Abstract
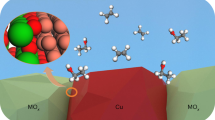
Efficient production of C2+ alcohols from the electrochemical CO2 reduction reaction (CO2RR) is of great interest. However, the CO2RR to C2+ alcohols has low selectivity and current density due to competing C2+ pathways that produce ethylene (C2H4). Here we report a stepwise precipitation and stepwise calcination strategy to create Pr–Cu oxide heterointerfaces (Pr6O11–Cu-SS) that produces an efficient CO2RR to C2+ alcohols. Pr6O11–Cu-SS exhibited high productivity and Faradaic efficiency (FE) for C2+ alcohols. At −1.08 V versus RHE, the current density and FE of C2+ alcohols reached 700 mA cm−2 and 71.3%, respectively, with the FEs of ethanol and n-propanol reaching 58.6% and 12.7%, respectively, under these conditions. The C2+ alcohols/C2H4 ratio was as high as 12:1. Experimental and theoretical studies indicated that the performance of the catalyst results from the existence of a Pr4+/Pr3+ structure in Pr6O11–Cu-SS, which is able to effectively stabilize Cuδ+/Cu0 via a unique Pr–O–Cu linkage and form stable oxide heterointerfaces. The binding strength and binding type of *CO were then altered on the heterointerfaces to form a mixed adsorption configuration, which induces asymmetric carbon–carbon coupling and selective hydrodeoxygenation to promote the generation of C2+ alcohols.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the findings of this study are available in the paper and its Supplementary Information. Source data are provided with this paper.
References
Mussatto, S. I. et al. Technological trends, global market, and challenges of bio-ethanol production. Biotechnol. Adv. 28, 817–830 (2010).
Birdja, Y. Y. et al. Advances and challenges in understanding the electrocatalytic conversion of carbon dioxide to fuels. Nat. Energy 4, 732–745 (2019).
Zhu, Q. et al. Carbon dioxide electroreduction to C2 products over copper–cuprous oxide derived from electrosynthesized copper complex. Nat. Commun. 10, 3851 (2019).
Ding, P. et al. Metal-based electrocatalytic conversion of CO2 to formic acid/formate. J. Mater. Chem. A 8, 21947–21960 (2020).
Shi, Y. et al. Unveiling hydrocerussite as an electrochemically stable active phase for efficient carbon dioxide electroreduction to formate. Nat. Commun. 11, 3415 (2020).
Calle-Vallejo, F. & Koper, M. T. M. Theoretical considerations on the electroreduction of CO to C2 species on Cu(100) electrodes. Angew. Chem. Int. Ed. 52, 7282–7285 (2013).
Lum, Y., Cheng, T., Goddard, W. A. & Ager, J. W. Electrochemical CO reduction builds solvent water into oxygenate products. J. Am. Chem. Soc. 140, 9337–9340 (2018).
Kortlever, R., Shen, J., Schouten, K. J. P., Calle-Vallejo, F. & Koper, M. T. M. Catalysts and reaction pathways for the electrochemical reduction of carbon dioxide. J. Phys. Chem. Lett. 6, 4073–4082 (2015).
Chen, C. et al. Highly efficient electroreduction of CO2 to C2+ alcohols on heterogeneous dual active sites. Angew. Chem. Int. Ed. 59, 16459–16464 (2020).
Liu, X. et al. Understanding trends in electrochemical carbon dioxide reduction rates. Nat. Commun. 8, 15438 (2017).
Liang, Y. et al. Efficient ethylene electrosynthesis through C–O cleavage promoted by water dissociation. Nat. Synth. 3, 1104–1112 (2024).
Kong, S. et al. Delocalization state-induced selective bond breaking for efficient methanol electrosynthesis from CO2. Nat. Catal. 6, 6–15 (2023).
Zhao, Z., Huang, J., Liao, P. & Chen, X. Highly efficient electroreduction of CO2 to ethanol via asymmetric C–C coupling by a metal–organic framework with heterodimetal dual sites. J. Am. Chem. Soc. 145, 26783–26790 (2023).
Liu, Z. et al. Switching CO2 electroreduction toward ethanol by delocalization state-tuned bond cleavage. J. Am. Chem. Soc. 146, 14260–14266 (2024).
Hoang, T. T. H. et al. Nanoporous copper–silver alloys by additive-controlled electrodeposition for the selective electroreduction of CO2 to ethylene and ethanol. J. Am. Chem. Soc. 140, 5791–5797 (2018).
Clark, E. L., Hahn, C., Jaramillo, T. F. & Bell, A. T. Electrochemical CO2 reduction over compressively strained CuAg surface alloys with enhanced multi-carbon oxygenate selectivity. J. Am. Chem. Soc. 139, 15848–15857 (2017).
Lee, S., Park, G. & Lee, J. Importance of Ag–Cu biphasic boundaries for selective electrochemical reduction of CO2 to ethanol. ACS Catal. 7, 8594–8604 (2017).
Hori, Y., Murata, A. & Takahashi, R. Formation of hydrocarbons in the electrochemical reduction of carbon dioxide at a copper electrode in aqueous solution. J. Chem. Soc. Faraday Trans. 1 85, 2309–2326 (1989).
Kuhl, K. P., Cave, E. R., Abram, D. N. & Jaramillo, T. F. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy Environ. Sci. 5, 7050–7059 (2012).
Wang, P. et al. Phase and structure engineering of copper tin heterostructures for efficient electrochemical carbon dioxide reduction. Nat. Commun. 9, 4933 (2018).
Zhuang, T. et al. Steering post-C–C coupling selectivity enables high efficiency electroreduction of carbon dioxide to multi-carbon alcohols. Nat. Catal. 1, 421–428 (2018).
Ma, W. et al. Electrocatalytic reduction of CO2 to ethylene and ethanol through hydrogen-assisted C–C coupling over fluorine-modified copper. Nat. Catal. 3, 478–487 (2020).
Hoang, T. T. H., Ma, S., Gold, J. I., Kenis, P. J. A. & Gewirth, A. A. Nanoporous copper films by additive-controlled electrodeposition: CO2 reduction catalysis. ACS Catal. 7, 3313–3321 (2017).
Soodi, S. et al. Selective electroreduction of CO2 to C2+ products on cobalt decorated copper catalysts. Chem. Synth. 4, 44 (2024).
Gu, Z. et al. Efficient electrocatalytic CO2 reduction to C2+ alcohols at defect-site-rich Cu surface. Joule 5, 429–440 (2021).
Xu, A. et al. Copper/alkaline earth metal oxide interfaces for electrochemical CO2-to-alcohol conversion by selective hydrogenation. Nat. Catal. 5, 1081–1088 (2022).
Sun, H. et al. Hierarchical palladium–copper–silver porous nanoflowers as efficient electrocatalysts for CO2 reduction to C2+ products. Acta Phys. -Chim. Sin. 40, 2307007 (2024).
Yang, B. et al. Electrocatalytic CO2 reduction to alcohols by modulating the molecular geometry and Cu coordination in bicentric copper complexes. Nat. Commun. 13, 5122 (2022).
Jiang, Y., Fu, H., Liang, Z., Zhang, Q. & Du, Y. Rare earth oxide based electrocatalysts: synthesis, properties and applications. Chem. Soc. Rev. 53, 714–763 (2024).
Liu, W., Bai, P., Wei, S., Yang, C. & Xu, L. Gadolinium changes the local electron densities of nickel 3d orbitals for efficient electrocatalytic CO2 reduction. Angew. Chem. Int. Ed. 61, e202201166 (2022).
Gao, Q., Zhang, W., Shi, Z., Yang, L. & Tang, Y. Structural design and electronic modulation of transition-metal-carbide electrocatalysts toward efficient hydrogen evolution. Adv. Mater. 31, 1802880 (2019).
Zheng, B. et al. Rare-earth doping in nanostructured inorganic materials. Chem. Rev. 122, 5519–5603 (2022).
Lv, J. J. et al. Microenvironment engineering for the electrocatalytic CO2 reduction reaction. Angew. Chem. Int. Ed. 61, e202207252 (2022).
Hahn, C. & Jaramillo, T. F. Using microenvironments to control reactivity in CO2 electrocatalysis. Joule 4, 292–294 (2020).
Su, L. et al. Pr6O11: temperature-dependent oxygen vacancy regulation and catalytic performance for lithium–oxygen batteries. ACS Appl. Mater. Interfaces 14, 40975–40984 (2022).
Abu-Zied, B. M. Controlled synthesis of praseodymium oxide nanoparticles obtained by combustion route: effect of calcination temperature and fuel to oxidizer ratio. Appl. Sur. Sci. 471, 246–255 (2019).
Zhang, Y. et al. Low-coordinated copper facilitates the *CH2CO affinity at enhanced rectifying interface of Cu/Cu2O for efficient CO2-to-multicarbon alcohols conversion. Nat. Commun. 15, 5172 (2024).
Feng, J. et al. Hydrophobic SiO2 armor: stabilizing Cuδ+ to enhance CO2 electroreduction toward C2+ products in strong acidic environments. ACS Nano 18, 15303–15311 (2024).
Liu, J. et al. Switching between C2+ products and CH4 in CO2 electrolysis by tuning the composition and structure of rare-earth/copper catalysts. J. Am. Chem. Soc. 145, 23037–23047 (2023).
Feng, J. et al. Improving CO2-to-C2+ product electroreduction efficiency via atomic lanthanide dopant-induced tensile-strained CuOx catalysts. J. Am. Chem. Soc. 145, 9857–9866 (2023).
Zhang, Z. et al. pH matters when reducing CO2 in an electrochemical flow cell. ACS Energy Lett. 5, 3101–3107 (2020).
Kim, J. Y. T., Sellers, C., Hao, S., Senftle, T. P. & Wang, H. Different distributions of multi-carbon products in CO2 and CO electroreduction under practical reaction conditions. Nat. Catal. 6, 1115–1124 (2023).
Peng, C. et al. (111) Facet-oriented Cu2Mg intermetallic compound with Cu3–Mg sites for CO2 electroreduction to ethanol with industrial current density. Angew. Chem. Int. Ed. 63, e202316907 (2024).
Li, H. et al. High-rate CO2 electroreduction to C2+ products over a copper–copper iodide catalyst. Angew. Chem. Int. Ed. 60, 14329–14333 (2021).
Zhuang, T. et al. Tunable CO2 electroreduction to ethanol and ethylene with controllable interfacial wettability. Nat. Commun. 14, 3575 (2023).
Sun, W. et al. V-doped Cu2Se hierarchical nanotubes enabling flow-cell CO2 electroreduction to ethanol with high efficiency and selectivity. Adv. Mater. 34, 2207691 (2022).
Wang, X. et al. Efficient electrically powered CO2-to-ethanol via suppression of deoxygenation. Nat. Energy 5, 478–486 (2020).
Li, F. et al. Cooperative CO2-to-ethanol conversion via enriched intermediates at molecule–metal catalyst interfaces. Nat. Catal. 3, 75–82 (2020).
Kim, J. Y. et al. Selective hydrocarbon or oxygenate production in CO2 electroreduction over metallurgical alloy catalysts. Nat. Synth. 3, 452–465 (2024).
Shayesteh Zeraati, A. et al. Carbon- and energy-efficient ethanol electrosynthesis via interfacial cation enrichment. Nat. Synth. 4, 75–83 (2025).
Li, J. et al. Electrochemical acetate production from high-pressure gaseous and liquid CO2. Nat. Catal. 6, 1151–1163 (2023).
Cai, Y. et al. Self-pressurizing nanoscale capsule catalysts for CO2 electroreduction to acetate or propanol. Nat. Synth. 3, 891–902 (2024).
Vos, R. E. & Koper, M. T. M. Nickel as electrocatalyst for CO(2) reduction: effect of temperature, potential, partial pressure, and electrolyte composition. ACS Catal. 14, 4432–4440 (2023).
Abdinejad, M. et al. Eliminating redox-mediated electron transfer mechanisms on a supported molecular catalyst enables CO2 conversion to ethanol. Nat. Catal. 7, 1109–1119 (2024).
Su, Z. et al. Probing the actual role and activity of oxygen vacancies in toluene catalytic oxidation: evidence from in situ XPS/NEXAFS and DFT + U calculation. ACS Catal. 13, 3444–3455 (2023).
Zhong, X. et al. Optimizing oxygen vacancies through grain boundary engineering to enhance electrocatalytic nitrogen reduction. Proc. Natl Acad. Sci. USA 120, e2306673120 (2023).
Gu, W., Liu, J., Hu, M., Wang, F. & Song, Y. La2O2CO3 encapsulated La2O3 nanoparticles supported on carbon as superior electrocatalysts for oxygen reduction reaction. ACS Appl. Mater. Interfaces 7, 26914–26922 (2015).
Zhong, X. et al. Autogenic synthesis of green- and red-emitting single-phase Pr2O2CO3 and PrO1.833 luminescent nanopowders. Inorg. Chem. 49, 10067–10073 (2010).
Zhang, H. et al. Three-dimensional inhomogeneity of zeolite structure and composition revealed by electron ptychography. Science 380, 633–638 (2023).
Zhao, Y. et al. Speciation of Cu surfaces during the electrochemical CO reduction reaction. J. Am. Chem. Soc. 142, 9735–9743 (2020).
Gunathunge, C. et al. Spectroscopic observation of reversible surface reconstruction of copper electrodes under CO2 reduction. J. Phys. Chem. C 121, 12337–12344 (2017).
Cai, R. et al. Engineering Cu(I)/Cu(0) interfaces for efficient ethanol production from CO2 electroreduction. Chem 10, 211–233 (2017).
She, X. et al. Pure-water-fed, electrocatalytic CO2 reduction to ethylene beyond 1,000 h stability at 10 Å. Nat. Energy 9, 81–91 (2024).
Tan, Y. et al. Discovery of hydrogen spillover-based binary electrocatalysts for hydrogen evolution: from theory to experiment. ACS Catal. 12, 11821–11829 (2022).
Ju, L. et al. Controllable CO2 electrocatalytic reduction via ferroelectric switching on single atom anchored In2Se3 monolayer. Nat. Commun. 12, 5128 (2021).
Xu, Q. et al. Understanding the synergistic mechanism of single atom Co-modified perovskite oxide for piezo-photocatalytic CO2 reduction. Appl. Catal. B 338, 123058 (2023).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169 (1996).
Kresse, G. & Furthmüller, J. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758 (1999).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865 (1997).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H–Pu. J. Chem. Phys. 132, 154104 (2010).
Wang, V. et al. VASPKIT: a user-friendly interface facilitating high-throughput computing and analysis using VASP code. Comput. Phys. Commun. 267, 108033 (2021).
Acknowledgements
The authors thank the National Key Research and Development Program of China (2020YFA0710203, 2023YFA1507400), the National Natural Science Foundation of China (22279146, 22102192, 22033009, 22293015 and 22121002), the CAS Project for Young Scientists in Basic Research (grant number YSBR-050) and the Photon Science Center for Carbon Neutrality, China Postdoctoral Science Foundation (BX20200336 and 2020M680680), S&T Program of Hebei (B2021208074).
Author information
Authors and Affiliations
Contributions
Q.Z. and B.H. conceived and supervised the project. J.L., Q.Z. and B.H. wrote the paper. J.L. conducted the experimental work. P.L., S.J., Y.W., L.J., Z.L., J.Z., Q.Q., X.K. and X.S. assisted with X-ray diffraction, SEM, NMR, TEM and XAFS measurements. All authors discussed the results and contributed to the final paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Alexandra Groves, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Notes 1–5, Figs. 1–80, Tables 1–7 and references 1–53.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, J., Li, P., Jia, S. et al. Electrocatalytic CO2 hydrogenation to C2+ alcohols catalysed by Pr–Cu oxide heterointerfaces. Nat. Synth 4, 730–743 (2025). https://doi.org/10.1038/s44160-025-00752-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-025-00752-4