Abstract
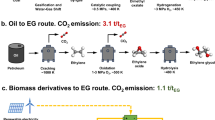
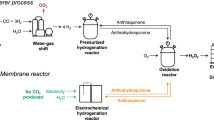
Hydrogen peroxide (H2O2) is not only a key eco-friendly oxidizer but also a promising energy carrier with an energy density comparable to that of compressed hydrogen. The industrial production of H2O2 relies on the energy-intensive and environmentally detrimental anthraquinone process, necessitating the exploration of greener alternatives. Here we demonstrate sustainable and unassisted electrochemical H2O2 production (via the two-electron oxygen reduction reaction) coupled to the oxidative valorization of glycerol, a biomass energy by-product, operating without external electric or solar energy inputs. We applied bismuth-loaded Pt and oxidized carbon nanotube electrocatalysts, for glycerol oxidation reaction and two-electron oxygen reduction reaction, respectively, which possess onset potentials close to the theoretical values for the electrochemical reactions. With this system, we achieved a high H2O2 production rate of approximately 8.475 μmol cm−2 min−1 and high glycerate selectivity for in situ glycerol oxidation reaction (74%), while producing renewable electricity on-site.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All relevant data are provided within this Article and its Supplementary Information.
References
Han, G. F. et al. Building and identifying highly active oxygenated groups in carbon materials for oxygen reduction to H2O2. Nat. Commun. 11, 2209 (2020).
Mehrotra, R., Oh, D. & Jang, J. W. Unassisted selective solar hydrogen peroxide production by an oxidised buckypaper-integrated perovskite photocathode. Nat. Commun. 12, 6644 (2021).
Mase, K., Yoneda, M., Yamada, Y. & Fukuzumi, S. Seawater usable for production and consumption of hydrogen peroxide as a solar fuel. Nat. Commun. 7, 11470 (2016).
Jung, E. et al. Atomic-level tuning of Co–N–C catalyst for high-performance electrochemical H2O2 production. Nat. Mater. 19, 436–442 (2020).
Perry, S. C. et al. Electrochemical synthesis of hydrogen peroxide from water and oxygen. Nat. Rev. Chem. 3, 442–458 (2019).
Xia, C., Xia, Y., Zhu, P., Fan, L. & Wang, H. Direct electrosynthesis of pure aqueous H2O2 solutions up to 20% by weight using a solid electrolyte. Science 366, 226–231 (2019).
Montoya, J. H. et al. Materials for solar fuels and chemicals. Nat. Mater. 16, 70–81 (2016).
Wang, Q., Pornrungroj, C., Linley, S. & Reisner, E. Strategies to improve light utilization in solar fuel synthesis. Nat. Energy 7, 13–24 (2021).
CO2 Emissions from Electricity Generation Factors, World 1990–2022 (IEA, 2025); https://www.iea.org/data-and-statistics/data-tools/energy-statistics-data-browser?country=WORLD&fuel=Energy%20supply&indicator=ElecGenByFuel
Verma, S., Lu, S. & Kenis, P. J. A. Co-electrolysis of CO2 and glycerol as a pathway to carbon chemicals with improved technoeconomics due to low electricity consumption. Nat. Energy 4, 466–474 (2019).
Global Biofuel Production in 2019 and Forecast to 2025 (IEA, 2020); https://www.iea.org/data-and-statistics/charts/global-biofuel-production-in-2019-and-forecast-to-2025
Low-Carbon Electricity Generation by Source, World 1990–2022 (IEA, 2025); https://www.iea.org/data-and-statistics/data-tools/energy-statistics-data-browser?country=WORLD&fuel=Energy%20supply&indicator=ElecGenByFuelLC
Liu, D. et al. Selective photoelectrochemical oxidation of glycerol to high value-added dihydroxyacetone. Nat. Commun. 10, 1779 (2019).
Luo, H. et al. Progress and perspectives in photo‐ and electrochemical‐oxidation of biomass for sustainable chemicals and hydrogen production. Adv. Energy Mater. 11, 2101180 (2021).
Li, T. & Harrington, D. A. An overview of glycerol electrooxidation mechanisms on Pt, Pd and Au. ChemSusChem 14, 1472–1495 (2021).
Kwon, Y., Birdja, Y., Spanos, I., Rodriguez, P. & Koper, M. T. M. Highly selective electro-oxidation of glycerol to dihydroxyacetone on platinum in the presence of bismuth. ACS Catal. 2, 759–764 (2012).
Wang, X. et al. The role of bismuth in suppressing the CO poisoning in alkaline methanol electrooxidation: switching the reaction from the CO to formate pathway. Nano Lett. 23, 685–693 (2023).
Wang, C. Y. et al. Intermetallic PtBi nanoplates with high catalytic activity towards electro‐oxidation of formic acid and glycerol. ChemElectroChem 7, 239–245 (2020).
de Souza, M. B. C. et al. Bi-modified Pt electrodes toward glycerol electrooxidation in alkaline solution: effects on activity and selectivity. ACS Catal. 9, 5104–5110 (2019).
Zope, B. N., Hibbitts, D. D., Neurock, M. & Davis, R. J. Reactivity of the gold/water interface during selective oxidation catalysis. Science 330, 74–78 (2010).
Li, Y., Wei, X., Han, S., Chen, L. & Shi, J. MnO2 electrocatalysts coordinating alcohol oxidation for ultra‐durable hydrogen and chemical productions in acidic solutions. Angew. Chem. Int. Ed. 60, 21464–21472 (2021).
Xi, N. et al. Polyhedral coordination determined Co–O activity for electrochemical oxidation of biomass alcohols. Adv. Energy Mater. 13, 2301572 (2023).
Nan, B. et al. Unique structure of active platinum–bismuth site for oxidation of carbon monoxide. Nat. Commun. 12, 3342 (2021).
Xie, J. et al. Influence of dioxygen on the promotional effect of Bi during Pt-catalyzed oxidation of 1,6-hexanediol. ACS Catal. 6, 4206–4217 (2016).
Fang, S. et al. Uncovering near-free platinum single-atom dynamics during electrochemical hydrogen evolution reaction. Nat. Commun. 11, 1029 (2020).
Jiang, K. et al. Single platinum atoms embedded in nanoporous cobalt selenide as electrocatalyst for accelerating hydrogen evolution reaction. Nat. Commun. 10, 1743 (2019).
Greczynski, G. & Hultman, L. Compromising science by ignorant instrument calibration-need to revisit half a century of published XPS data. Angew. Chem. Int. Ed. 59, 5002–5006 (2020).
Stamenkovic, V. R. et al. Improved oxygen reduction activity on Pt3Ni(111) via increased surface site availability. Science 315, 493–497 (2007).
Hao, Y.-C. et al. Promoting nitrogen electroreduction to ammonia with bismuth nanocrystals and potassium cations in water. Nat. Catal. 2, 448–456 (2019).
Kang, T. S. et al. High-performance amorphous InGaZnO thin-film transistors via staked ultrathin high-k TaOx buffer layer grown on low-k SiO2 gate oxide. Adv. Electron. Mater. 3, 1600452 (2017).
Zheng, W., Liu, M. & Lee, L. Y. S. Best practices in using foam-type electrodes for electrocatalytic performance benchmark. ACS Energy Lett. 5, 3260–3264 (2020).
Kwon, Y., Lai, S. C., Rodriguez, P. & Koper, M. T. Electrocatalytic oxidation of alcohols on gold in alkaline media: base or gold catalysis? J. Am. Chem. Soc. 133, 6914–6917 (2011).
Lu, Z. et al. High-efficiency oxygen reduction to hydrogen peroxide catalysed by oxidized carbon materials. Nat. Catal. 1, 156–162 (2018).
Pembere, A. M. & Luo, Z. Jones oxidation of glycerol catalysed by small gold clusters. Phys. Chem. Chem. Phys. 19, 6620–6625 (2017).
Avilés Acosta, J. E., Lin, J. C., Un Lee, D., Jaramillo, T. F. & Hahn, C. Electrochemical flow reactor design allows tunable mass transport conditions for operando surface enhanced infrared absorption spectroscopy. ChemCatChem 15, e202300520 (2023).
Kresse, G. & Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H–Pu. J. Chem. Phys. 132, 154104 (2010).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Nørskov, J. K. et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 108, 17886–17892 (2004).
Acknowledgements
This work was supported by the National Research Foundation of Korea for the grant by the South Korea Ministry of Science and ICT (MSIT) (grant nos. RS-2023-00222006, 2022H1D3A3A01081140 and RS-2024-00456139) (J.-W.J.), Basic Science Research Program (grant no. RS-2024-00451160), National Research Council of Science and Technology (NST) grant by the Korea government (MSIT) (grant no. GTL24011-102) (D.-H.S), US Department of Energy, Office of Science, Office of Basic Energy Sciences, Chemical Sciences, Geosciences, and Biosciences Division, and Catalysis Science Program to the SUNCAT Center for Interface Science and Catalysis, for ATR-FTIR studies, sample preparation, and TEM (T.F.J., S.-W.L.). The computational work was supported by the Supercomputing Center/Korea Institute of Science and Technology Information with supercomputing resources, including technical support (grant no. KSC-2024-CRE-0440). D.U.L., A.C., J.E.A.A. and J.E.M. acknowledge a cooperative research and development agreement sponsored by TotalEnergies American Services, Inc. (affiliate of TotalEnergies SE) under agreement number TC02307 for electrochemical cell development and DFT calculations. J.E.M. acknowledges a graduate fellowship through the National Science Foundation Graduate Research Fellowship under grant no. DGE-1656518. Y.X. acknowledges NSERC for their support in the form of a Banting postdoctoral fellowship.
Author information
Authors and Affiliations
Contributions
D.O., S.W.H., D.Y.K., J.E.M., T.F.J., D.-H.S. and J.-W.J. conceptualized this study. D.O., S.W.H., D.Y.K. and J.E.M. curated data. D.O., J.E.M., J.E.A.A., S.-W.L., Y.X. and D.U.L. established methodology. J.L. helped with HPLC measurements. A.C. helped with DFT calculations. T.F.J., D.-H.S. and J.-W.J. directed the research. D.O. visualized the data. D.O., S.W.H., D.Y.K., J.E.M., T.F.J., D.-H.S. and J.-W.J. cowrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks José Solla-Gullón, Kan Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Alexandra Groves, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Notes 1–3, Figs. 1–32 and Tables 1–3.
Supplementary Data 1
Numerical data for Supplementary Figs. 1–32 and Tables 1–3.
Source data
Source Data Fig. 2
Source data for Fig. 2.
Source Data Fig. 3
Source data for Fig. 3.
Source Data Fig. 4
Source data for Fig. 4.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Oh, D., Hwang, S.W., Kim, D.Y. et al. Unassisted electrochemical H2O2 production coupled to glycerol oxidation. Nat. Synth (2025). https://doi.org/10.1038/s44160-025-00774-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44160-025-00774-y
This article is cited by
-
Combining oxygen reduction with glycerol oxidation in a galvanic device
Nature Synthesis (2025)
-
Membrane electrode assembly for hydrogen peroxide electrosynthesis
Nature Reviews Clean Technology (2025)