Abstract
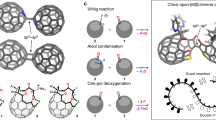
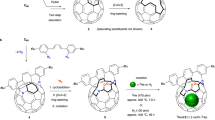
The discovery of buckminsterfullerene (C60) marked a milestone in exploring three-dimensional carbon materials. However, with the exponential increase in the number of isomers for higher fullerenes, it has become challenging to realize the enrichment of the isomers by molecular recognition. Here we report two pseudo-cubic metal–organic cages, T and S4, with distinct cavity microenvironments, that showcase recognition specificity towards higher fullerene isomers. Compared with cage T, a symmetry shift from S4 to C2 emerges upon encapsulating an ellipsoidal D2-C76 guest, owing to the precise shape matching that curtails guest rotation. Furthermore, the low-symmetry cage S4 shows exceptional sensitivity in distinguishing between closely related isomers, such as a pair of C2v-symmetric C78 isomers, and shows promise for the selective enrichment of higher fullerenes. The approach of reducing symmetry positions metal–organic cages as promising candidates for encapsulating and identifying a broader spectrum of fullerene isomers, paralleling the specificity observed in biological systems.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Crystallographic data are available free of charge from the Cambridge Crystallographic Data Centre under reference CCDC no. 2278920 (C2v-C78), 2278921 (C2v′-C78), 2307962 (C3-1), 2307963 (P-1), 2307964(4), 2307965 (T-3), 2307966 (C60⊂S4-4) and 2307967 (C70⊂S4-4). These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via https://www.ccdc.cam.ac.uk/structures/. All other data supporting the findings of this study are available in the Article or the Supplementary Information.
References
Ringe, D. & Petsko, G. A. How enzymes work. Science 320, 1428–1429 (2008).
Lehn, J.-M. Supramolecular chemistry: receptors, catalysts, and carriers. Science 227, 849–856 (1985).
Fujita, D. et al. Self-assembly of tetravalent Goldberg polyhedra from 144 small components. Nature 540, 563–566 (2016).
Cullen, W., Misuraca, M. C., Hunter, C. A., Williams, N. H. & Ward, M. D. Highly efficient catalysis of the Kemp elimination in the cavity of a cubic coordination cage. Nat. Chem. 8, 231–236 (2016).
Ke, X. S., Kim, T., Lynch, V. M., Kim, D. & Sessler, J. L. Flattened calixarene-like cyclic BODIPY array: a new photosynthetic antenna model. J. Am. Chem. Soc. 139, 13950–13956 (2017).
Wu, T. et al. Supramolecular triangular orthobicupola: self-assembly of a giant Johnson solid J27. Chem 7, 2429–2441 (2021).
Lu, Y.-L. et al. A robust protein-mimicking metallo-amine cage showing proton-driven allostery with water as the effector. Chem 9, 2144–2160 (2023).
Wu, K. et al. Systematic construction of progressively larger capsules from a fivefold linking pyrrole-based subcomponent. Nat. Synth. 2, 789–797 (2023).
Kroto, H. W., Heath, J. R., O’Brien, S. C., Curl, R. F. & Smalley, R. E. C60: buckminsterfullerene. Nature 318, 162–163 (1985).
Effing, J. et al. C60 and C70 in a basket?—Investigations of mono- and multilayers from azacrown compounds and fullerenes. Angew. Chem. Int. Ed. 31, 1599–1602 (1992).
Boyd, P. D. W. & Reed, C. A. Fullerene–porphyrin constructs. Acc. Chem. Res. 38, 235–242 (2005).
Kawase, T. & Kurata, H. Ball-, bowl-, and belt-shaped conjugated systems and their complexing abilities: exploration of the concave–convex π–π interaction. Chem. Rev. 106, 5250–5273 (2006).
Tashiro, K. & Aida, T. Metalloporphyrin hosts for supramolecular chemistry of fullerenes. Chem. Soc. Rev. 36, 189–197 (2007).
Canevet, D., Pérez, E. M. & Martín, N. Wraparound hosts for fullerenes: tailored macrocycles and cages. Angew. Chem. Int. Ed. 50, 9248–9259 (2011).
Shi, Y. et al. Selective extraction of C70 by a tetragonal prismatic porphyrin cage. J. Am. Chem. Soc. 140, 13835–13842 (2018).
Xu, Y.-Y. et al. Flexible decapyrrylcorannulene hosts. Nat. Commun. 10, 485 (2019).
Matsumoto, K. et al. A peanut-shaped polyaromatic capsule: solvent-dependent transformation and electronic properties of a non-contacted fullerene dimer. Angew. Chem. Int. Ed. 58, 8463–8467 (2019).
Chang, X. et al. Self-assembled perylene bisimide-cored trigonal prism as an electron-deficient host for C60 and C70 driven by ‘like dissolves like’. J. Am. Chem. Soc. 142, 15950–15960 (2020).
Bera, S., Das, S., Melle-Franco, M. & Mateo-Alonso, A. An organic molecular nanobarrel that hosts and solubilizes C60. Angew. Chem. Int. Ed. 62, e202216540 (2023).
Atwood, J. L., Koutsantonis, G. A. & Raston, C. L. Purification of C60 and C70 by selective complexation with calixarenes. Nature 368, 229–231 (1994).
Suzuki, T., Nakashima, K. & Shinkai, S. Very convenient and efficient purification method for fullerene (C60) with 5,11,17,23,29,35,41,47-octa-tert-bultylcalix[8]arene-49,50,51,52,53,54,55,56-octol. Chem. Lett. 23, 699–702 (1994).
Huang, H. et al. Third-order nonlinear optical response of fullerenes as a function of the carbon cage size (C60 to C96) at 0.532 μm. J. Phys. Chem. B 102, 61–66 (1998).
Umeyama, T. & Imahori, H. Isomer effects of fullerene derivatives on organic photovoltaics and perovskite solar cells. Acc. Chem. Res. 52, 2046–2055 (2019).
Li, T. et al. A new interleukin-13 amino-coated gadolinium metallofullerene nanoparticle for targeted MRI detection of glioblastoma tumor cells. J. Am. Chem. Soc. 137, 7881–7888 (2015).
Puente Santiago, A. R. et al. A new class of molecular electrocatalysts for hydrogen evolution: catalytic activity of M3N@C2n (2n = 68, 78, and 80) fullerenes. J. Am. Chem. Soc. 143, 6037–6042 (2021).
Ettl, R., Chao, I., Diederich, F. & Whetten, R. L. Isolation of C76, a chiral (D2) allotrope of carbon. Nature 353, 149–153 (1991).
Hawkins, J. M. & Meyer, A. Optically active carbon: kinetic resolution of C76 by asymmetric osmylation. Science 260, 1918–1920 (1993).
Shoji, Y., Tashiro, K. & Aida, T. One-pot enantioselective extraction of chiral fullerene C76 using a cyclic host carrying an asymmetrically distorted, highly π-basic porphyrin module. J. Am. Chem. Soc. 132, 5928–5929 (2010).
Inokuma, Y., Arai, T. & Fujita, M. Networked molecular cages as crystalline sponges for fullerenes and other guests. Nat. Chem. 2, 780–783 (2010).
García-Simón, C., Costas, M. & Ribas, X. Metallosupramolecular receptors for fullerene binding and release. Chem. Soc. Rev. 45, 40–62 (2016).
Kishi, N. et al. Facile catch and release of fullerenes using a photoresponsive molecular tube. J. Am. Chem. Soc. 135, 12976–12979 (2013).
Sanchez-Molina, I. et al. Self-assembly, host–guest chemistry, and photophysical properties of subphthalocyanine-based metallosupramolecular capsules. J. Am. Chem. Soc. 135, 10503–10511 (2013).
Liu, K. S., Li, M. J., Lai, C. C. & Chiu, S. H. Incarceration of higher-order fullerenes within cyclotriveratrylene-based hemicarcerands allows selective isolation of C76, C78, and C84 from a commercial fullerene mixture. Chem. Eur. J. 22, 17468–17476 (2016).
Sun, W. D. et al. Self-assembled carcerand-like cage with a thermoregulated selective binding preference for purification of high-purity C60 and C70. J. Org. Chem. 83, 14667–14675 (2018).
Kawano, S.-I., Fukushima, T. & Tanaka, K. Specific and oriented encapsulation of fullerene C70 into a supramolecular double-decker cage composed of shape-persistent macrocycles. Angew. Chem. Int. Ed. 57, 14827–14831 (2018).
García-Simón, C. et al. Sponge-like molecular cage for purification of fullerenes. Nat. Commun. 5, 5557 (2014).
Meng, W. et al. A self-assembled M8L6 cubic cage that selectively encapsulates large aromatic guests. Angew. Chem. Int. Ed. 50, 3479–3483 (2011).
Ubasart, E. et al. Straightforward supramolecular purification of C84 from a fullerene extract. Org. Chem. Front. 8, 4101–4105 (2021).
Shoji, Y., Tashiro, K. & Aida, T. Selective extraction of higher fullerenes using cyclic dimers of zinc porphyrins. J. Am. Chem. Soc. 126, 6570–6571 (2004).
Leonhardt, V., Fimmel, S., Krause, A.-M. & Beuerle, F. A covalent organic cage compound acting as a supramolecular shadow mask for the regioselective functionalization of C60. Chem. Sci. 11, 8409–8415 (2020).
Ubasart, E. et al. A three-shell supramolecular complex enables the symmetry-mismatched chemo- and regioselective bis-functionalization of C60. Nat. Chem. 13, 420–427 (2021).
Lu, Z. et al. Enantioselective fullerene functionalization through stereochemical information transfer from a self-assembled cage. Nat. Chem. 15, 405–412 (2023).
Rizzuto, F. J., Wood, D. M., Ronson, T. K. & Nitschke, J. R. Tuning the redox properties of fullerene clusters within a metal-organic capsule. J. Am. Chem. Soc. 139, 11008–11011 (2017).
Hasegawa, S. et al. Long-lived C60 radical anion stabilized inside an electron-deficient coordination cage. J. Am. Chem. Soc. 143, 9718–9723 (2021).
Tsutsui, T., Catti, L., Yoza, K. & Yoshizawa, M. An atropisomeric M2L4 cage mixture displaying guest-induced convergence and strong guest emission in water. Chem. Sci. 11, 8145–8150 (2020).
Martínez-Agramunt, V. & Peris, E. Photocatalytic properties of a palladium metallosquare with encapsulated fullerenes via singlet oxygen generation. Inorg. Chem. 58, 11836–11842 (2019).
Banerjee, R., Chakraborty, D., Jhang, W.-T., Chan, Y.-T. & Mukherjee, P. S. Structural switching of a distorted trigonal metal–organic cage to a tetragonal cage and singlet oxygen mediated oxidations. Angew. Chem. Int. Ed. 62, e202305338 (2023).
Sun, Q. F., Sato, S. & Fujita, M. An M12(L1)12(L2)12 cantellated tetrahedron: a case study on mixed-ligand self-assembly. Angew. Chem. Int. Ed. 53, 13510–13513 (2014).
Preston, D., Barnsley, J. E., Gordon, K. C. & Crowley, J. D. Controlled formation of heteroleptic [Pd2(La)2(Lb)2]4+ cages. J. Am. Chem. Soc. 138, 10578–10585 (2016).
Zhang, L. et al. Desymmetrized vertex design toward a molecular cage with unusual topology. Angew. Chem. Int. Ed. 59, 20846–20851 (2020).
Tarzia, A., Lewis, J. E. M. & Jelfs, K. E. High-throughput computational evaluation of low symmetry Pd2L4 cages to aid in system design. Angew. Chem. Int. Ed. 60, 20879–20887 (2021).
Tang, X., Chu, D., Gong, W., Cui, Y. & Liu, Y. Metal–organic cages with missing linker defects. Angew. Chem. Int. Ed. 60, 9099–9105 (2021).
Wang, J. H. et al. Altering the properties of spiropyran switches using coordination cages with different symmetries. J. Am. Chem. Soc. 144, 21244–21254 (2022).
Chen, B. et al. Cooperativity of steric bulk and H-bonding in coordination sphere engineering: heteroleptic Pd-II cages and bowls by design. Chem. Sci. 13, 1829–1834 (2022).
Liu, Y. et al. Controlled construction of heteroleptic [Pd2(LA)2(LB)(LC)]4+ cages: a facile approach for site-selective endo-functionalization of supramolecular cavities. Angew. Chem. Int. Ed. 62, e202217215 (2023).
Kuck, D. A facile route to benzoannelated centrotriquinanes. Angew. Chem. Int. Ed. 23, 508–509 (1984).
Markopoulos, G. et al. Tribenzotriquinacene: a versatile synthesis and C3-chiral platforms. Angew. Chem. Int. Ed. 51, 12884–12887 (2012).
Wagner, P. et al. Chiral self-sorting of giant cubic [8+12] salicylimine cage compounds. Angew. Chem. Int. Ed. 60, 8896–8904 (2021).
Benke, B. P., Kirschbaum, T., Graf, J., Gross, J. H. & Mastalerz, M. Dimeric and trimeric catenation of giant chiral [8+12] imine cubes driven by weak supramolecular interactions. Nat. Chem. 15, 413–423 (2023).
Kirchner, P. H. et al. A water-stable boronate ester cage. J. Am. Chem. Soc. 146, 5305–5315 (2024).
Beaudoin, D., Rominger, F. & Mastalerz, M. Chiral self-sorting of [2+3] salicylimine cage compounds. Angew. Chem. Int. Ed. 56, 1244–1248 (2017).
Wagner, P., Rominger, F., Gross, J. H. & Mastalerz, M. Solvent-controlled quadruple catenation of giant chiral [8+12] salicylimine cubes driven by weak hydrogen bonding. Angew. Chem. Int. Ed. 62, e202217251 (2023).
Strübe, J., Neumann, B., Stammler, H.-G. & Kuck, D. Solid-state enantiopure organic nanocubes formed by self organization of a C3-symmetrical tribenzotriquinacene. Chem. Eur. J. 15, 2256–2260 (2009).
Mughal, E. U. & Kuck, D. Merging tribenzotriquinacene with hexa-peri-hexabenzocoronene: a cycloheptatriene unit generated by Scholl reaction. Chem. Commun. 48, 8880–8882 (2012).
Qu, H. et al. Molecular face-rotating cube with emergent chiral and fluorescence properties. J. Am. Chem. Soc. 139, 18142–18145 (2017).
Li, Y. & Flood, A. H. Pure C–H hydrogen bonding to chloride ions: a preorganized and rigid macrocyclic receptor. Angew. Chem. Int. Ed. 47, 2649–2652 (2008).
Maglic, J. B. & Lavendomme, R. MoloVol: an easy-to-use program for analyzing cavities, volumes and surface areas of chemical structures. J. Appl. Crystallogr. 55, 1033–1044 (2022).
Dobashi, H., Catti, L., Tanaka, Y., Akita, M. & Yoshizawa, M. N-doping of polyaromatic capsules: small cavity modification leads to large change in host–guest interactions. Angew. Chem. Int. Ed. 59, 11881–11885 (2020).
Diederich, F. et al. Fullerene isomerism: isolation of C2v-C78 and D3-C78. Science 254, 1768–1770 (1991).
Kikuchi, K. et al. NMR characterization of isomers of C78, C82 and C84 fullerenes. Nature 357, 142–145 (1992).
Acknowledgements
This work was supported by the National Key Research and Development Program of China (grants 2022YFA1503300 and 2021YFA1500400 to Q.-F.S. and grant 2022YFB3807700 to X.L.), National Natural Science Foundation of China (grant 22201285 to X.-Q.G., grant 22171264 to Q.-F.S., grants 21925104 and 92261204 to X.L. and grant 61825107 to Q.-F.S.) and Science Foundation of Fujian Province (grant 202lJ02016 to Q.-F.S.). We thank the staff of BL17B1 beamline at National Centre for Protein Sciences Shanghai and Shanghai Synchrotron Radiation Facility (SSRF), Shanghai, People’s Republic of China, for assistance during data collection.
Author information
Authors and Affiliations
Contributions
Q.-F.S., X.L., X.-Q.G., P.Y. and L.B. conceived the project, carried out research, analysed all experiments and wrote the manuscript. L.-P.Z. performed the mass measurements. L.-X.C. and S.-J.H. contributed to X-ray crystallographic analysis. X.-F.D. contributed to the figure production. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Jonathan Charmant, Xavi Ribas, Giuseppe Trusso Sfrazzetto and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Alison Stoddart, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–162 and Tables 1–27.
Supplementary Data 1
Crystallographic data for C2v-C78 (CCDC reference 2278920).
Supplementary Data 2
Crystallographic data for C2v′-C78 (CCDC reference 2278921).
Supplementary Data 3
Crystallographic data for C3-1 (CCDC reference 2307962).
Supplementary Data 4
Crystallographic data for P-1 (CCDC reference 2307963).
Supplementary Data 5
Crystallographic data for 4 (CCDC reference 2307964).
Supplementary Data 6
Crystallographic data for T-3 (CCDC reference 2307965).
Supplementary Data 7
Crystallographic data for C60⊂S4-4 (CCDC reference 2307966).
Supplementary Data 8
Crystallographic data for C70⊂S4-4 (CCDC reference 2307967).
Source data
Source Data Fig. 2
ESI-TOF-MS of T-3.
Source Data Fig. 3
ESI-TOF-MS of S4-4 and C60⊂S4-4.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, XQ., Yu, P., Zhou, LP. et al. Low-symmetry coordination cages enable recognition specificity and selective enrichment of higher fullerene isomers. Nat. Synth 4, 359–369 (2025). https://doi.org/10.1038/s44160-024-00697-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-024-00697-0