Abstract
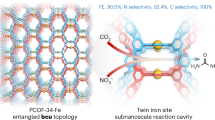
The synthesis of urea by the electrochemical co-reduction of CO2 and nitrate is a crucial and challenging task. Catalysts typically suffer from either low Faradaic efficiency (FE) or inadequate current density, leading to a restricted yield rate of urea. Here we report ultrasmall γ-Fe2O3 nanoparticles (<2 nm) encapsulated in the pores of a conductive (40 S cm−1) metal–organic framework Ni-HITP (HITP = 2,3,6,7,10,11-hexaaminotriphenylene), resulting in a composite material, γ-Fe2O3@Ni-HITP. Under neutral conditions, γ-Fe2O3@Ni-HITP exhibited a state-of-art electrocatalytic performance for urea synthesis through the co-reduction of CO2 and nitrate in CO2-saturated 1 M KHCO3 and 0.1 M KNO3 aqueous solutions, achieving a FEurea of 67.2(6)%, a current density of −90 mA cm−2 and an high yield rate of \(20.4(2)\,{\mathrm{g}}\,{\mathrm{h}}^{-1}\,{\mathrm{g}}_{\mathrm{cat}}^{-1}\) (7.7(1) mg h−1 cm−2), which is about five times higher than the rates of previously reported catalysts. No degradation was observed over 150 h of continuous operation at such a high yield rate. Enlarging the electrode area by 125 times yielded about 1.05(4) g of high-purity urea over 8 h. A mechanistic study revealed that Fe(III) ions in the γ-Fe2O3 nanoparticles exhibit high activity, generating the key intermediates *NH2 and *COOH. Furthermore, pairs of adjacent Fe(III) ions in the γ-Fe2O3 nanoparticles can act as highly active catalytic sites for catalysing the C–N coupling between *NH2 and *COOH, resulting in the formation of the subsequent key intermediate *CONH2, thereby contributing to the exceptionally high performance of γ-Fe2O3@Ni-HITP for urea production.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data supporting the finding of the study are available in the paper and its Supplementary Information. Source data are provided with this paper.
References
Xia, M. et al. Solar urea: towards a sustainable fertilizer industry. Angew. Chem. Int. Ed. 61, e202110158 (2022).
Lim, J., Fernández, C. A., Lee, S. W. & Hatzell, M. C. Ammonia and nitric acid demands for fertilizer use in 2050. ACS Energy Lett. 6, 3676–3685 (2021).
Li, J., Zhang, Y., Kuruvinashetti, K. & Kornienko, N. Construction of C–N bonds from small-molecule precursors through heterogeneous electrocatalysis. Nat. Rev. Chem. 6, 303–319 (2022).
Giddey, S., Badwal, S. P. S. & Kulkarni, A. Review of electrochemical ammonia production technologies and materials. Int. J. Hydrogen Energy 38, 14576–14594 (2013).
Tang, C., Zheng, Y., Jaroniec, M. & Qiao, S. Z. Electrocatalytic refinery for sustainable production of fuels and chemicals. Angew. Chem. Int. Ed. 60, 19572–19590 (2021).
Wei, X. et al. Oxygen vacancy-mediated selective C–N coupling toward electrocatalytic urea synthesis. J. Am. Chem. Soc. 144, 11530–11535 (2022).
Geng, J. et al. Ambient electrosynthesis of urea with nitrate and carbon dioxide over iron-based dual-sites. Angew. Chem. Int. Ed. 62, e202210958 (2023).
Lv, C. et al. A defect engineered electrocatalyst that promotes high-efficiency urea synthesis under ambient conditions. ACS Nano 16, 8213–8222 (2022).
Leverett, J. et al. Tuning the coordination structure of Cu–N–C single atom catalysts for simultaneous electrochemical reduction of CO2 and NO3− to urea. Adv. Energy Mater. 12, 2201500 (2022).
Huang, Y. et al. Unveiling the quantification minefield in electrocatalytic urea synthesis. Chem. Eng. J. 453, 139836–139842 (2023).
Zhang, S. et al. High-efficiency electrosynthesis of urea over bacterial cellulose regulated Pd–Cu bimetallic catalyst. EES Catal. 1, 45–53 (2023).
Zhang, X. et al. Identifying and tailoring C−N coupling site for efficient urea synthesis over diatomic Fe−Ni catalyst. Nat. Commun. 13, 5337–5345 (2022).
Zhao, Y. et al. Efficient urea electrosynthesis from carbon dioxide and nitrate via alternating Cu–W bimetallic C–N coupling sites. Nat. Commun. 14, 4491–4502 (2023).
Li, N. et al. Metalphthalocyanine frameworks grown on TiO2 nanotubes for synergistically and efficiently electrocatalyzing urea production from CO2 and nitrate. Sci. China Chem. 66, 1417–1424 (2023).
Meng, N. et al. Oxide-derived core–shell Cu@Zn nanowires for urea electrosynthesis from carbon dioxide and nitrate in water. ACS Nano 16, 9095–9104 (2022).
Lv, C. et al. Selective electrocatalytic synthesis of urea with nitrate and carbon dioxide. Nat. Sustain. 4, 868–876 (2021).
Tu, X. et al. A universal approach for sustainable urea synthesis via intermediate assembly at the electrode/electrolyte interface. Angew. Chem. Int. Ed. 63, e202317087 (2024).
Yang, Q., Xu, Q. & Jiang, H. L. Metal–organic frameworks meet metal nanoparticles: synergistic effect for enhanced catalysis. Chem. Soc. Rev. 46, 4774–4808 (2017).
Gao, C., Lyu, F. & Yin, Y. Encapsulated metal nanoparticles for catalysis. Chem. Rev. 121, 834–881 (2021).
Lin, L. et al. Rational design and synthesis of two-dimensional conjugated metal–organic polymers for electrocatalysis applications. Chem 8, 1822–1854 (2022).
Sheberla, D. et al. High electrical conductivity in Ni3(2,3,6,7,10,11-hexaiminotriphenylene)2, a semiconducting metal–organic graphene analogue. J. Am. Chem. Soc. 136, 8859–8862 (2014).
Xu, J. et al. Breaking local charge symmetry of iron single atoms for efficient electrocatalytic nitrate reduction to ammonia. Angew. Chem. Int. Ed. 62, e202308044 (2023).
Gu, J., Hsu, C.-S., Bai, L., Chen, H. M. & Hu, X. Atomically dispersed Fe3+ sites catalyze efficient CO2 electroreduction to CO. Science 364, 1091–1094 (2019).
Zhao, D. et al. Atomic-level engineering Fe1N2O2 interfacial structure derived from oxygen-abundant metal–organic frameworks to promote electrochemical CO2 reduction. Energy Environ. Sci. 15, 3795–3804 (2022).
Holder, C. F. & Schaak, R. E. Tutorial on powder X-ray diffraction for characterizing nanoscale materials. ACS Nano 13, 7359–7365 (2019).
Ke, F. et al. Facile fabrication of magnetic metal–organic framework nanocomposites for potential targeted drug delivery. J. Mater. Chem. 21, 3843–3848 (2011).
Louie, M. W. & Bell, A. T. An investigation of thin-film Ni–Fe oxide catalysts for the electrochemical evolution of oxygen. J. Am. Chem. Soc. 135, 12329–12337 (2013).
Wei, C. et al. Approaches for measuring the surface areas of metal oxide electrocatalysts for determining their intrinsic electrocatalytic activity. Chem. Soc. Rev. 48, 2518–2534 (2019).
Xu, Z., Liang, Z., Guo, W. & Zou, R. In situ/operando vibrational spectroscopy for the investigation of advanced nanostructured electrocatalysts. Coord. Chem. Rev. 436, 213824–213850 (2021).
Perez-Gallent, E., Figueiredo, M. C., Calle-Vallejo, F. & Koper, M. T. Spectroscopic observation of a hydrogenated CO dimer intermediate during CO reduction on Cu(100) electrodes. Angew. Chem. Int. Ed. 56, 3621–3624 (2017).
Chen, C. et al. Coupling N2 and CO2 in H2O to synthesize urea under ambient conditions. Nat. Chem. 12, 717–724 (2020).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–541 (2005).
Chen, T. et al. Continuous electrical conductivity variation in M3(hexaiminotriphenylene)2 (M = Co, Ni, Cu) MOF alloys. J. Am. Chem. Soc. 142, 12367–12373 (2020).
Hmadeh, M. et al. New porous crystals of extended metal–catecholates. Chem. Mater. 24, 3511–3513 (2012).
Kang, Y. S., Risbud, S., Rabolt, J. F. & Stroeve, P. Synthesis and characterization of nanometer-size Fe3O4 and γ-Fe2O3 particles. Chem. Mater. 8, 2209–2211 (1996).
Ding, P. et al. Elucidating the roles of Nafion/solvent formulations in copper-catalyzed CO2 electrolysis. ACS Catal. 13, 5336–5347 (2023).
Zhu, D., Zhang, L., Ruther, R. E. & Hamers, R. J. Photo-illuminated diamond as a solid-state source of solvated electrons in water for nitrogen reduction. Nat. Mater. 12, 836–841 (2013).
Chen, G. et al. Electrochemical reduction of nitrate to ammonia via direct eight-electron transfer using a copper-molecular solid catalyst. Nat. Energy 5, 605–613 (2020).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Hamann, D. R., Schlüter, M. & Chiang, C. Norm-conserving pseudopotentials. Phys. Rev. Lett. 43, 1494–1497 (1979).
Acknowledgements
This work was supported by the National Key Research and Development Program of China (grant number 2021YFA1500401 to P.-Q.L.), the National Natural Science Foundation of China (grant number 21890380 to X.-M.C., grant numbers 22371304 and 21821003 to P.-Q.L. and grant number 223B2123 to Z.-H.Z.), the Special Fund Project for Science and Technology Innovation Strategy of Guangdong Province (grant number STKJ2023078 to X.-M.C. and P.-Q.L.) and the Guangzhou Science and Technology Program (grant number SL2023A04J01767 to P.-Q.L.).
Author information
Authors and Affiliations
Contributions
P.-Q.L. designed the research. D.-S.H. performed the syntheses and measurements. X.-F.Q. performed the PDFT calculations. Y.H., M.M. and L.L. performed the STEM measurements. D.-S.H., J.-R.H., Z.-H.Z., P.-Q.L. and X.-M.C. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Marta Hatzell, Licheng Sun, Haihui Wang, Haimin Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Alison Stoddart, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–63 and Tables 1–6.
Source data
Source Data Fig. 2
Source data.
Source Data Fig. 3
Source data.
Source Data Fig. 4
Source data.
Source Data Fig. 5
Source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, DS., Qiu, XF., Huang, JR. et al. Electrosynthesis of urea by using Fe2O3 nanoparticles encapsulated in a conductive metal–organic framework. Nat. Synth 3, 1404–1413 (2024). https://doi.org/10.1038/s44160-024-00603-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-024-00603-8
This article is cited by
-
Floatable artificial leaf to couple oxygen-tolerant CO2 conversion with water purification
Nature Communications (2025)
-
Acetonitrile-driven universal CO production across diverse metal catalysts
Science China Chemistry (2025)
-
Recent progress in electrocatalytic C-N coupling of CO2 and inorganic N-containing small molecules
Science China Chemistry (2025)