruited. At baseline, trained health workers administered laptop-based questionnaires on socio-demographics, lifestyle behaviors, medical history, and females’ reproductive history, measured height, weight, waist circumference (WC), and blood pressure, and collected blood samples. For long-term follow-up (2004–2015), all participants were followed by hospital admission, while disease diagnoses were collected through electronic linkage with local chronic disease registries and the new national health insurance claim databases.
Ethics approvals for the CKB study were obtained from the Ethical Review Committee of the Chinese Center for Disease Control and Prevention (Beijing, China) (Approval No. 005/2004), and the Oxford Tropical Research Ethics Committee, University of Oxford (UK) (Approval No. 025-04). All participants provided written informed consent for participation.
Among the 512,726 participants, we excluded females with a history of breast lump removal, hysterectomy, ovary removal, cancer, coronary heart disease, or ischemic heart disease at baseline, 272,052 parous females were included in our study (Supplementary Fig. S1). We further excluded females with exceptionally long each-child lactation duration (>60 months) or missing data on socio-demographics and lifestyle factors, resulting 271,435 included. Among them, 134,094 were postmenopausal. After excluding those with abnormal menopausal age (<40 or ≥baseline age)31, 130,147 parous postmenopausal females were our study sample for the analysis.
Assessment of lactation duration
At baseline, females completed an interviewer-administered electronic questionnaire on lactation duration for each live birth using the specific question “Age and length of lactation at each live birth”. Lifetime lactation duration was the summation of the lactation duration for all live births and was categorized into never, 1–12 months, 13–24 months, 25–36 months, 37–48 months, and >48 months32. Per-child lactation duration was calculated as lifetime lactation duration divided by live birth counts. Lactation duration for the first child was also assessed in our analysis. Per-child lactation duration and first-child lactation duration were divided into six categories, respectively (never, 1–6 months, 7–12 months, 13–18 months, 19–24 months, and >24 months), based on the infant feeding guideline 2022 in China25. Parous postmenopausal females who had never lactated were considered as references.
Ascertainment of incident IHD
During the follow-up (2004–2015), IHD was assessed through hospital admission, established disease registries, and health insurance databases in China28. IHD was coded by trained staff ‘blinded’ to baseline information according to the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) (I20–I25)33.
Covariates
Baseline information on covariates included socio-demographics, lifestyle factors, anthropometric measurements, medical history, and reproductive factors.
Socio-demographics included age at baseline, marital status, residence, and socioeconomic factors (highest education attainment, occupation, and annual household income). Marital status was classified as married and unmarried (never married or separated or widowed or divorced). Residence was divided into rural and urban. Highest education attainment was categorized into less than primary school, middle school, high school or above. Occupation was classified into three categories: unemployed, retired or others; farmer or worker; sales, self-employed, manager or professional. Annual household income was divided into three levels: low (<¥10,000), middle-low (¥10,000–34,999), and high (≥¥35,000). 1 CNY was approximately equal to 0.125 USD at the time of the survey.
Lifestyle factors included smoking status, passive smoking status, alcohol intake, and physical activity in metabolic-equivalents of task (MET). Smoking status was divided into non-smoker and smoker (occasional, ex-regular, current smoker), while passive smoking, also named second-hand smoking, was classified as occasionally and most days. Alcohol intake status was categorized into non-drinker and drinker (ex-regular, occasional, monthly, reduced intake, weekly drinker). Physical activity was defined as a continuous variable and measured by MET. The information on physical activity, including occupational tasks, commuting, leisure time activities, and household tasks, was collected at baseline34. METs of different physical activities were validated from the 2011 compendium of physical activities, and separately multiplied by the frequency and duration of physical activity to calculate physical activity in MET (hours/day)35.
Anthropometric measurements included height, weight, and WC. Body mass index (BMI) was calculated as the body weight in kilograms divided by the square of the height in meters (kg/m2), and was further divided into underweight ( < 18.5 kg/m2), normal weight (18.5–23.9 kg/m2), overweight (24.0–27.9 kg/m2), and obesity ( ≥ 28.0 kg/m2)36. While WC > 85 cm for females was defined as central obesity37.
Medical history contained the history of stroke, history of diabetes, history of hypertension, and medication use, such as anticoagulation therapy, hypolipidemic therapy, and they were all dichotomous. Random glucose was assessed using the Sure Step Plus System (Johnson & Johnson, New Brunswick, NJ, USA). If the value was between 7.8 mmol/L and 11.0 mmol/L, a fasting glucose test was required29. Diabetes was defined as fasting glucose ≥7.0 mmol/L, random glucose ≥11.1 mmol/L, or self-reported physician diagnosis or under treatment38. Blood pressure was measured twice, and the average of the two measurements was recorded. If the difference in systolic blood pressure was greater than 10 mmHg, a third measurement would be carried out, and the average of the last two values would be recorded. Hypertension was defined as blood pressure ≥140/90 mmHg, self-reported physician diagnosis, or using drugs to lower blood pressure39.
Reproductive events included history of taking oral contraceptive pills, gravidity, parity, age at first live birth, and age at menopause. Gravidity was classified into three levels: ≤3, 4, and ≥5. Parity was divided into ≤3, 4–6, and 7–10. Age at first live birth was classified into <20 years, 20-24 years, and >24 years40. Age at menopause was divided into <45 years, 45–54 years, and >54 years41.
Statistics and reproducibility
Latent class analysis (LCA) was employed to identify underlying socioeconomic status (SES) classes based on three individual socioeconomic factors: highest education attainment, occupation, and annual household income. Each factor was categorized into three levels for practical interpretation and considering sample size. The selection criteria of various LCA models included theoretical interpretability and fit statistics, such as low absolute Akaike information criterion and Bayesian information criterion values, likelihood ratio statistic, and high-scaled relative entropy42,43,44. The scaled relative entropy measures the certainty of classification, where 0% indicates poor certainty and 100% represents perfect certainty. Finally, two latent SES classes (low, high) were identified according to the item-response probabilities, and the class memberships were determined by the posterior item probabilities (Supplementary Table S1). The study used the R package poLCA (v1.6.0) to implement the LCA procedure45.
Baseline characteristics of the included parous postmenopausal females were described in the form of means and standard deviations (SDs) for normally distributed continuous variables, and medians and interquartile ranges (IQRs) for non-normally distributed continuous variables. Differences were examined by using two samples t-test or Wilcoxon rank sum test, accordingly. Numbers (N) and percentages (%) were presented for categorical variables, with differences calculated using Chi-square test. The incidence density of incident IHD was calculated as the number of events per 100,000 person-years.
Cox proportional hazard regression models were used to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) of lactation durations (lifetime lactation duration, per-child lactation duration, and first-child lactation duration) with incident IHD during follow-up. The proportional hazards assumption was examined by Schoenfeld residuals plot (Supplementary Fig. S2). To address the time-varying effects, Cox models used age as the underlying time scale and adopted a strata function of birth cohort (in 1-year intervals). The time to event was determined as the period from the baseline survey (2004 to 2008) to the date of the IHD event, death, loss to follow-up, or the end of follow-up (December 31, 2015), whichever came first. Model 1 was adjusted for age at baseline. Model 2 was additionally adjusted for socio-demographics (marital status, residence, highest education attainment, occupation, annual household income), lifestyle factors (smoking status, passive smoking status, alcohol intake, physical activity), anthropometric measurements (BMI, WC), medical history (history of stroke, history of diabetes, history of hypertension, history of anticoagulation therapy, history of hypolipidemic therapy), and reproductive factors (history of taking oral contraceptive pills, parity, age at first live birth, age at menopause). A competing risk model analysis was conducted to account for the influence of death as a competing event on IHD. Restricted cubic splines (RCS) were used to investigate the dose-risk associations of various forms of lactation durations with incident IHD.
To examine potential variations in different subgroups, we conducted stratified analyses based on various factors, including SES-residence, SES, residence, SES components, and parity. Furthermore, we performed stratification analysis by menopausal status to assess the variations in premenopausal and perimenopausal females. P for interaction was evaluated by comparing models with and without a multiplicative interaction term, which included one each for lactation and stratification variables. In addition, we conducted two sensitivity analyses to examine the potential confounding effects of these factors on the model performance. Firstly, we excluded participants who were taking cardiovascular drugs such as angiotensin-converting enzyme inhibitors, beta-blockers, calcium antagonists, diuretics, aspirin, and statins. Secondly, we excluded participants with cardiovascular diseases (stroke, diabetes, hypertension, rheumatic heart disease, kidney disease).
To provide a population perspective of how scaling up breastfeeding duration practice may impact the number of IHD cases in China, we calculated the number of preventable IHD cases among Chinese females over 40 in 2019 based on three hypothetical scenarios for average lactation duration per child: (I) all females lactating for an optimal duration (7–12 months as indicated in CKB); (II) 50% of non-lactation females switching to lactating for an optimal duration (7–12 months); (III) 50% of females who lactate for less than 7 months or not at all extending their lactation duration to an optimal duration (7–12 months). In contrast to PAF, which is typically based on an impractical counterfactual scenario assuming a 100% reduction in the risk factor, PIF is estimated based on reasonably achievable theoretical counterfactual scenarios46,47. This study used PAF for scenario I and PIF for scenarios II and III to estimate the preventable IHD cases.
The calculation of PAF was based on the following equation48:
Where Pei = the fraction of the IHD cases in the lactation duration category i, HRi = the relative hazard ratio for IHD at lactation duration category i, n = the number of lactation duration categories.
The PIF was calculated by the following formula:
Where Pe = the fraction of the IHD cases in lactation duration category, P’e = the counterfactual fraction of the IHD cases that lactation duration category adherent to the optimal duration, HR = the relative hazard ratio for IHD at lactation duration category.
The fraction of IHD cases in each category of average lactation duration per child and relative HRs of IHD were derived from CKB. The 95% CI of PAF and PIF were calculated using bootstrap49. We further calculated how many fewer IHD cases could be expected based on PAF or PIF. The preventable IHD cases were estimated based on the number of incidents of IHD among Chinese females above 40 years old in 2019 in Global Burden of Disease 20191.
All analyses were performed using R statistical software (version 4.2.2). The statistical significance was set at two-tailed P < 0.05. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.
Results
Baseline characteristics
A total of 130,147 parous postmenopausal females were finally included in our study (median age: 58.4 [IQR 54.0–64.8]), of whom 14,268 (11.0%) developed IHD during follow-up, showing an incidence density of 1276.2 per 100,000 person-years. The baseline characteristics of the 130,147 parous postmenopausal females are shown in Supplementary Data 1. Generally, most participants were rural residents (55.4%), had an education level of primary school or below (74.5%), unemployed or retired (53.5%), and belonging to middle-income households (51.0%). These participants with incident IHD tended to have gravidity of ≥5 (41.2% vs. 30.3%) and have parity of ≥4 (37.4% vs. vs. 26.1%), compared to non-IHD ones. In a non-IHD subgroup, the proportions of participants with age at first live birth between 20 and 24 (53.4%) or with age at menopause between 45 and 54 (84.4%) are larger. Compared with those who did not develop IHD, parous postmenopausal females who developed IHD have significantly longer lifetime lactation duration (44.0 [IQR 24.0–72.0] months vs. 36.0 [IQR 24.0–60.0] months) and per-child lactation duration (13.0 [IQR 12.0–19.5] months vs. 12.2 [IQR 12.0–19.3] months). While for the first-child lactation duration, this difference was not statistically significant. More baseline characteristics of females by SES or residence are shown in Supplementary Data 2.
The association between lactation duration and IHD
As shown in Fig. 1, after full adjustment for potential covariates, compared with parous postmenopausal females who had never breastfed, females who lactated for 1–12 months (adjusted hazard ratio [aHR]: 0.71, 95% CI: 0.63–0.80), 13–24 months (aHR: 0.73, 95% CI: 0.65–0.81), 25–36 months (aHR: 0.75, 95% CI: 0.67–0.84), 37–48 months (aHR: 0.79, 95% CI: 0.70–0.89), and >48 months (aHR: 0.85, 95% CI: 0.75–0.96) during lifetime had lower risks of IHD. Similarly, lower risks of IHD were observed in parous postmenopausal females who had lactated per child for 1–6 months (aHR: 0.82, 95% CI: 0.72–0.93), 7–12 months (aHR: 0.70, 95% CI: 0.63–0.78), 13–18 months (aHR: 0.79, 95% CI: 0.71–0.89), 19–24 months (aHR: 0.78, 95% CI: 0.70–0.88), and >24 months (aHR: 0.78, 95% CI: 0.69–0.88). First-child lactation duration of 7–12 months (aHR: 0.80, 95% CI: 0.74–0.87), 13–18 months (aHR: 0.92, 95% CI: 0.85–0.99), 19–24 months (aHR: 0.86, 95% CI: 0.79–0.94), and >24months (aHR: 0.81, 95% CI: 0.73–0.90) were associated with lower risks of IHD. Moreover, competing risk model analysis showed similar results (Supplementary Table S2).
Notes: IHD ischemic heart disease, aHR adjusted hazard ratio, CI confidence interval. Colors in yellow, blue, and purple represent the lactation duration of a lifetime per child and first child, respectively. Cases (%) were expressed in numbers with percent. Incidence density was expressed per 100,000 person-years. The model was adjusted for age, marital status, residence, highest education attainment, occupation, annual household income, body mass index, waist circumference, smoking status, passive smoking, alcohol intake status, physical activity in metabolic equivalents of task hours/day, history of anticoagulation therapy, history of hypolipidemic therapy, history of stroke, history of diabetes, history of hypertension, history of oral contraceptive pill use, parity, age of first birth, age of menopause.
The RCS curves (Fig. 2) exhibit non-linear dose-response associations between three lactation duration measurements (lifetime lactation duration, per-child lactation duration, and first-child lactation duration) and IHD. The associations appeared to be U-shaped, with the nadir of IHD risk occurring at the lactation duration of 24.06 months for lifetime, 9.95 months for per child, and 10.85 months for the first child.
Notes: IHD ischemic heart disease, aHR adjusted hazard ratio, CI confidence interval. a–c represent the dose–risk association of IHD with lifetime, per-child, and first-child lactation durations, respectively. Colors in yellow, blue, and purple represent lactation duration of a lifetime, per child, and first child, respectively. The shading indicates the 95% CI of the aHR for IHD. Model was adjusted for age, marital status, residence, highest education attainment, occupation, annual household income, body mass index, waist circumference, smoking status, passive smoking, alcohol intake status, physical activity in metabolic equivalents of task hours/day, history of anticoagulation therapy, history of hypolipidemic therapy, history of stroke, history of diabetes, history of hypertension, history of oral contraceptive pill use, parity, age of first birth, age of menopause.
In the stratified analysis by SES-residence, SES, and residence (Fig. 3), significantly inverse associations of lifetime or per-child lactation duration with IHD were observed in females with low SES and urban residence. When stratified by each SES component (Supplementary Fig. S3) and parity (Supplementary Fig. S4), similar results were found in females with primary school or below, unemployed or retired, middle annual household income, parity of ≤3. In addition, we found that the associations of longer lactation duration (lifetime: ≥37 months; first-child: ≥13 months) with IHD tended to be attenuated among females who were premenopausal when stratified by menopausal status (Supplementary Fig. S5).
Notes: SES socioeconomic status, IHD ischemic heart disease, aHR adjusted hazard ratio, CI confidence interval. Colors in yellow, blue, and purple represent lactation duration of lifetime, per child, and first child, respectively. All models adjusted for the following covariates but excluded stratification variables involved in the model itself: age, marital status, residence, highest education attainment, occupation, annual household income, body mass index, waist circumference, smoking status, passive smoking, alcohol intake status, physical activity in metabolic equivalents of task-hours/day, history of anticoagulation therapy, history of hypolipidemic therapy, history of stroke, history of diabetes, history of hypertension, history of oral contraceptive pill use, parity, age of first birth, age of menopause.
Sensitivity analyses were conducted among individuals who did not take cardiovascular drugs (N = 121,414), without pre-existing CVDs (N = 65,808). The findings from sensitivity analyses, as presented in Supplementary Table S3, also demonstrated significantly inverse correlations of cumulative lactation duration with incident IHD. Specifically, parous postmenopausal females with a lifetime lactation duration of ≥1 months and a per-child lactation duration ≥7 months were at a lower risk of IHD.
Scenario-based projections
The estimated numbers of preventable IHD cases among Chinese females over 40 in 2019 are shown in Table 1. using PAF or PIF in various modeled scenarios of average lactation duration per child.
Scenario I, which assumed that all females could lactate for an optimal duration of 7–12 months, showed a potential prevention of 7.50% (95% CI: 5.81%-9.19%) or 115,196 IHD cases. In scenario II, which assumed that 50% of non-lactating females could switch to lactating for an optimal duration (7–12 months), a potential prevention of 0.54% (95% CI: 0.08%-1.00%) or 8,296 IHD cases was estimated. Scenario III, which assumed that 50% of females who lactated for less than 7 months or not at all could extend their lactation duration to 7–12 months, showed a potential prevention of 0.92% (95% CI: 0.32%-1.52%) or 14,126 IHD cases.
Discussion
Our study contributes to the growing body of evidence on the cardioprotective benefits of lactation, revealing that parous postmenopausal females in China who lactated had lower risks of IHD compared to those never breastfed. Such associations were U-shaped, with the lowest IHD risk identified among females with lifetime lactation durations around 24.06 months, or an average duration of 9.95 months per child, or for the first child at about 10.85 months. Such associations were particularly pronounced among females with low SES residing in urban areas. Our findings suggest that scaling up breastfeeding practice to a near-universal level in China could have potentially prevented up to 115,000 IHD cases among females over 40 years in 2019.
The associations between lactation duration and the risk of CVDs have been established previously. In a random-effects meta-analysis involving data from over a million parous women found an 11% risk reduction for CVD and a 14% reduction for CHD among those who breastfed compared to those who did not18. Similarly, in a case-control study conducted in Europe, a 33% lower risk of CHD was observed among females who breastfed for six months or longer per child50. Another prospective study of 300,000 Chinese females highlighted that every additional six months of breastfeeding per child was associated with a 4% decreased risk of CHD37. Consistent with these findings, our study further revealed risk reductions between 8% and 30% across various lactation subgroups, underscoring the importance of lactation for cardiovascular health.
For maternal metabolism, pregnancy ends not with delivery, but with weaning. During pregnancy, the maternal metabolic system undergoes several changes (including the accumulation of fat, increased insulin resistance, higher atherogenic lipid levels and blood pressure) to support fetal growth and prepare for breastfeeding51,52,53. While these changes, which increase the risk of CVDs and metabolic diseases, may be impacted by breastfeeding. One potential mechanism for the reduced risk of IHD associated with lactation is the “reset hypothesis”54. This hypothesis posits that lactation may drain calories and cholesterol from the lactating mother, thereby resetting maternal metabolism post-pregnancy, which may reduce the adverse metabolic risk incurred pregnancy19. Support for this hypothesis is found in studies indicating that non-lactating women have higher BMI, lipid levels, and blood pressure than those who lactated for extended periods15,55. Others found that maternal obesity is associated with discontinuation of breastfeeding and delayed onset of lactogenesis following delivery56,57. Additionally, the roles of lactation-associated hormones such as prolactin and oxytocin are also implicated in providing cardiovascular and metabolic benefits58,59,60.
Prior studies have reported various patterns of association between lactation and cardiovascular health outcomes, including linear, plateauing, and U-shaped relationships for cardiovascular disease (CVD) mortality and morbidity18,19,20. For instance, a comprehensive systematic review and meta-analysis encompassing data from over one million parous women reported a substantially decreased risk of maternal cardiovascular disease and coronary heart disease (CHD) with lactation durations of up to 12 months, with the effect on CHD appearing to plateau between 12 and 48 months18. Additionally, a Norwegian study disclosed a U-shaped association between lifetime lactation duration and CVD mortality risk among parous women, whereas a large-scale prospective cohort study in China identified an inverse log‐linear association between lactation duration per child and the risk of major CVDs19,61. Our study identified a U-shaped association, suggesting a nuanced interplay between lactation and cardiovascular health. Lactation has been suggested to have beneficial effects on cardiovascular and metabolic system by reducing inflammation, improving lipid metabolism, and lowering blood pressure19. This association may be also influenced by CVD risk factors such as parity and pregnancy complications17,62,63. Notably, our study found that the beneficial effects of lactation on IHD were most pronounced among females with parity of ≤3, indicating that the increased cardiovascular risk associated with multiparity may diminish lactation’s benefits, then appearing in U-shaped associations between lactation and the risk of IHD. Besides, longer lifetime lactation duration, commonly accompanied by multiparity, often correlates with a higher risk of pregnancy complications, including gestational diabetes, hypertensive disorders of pregnancy, and pre-eclampsia64. These pregnancy complications, in turn, can increase the overall risk of cardiovascular diseases, including IHD16. Therefore, the protective effect of longer lactation duration on IHD risk may be diluted by the potential adverse impact of pregnancy complications.
Our study has additionally revealed the modification effect of socioeconomic disparities and residential settings in the association between lactation and IHD risk. Females with low SES often face multifaceted challenges that include, but are not limited to, restricted access to healthcare, higher prevalence of unhealthy lifestyles, and increased exposure to environmental stressors. These challenges collectively contribute to an elevated risk of CVDs21. In this context, lactation stands out as an accessible and cost-effective intervention that can potentially mitigate the IHD burden among these disadvantaged populations. Furthermore, our findings highlighted the differential impact of residential settings on modifying the association between lactation and IHD risk. Specifically, we observed that significant associations between lactation and IHD were discernible primarily among urban residents. In contrast, females living in rural settings typically had higher parity, which might diminish the cardioprotective benefits of lactation, thereby rendering the association less significant in these populations17,62,63.
Our findings reveal the positive impact of lactation duration, regardless of the measures employed (lifetime, average per child, or first-child). The findings regarding cumulative lactation duration across multiple children provide valuable insights into the promotion of sustained breastfeeding practices in subsequent pregnancies. While lactation duration for one child could serve as a practical and widely communicable measure in public health, making it suitable for setting achievable breastfeeding goals. With the application of PAF and PIF, this study successfully revealed that by scaling up lactation to adequate durations, there is potential for a notable reduction in IHD events. Scaling up breastfeeding practices by promoting and encouraging women to lactate for adequate duration could be recommended in reducing IHD events. These insights could inform future public health strategies aimed at advocating for integrating lactation optimization into IHD preventive strategies from a maternal health standpoint. Several strategies may be effective to promote breastfeeding practice according to previous studies, e.g. ensuring stable funding and resources for a successful national strategy for protection, promotion, and support of breastfeeding, calling for strong governmental commitment to develop an effective national breastfeeding program65. This study thereby provides a foundation for policy initiatives that prioritize maternal lactation as a modifiable risk factor for IHD, with essential implications for public health outcomes.
Our study represents a considerable advancement in the exploration of lactation’s protective effects against ischemic heart disease (IHD) in parous postmenopausal women, within the Chinese demographic. This study, to the best of our knowledge, is the first large-scale prospective cohort study examining the associations of lactation duration, using three indicators, with the risk of IHD, among parous postmenopausal females in China. The robustness of our findings is supported by the extensive follow-up period, the high follow-up rate from the CKB study. Our use of ICD-10 diagnosis, made by trained healthcare professionals, rather than self-reporting, enhances the accuracy of our diagnosis of IHD. Stratification and sensitivity analyses have been employed to confirm the credibility of our primary outcomes. Furthermore, the application of PAF and PIF in our study not only underscores the preventive potential of prolonged lactation but also provides a quantifiable framework for public health initiatives aimed at elevating breastfeeding practices to reduce the burden of IHD among postmenopausal women.
Despite valuable strengths, it is important to acknowledge the limitations. First, we excluded females who had developed IHD prior to the set baseline age, which may introduce a potential bias towards those who were older at baseline. Second, since the information on lactation duration was collected through self-reported questionnaires, the possibility of recall bias cannot be disregarded. Third, although comprehensive adjustment has been made for a wide range of covariates, the influence of certain confounding factors, such as the family history of IHD, dietary variables were not taken into account, limiting our ability to establish causality. Important data on pregnancy complications, such as pre-eclampsia or gestational diabetes, that may affect lactation success, were not captured either. Fourth, our study is also unable to assess the risk of IHD during the childbearing years, restricting our findings to a postmenopausal cohort.
In conclusion, our study indicates that lactation durations of at least six months may be beneficial in reducing the risk of IHD, especially for those with low SES and urban residence. These findings underscore the positive population-level impact achievable through the promotion of extended breastfeeding practices, aligning with the existing guidelines that advocate for prolonged optimal breastfeeding. Public health initiatives should prioritize the enhancement of breastfeeding support structures, particularly targeting urban women of low SES, to facilitate these recommended lactation durations. Such targeted strategies have the potential to a decrease in IHD burden, providing long-term health benefits and reducing economic burdens associated with cardiovascular diseases.
Data availability
This research has been conducted using the China Kadoorie Biobank (CKB) resource (www.ckbiobank.org). The raw China Kadoorie Biobank data underlying this article can be accessed via https://www.ckbiobank.org/CKBDataAccess, following the institution’s data-access policies. Preliminary event adjudication data are not publicly available. Publication of results does not require or imply approval by the membership of the CKB Collaborative Group. The source data for Figs. 1–3 can be found in Supplementary Data 3, and the source data for Supplementary Figs. S3-S5 can be found in Supplementary Data 4.
Code availability
The codes of the R statistical software (version 4.2.2) used for the data analyses in this study have been independently reviewed and checked for quality control. Since the code is only meaningful for those with access to the CKB dataset, it can be provided upon request to researchers with access to the dataset by contacting the corresponding author. Access to the code will be granted for academic use within four weeks of application.
References
Roth, G. A. et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 76, 2982–3021 (2020).
India State-Level Disease Burden Initiative CVD Collaborators. The changing patterns of cardiovascular diseases and their risk factors in the states of India: the Global Burden of Disease Study 1990-2016. Lancet Glob. Health 6, e1339-e1351 (2018).
GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396, 1204–1222 (2020).
Zhou, M. et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 394, 1145–1158 (2019).
Wang, W. et al. Mortality and years of life lost of cardiovascular diseases in China, 2005-2020: Empirical evidence from national mortality surveillance system. Int. J. Cardiol. 340, 105–112 (2021).
China, O. O. T. S. C. O. Notice of the General Office of the State Council on printing and distributing China’s Medium and Long-term Plan for Prevention and Treatment of Chronic Diseases (2017–2025), http://www.gov.cn/zhengce/content/2017-02/14/content_5167886.htm (Jan 22, 2017).
Wang, B. et al. Spatiotemporal variations in ischemic heart disease mortality and related risk factors in China between 2010 and 2015: a multilevel analysis. BMC Public Health 21, 9 (2021).
Wei, D. et al. Age-period-cohort analysis of ischemic heart disease morbidity and mortality in China, 1990-2019. Circ. J. Off. J. Jpn. Circ. Soc. 86, 1437–1443 (2022).
Vogel, B. et al. The Lancet women and cardiovascular disease Commission: reducing the global burden by 2030. Lancet 397, 2385–2438 (2021).
Mendirichaga, R. & Jacobs, A. K. Sex differences in ischemic heart disease-the paradox persists. JAMA Cardiol. 5, 754–756 (2020).
Vaccarino, V. et al. Ischaemic heart disease in women: are there sex differences in pathophysiology and risk factors? Position paper from the working group on coronary pathophysiology and microcirculation of the European Society of Cardiology. Cardiovasc. Res. 90, 9–17 (2011).
Shufelt, C., Waldman, T., Wang, E. & Merz, C. N. Female-specific factors for IHD: across the reproductive lifespan. Curr. Atherosclerosis Rep. 17, 481 (2015).
Nguyen, B. et al. Breastfeeding and cardiovascular disease hospitalization and mortality in parous women: evidence From a Large Australian Cohort Study. J. Am. Heart Assoc. 8, e011056 (2019).
Zachou, G., Armeni, E. & Lambrinoudaki, I. Lactation and maternal cardiovascular disease risk in later life. Maturitas 122, 73–79 (2019).
Victora, C. G. et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet 387, 475–490 (2016).
Smith, G. N. & Dayan, N. Management of cardiac and cardiovascular dysfunction postpartum should include support to initiate and continue breastfeeding. Eur. J. Prevent. Cardiol. 31, e45-e46 (2024).
Gallagher, L. G. et al. Reproductive history and mortality from cardiovascular disease among women textile workers in Shanghai, China. Int. J. Epidemiol. 40, 1510–1518 (2011).
Tschiderer, L. et al. Breastfeeding is associated with a reduced maternal cardiovascular risk: Systematic review and meta‐analysis involving data from 8 studies and 1 192 700 parous women. J. Am. Heart Assoc. 11, e022746 (2022).
Natland Fagerhaug, T. et al. A prospective population-based cohort study of lactation and cardiovascular disease mortality: the HUNT study. BMC Public Health 13, 1070 (2013).
Peters, S. A. et al. Breastfeeding and the risk of maternal cardiovascular disease: a prospective study of 300 000 Chinese women. J. Am. Heart Assoc. 6, e006081 (2017).
Schultz, W. M. et al. Socioeconomic status and cardiovascular outcomes: challenges and interventions. Circulation 137, 2166–2178 (2018).
Alston, L., Jacobs, J., Allender, S. & Nichols, M. A comparison of the modelled impacts on CVD mortality if attainment of public health recommendations was achieved in metropolitan and rural Australia. Public Health Nutr. 23, 339–347 (2020).
Thompson, F. et al. Potentially preventable dementia in a First Nations population in the Torres Strait and Northern Peninsula Area of North Queensland, Australia: a cross sectional analysis using population attributable fractions. Lancet Region. Health West. Pac. 26, 100532 (2022).
Saadeh, M. R. A new global strategy for infant and young child feeding. Forum Nutr. 56, 236–238 (2003).
Chinese Nutrition Society. China Infant Feeding Guideline (2022), https://www.cnsoc.org/learnnews/2622002016.html (June 1st, 2022).
China Development Research Foundation. Report on National Survey into Factors Influencing Breastfeeding, https://cdrf-en.cdrf.org.cn/jjhdt/5273.htm (January 25, 2022).
Chinese Nutrition Society. Scientific Research Report on dietary guidelines for Chinese residents (2021), http://dg.cnsoc.org (March 8th, 2022).
Chen, Z. et al. China Kadoorie Biobank of 0.5 million people: survey methods, baseline characteristics and long-term follow-up. Int. J. Epidemiol. 40, 1652–1666 (2011).
Chen, Z. et al. Cohort profile: the Kadoorie Study of Chronic Disease in China (KSCDC). Int. J. Epidemiol. 34, 1243–1249 (2005).
Li, L. M. et al. [The China Kadoorie Biobank: related methodology and baseline characteristics of the participants]. Zhonghua Liu Xing Bing Xue Za Zhi 33, 249–255 (2012).
The 2023 Chinese menopause symptom management and menopausal hormone therapy guidelines. Zhonghua Fu Chan Ke Za Zhi 58, 4–21, https://doi.org/10.3760/cma.j.cn112141-20221118-00706 (2023).
Lee, S. Y., Kim, M. T., Kim, S. W., Song, M. S. & Yoon, S. J. Effect of lifetime lactation on breast cancer risk: a Korean women’s cohort study. Int. J. cancer 105, 390–393 (2003).
Brämer, G. R. International statistical classification of diseases and related health problems. Tenth revision. World Health Stat. Q. 41, 32–36 (1988).
Du, H. et al. Physical activity and sedentary leisure time and their associations with BMI, waist circumference, and percentage body fat in 0.5 million adults: the China Kadoorie Biobank study. Am. J. Clin. Nutr. 97, 487–496 (2013).
Ainsworth, B. E. et al. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med. Sci. Sports Exerc. 43, 1575–1581 (2011).
Gao, M. et al. Metabolically healthy obesity, transition to unhealthy metabolic status, and vascular disease in Chinese adults: a cohort study. PLoS Med. 17, e1003351 (2020).
Yang, R. et al. Modification effect of ideal cardiovascular health metrics on genetic association with incident heart failure in the China Kadoorie Biobank and the UK Biobank. BMC Med. 19, 259 (2021).
Jia, W. et al. Standards of medical care for type 2 diabetes in China 2019. Diab./Metab. Res. Rev. 35, e3158 (2019).
Center, C. et al. National guideline for hypertension management in China (2019). Zhonghua Xin Xue Guan Bing. Za Zhi 48, 10–46 (2020).
Qu, X. et al. Association between age at first childbirth and type 2 diabetes in Chinese women. J. Diab. Investig. 11, 223–231 (2020).
Muka, T. et al. Association of age at onset of menopause and time since onset of menopause with cardiovascular outcomes, intermediate vascular traits, and all-cause mortality: a systematic review and meta-analysis. JAMA Cardiol. 1, 767–776 (2016).
Melendez-Torres, G. et al. Measurement invariance properties and external construct validity of the short Warwick-Edinburgh mental wellbeing scale in a large national sample of secondary school students in Wales. Health Qual. Life Outcomes 17, 1–9 (2019).
Collins, L. M. & Lanza, S. T. Latent Class and Latent Transition Analysis: with Applications in the Social, Behavioral, and Health Sciences. Vol. 718 (John Wiley & Sons, 2009).
Lowthian, E. et al. Using latent class analysis to explore complex associations between socioeconomic status and adolescent health and well-being. J. Adolesc. Health 69, 774–781 (2021).
Linzer, D. A. & Lewis, J. B. poLCA: An R package for polytomous variable latent class analysis. J. Stat. Softw. 42, 1–29 (2011).
Naz, S., Page, A., Agho, K. E. J. G. H. R. & Policy. Attributable risk and potential impact of interventions to reduce household air pollution associated with under-five mortality in South Asia. Glob. Health Res. Policy 3, 1–9 (2018).
Mitsakakis, N., Wijeysundera, H. C. & Krahn, M. Beyond case fatality rate: using potential impact fraction to estimate the effect of increasing treatment uptake on mortality. BMC Med. Res. Methodol. 13, 109 (2013).
Lionello, L., Counil, E. & Henry, E. Ranking the burden of disease attributed to known risk factors. Preprint at https://hal.science/hal-03330085/ (2021).
Efron, B. & Tibshirani, R. J. An Introduction to the Bootstrap. (CRC press, 1994).
Peters, S. A. et al. Parity, breastfeeding and risk of coronary heart disease: a pan-European case-cohort study. Eur. J. Prevent. Cardiol. 23, 1755–1765 (2016).
Sidebottom, A. C., Brown, J. E. & Jacobs, D. R. Jr Pregnancy-related changes in body fat. Eur. J. Obstet. Gynecol. Reprod. Biol. 94, 216–223 (2001).
Magee, L. A., Abalos, E., von Dadelszen, P., Sibai, B. & Walkinshaw, S. A. Control of hypertension in pregnancy. Curr. Hypertens. Rep. 11, 429–436 (2009).
Lain, K. Y. & Catalano, P. M. Metabolic changes in pregnancy. Clin. Obstet. Gynecol. 50, 938–948 (2007).
Stuebe, A. M. & Rich-Edwards, J. W. The reset hypothesis: lactation and maternal metabolism. Am. J. Perinatol. 26, 81–88 (2009).
Natland, S. T., Nilsen, T. I., Midthjell, K., Andersen, L. F. & Forsmo, S. Lactation and cardiovascular risk factors in mothers in a population-based study: the HUNT-study. Int. Breastfeed. J. 7, 8 (2012).
Baker, J. L., Michaelsen, K. F., Sørensen, T. I. & Rasmussen, K. M. High prepregnant body mass index is associated with early termination of full and any breastfeeding in Danish women. Am. J. Clin. Nutr. 86, 404–411 (2007).
Nommsen-Rivers, L. A., Chantry, C. J., Peerson, J. M., Cohen, R. J. & Dewey, K. G. Delayed onset of lactogenesis among first-time mothers is related to maternal obesity and factors associated with ineffective breastfeeding. Am. J. Clin. Nutr. 92, 574–584 (2010).
Light, K. C. et al. Oxytocin responsivity in mothers of infants: a preliminary study of relationships with blood pressure during laboratory stress and normal ambulatory activity. Health Psychol. 19, 560–567 (2000).
Jonas, W. et al. Short- and long-term decrease of blood pressure in women during breastfeeding. Breastfeed. Med. 3, 103–109 (2008).
Augoulea, A. et al. Breastfeeding is associated with lower subclinical atherosclerosis in postmenopausal women. Gynecol. Endocrinol. 36, 796–799 (2020).
Peters, S. A. E. et al. Breastfeeding and the risk of maternal cardiovascular disease: a prospective study of 300 000 Chinese Women. J. Am. Heart Assoc. 6, https://doi.org/10.1161/jaha.117.006081 (2017).
Koski-Rahikkala, H., Pouta, A., Pietiläinen, K. & Hartikainen, A. L. Does parity affect mortality among parous women? J. Epidemiol. Community Health 60, 968–973 (2006).
Okamoto, K. et al. Menstrual and reproductive factors for subarachnoid hemorrhage risk in women: a case-control study in Nagoya, Japan. Stroke 32, 2841–2844 (2001).
Bai, J., Wong, F. W., Bauman, A. & Mohsin, M. Parity and pregnancy outcomes. Am. J. Obstet. Gynecol. 186, 274–278 (2002).
Mazariegos, M. et al. Lactation and maternal risk of diabetes: evidence from the Mexican Teachers’ Cohort. Matern. Child Nutr. 15, e12880 (2019).
Acknowledgements
We thank all CKB participants, project staff, the China National Center for Disease Control and Prevention and its regional offices (for access to death and disease registries) and the Chinese National Health Insurance scheme (for linkage to hospitalization data). We appreciate CKB collaborative group providing data for this study (Application number: DAR-2020-00212). Funding for the development of and maintenance of the CKB resource has been received from the British Heart Foundation, Cancer Research UK, Chinese Ministry of Science and Technology, Chinese National Natural Science Foundation, the Kadoorie Charitable Foundation in Hong Kong, Medical Research Council UK and the Wellcome Trust. Unless specified elsewhere, these funders have not supported the research work required for the preparation of this paper.
Author information
Authors and Affiliations
Contributions
P.S. designed the study. L.H. managed, analyzed the data. L.H., S.S., and K.L. prepared the first draft. L.H., S.S., K.L., W.S., and W.L. reviewed and edited the manuscript, with comments from C.Y., X.L., and P.S. All authors were involved in revising the paper, had full access to the data and gave final approval of the submitted versions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Medicine thanks Graeme N. Smith and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. [Peer review reports are available].
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hou, L., Shan, S., Lu, K. et al. Lactation duration and ischemic heart disease among parous postmenopausal females from a prospective cohort study. Commun Med 5, 86 (2025). https://doi.org/10.1038/s43856-025-00806-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43856-025-00806-w