Abstract
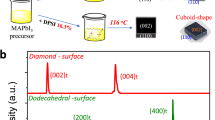
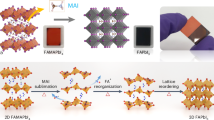
Solution growth of metal halide perovskites has enabled the development of applications including solar cells, light-emitting diodes and detectors, but the crystal growth mechanism remains unclear. Herein we studied the in situ transition of solute to crystals at the solid–liquid interface of methylammonium lead triiodide single crystals in γ-butyrolactone solution using microscopic spectroscopy. By establishing a temperature–bandgap relationship of the precursor solution, we observe a cooler interfacial region (1.5–4 μm from the crystal edge), explained by endothermic particle dissolution. This cooler region serves as a protective layer, hindering the attachment of particles with random orientations, maintaining the crystal facet orientation. The cooler interfacial protective layer is formed by the dissolution of particles driven by latent heat from crystallization and the concentration gradient of monomers at the interface. Disruption of the protective layer results in polycrystals with irregular facets. The understanding of the growth mechanisms of perovskite crystals provides insights for further improving the quality of solution-grown crystals.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The main data supporting the findings of this study are available within the published article and its Supplementary Information and source data files. Source data are provided with this paper.
References
Dong, Q. et al. Electron-hole diffusion lengths >175 μm in solution-grown CH3NH3PbI3 single crystals. Science 347, 967–970 (2015).
Shi, D. et al. Low trap-state density and long carrier diffusion in organolead trihalide perovskite single crystals. Science 347, 519–522 (2015).
Kojima, A., Teshima, K., Shirai, Y. & Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 131, 6050–6051 (2009).
Saidaminov, M. I. et al. High-quality bulk hybrid perovskite single crystals within minutes by inverse temperature crystallization. Nat. Commun. 6, 7586 (2015).
National Renewable Energy Laboratory. Best research-cell efficiency chart (2024); https://www.nrel.gov/pv/cell-efficiency.html
Fakharuddin, A. et al. Perovskite light-emitting diodes. Nat. Electron. 5, 203–216 (2022).
Jiang, J. et al. Efficient pure-red perovskite light-emitting diodes with strong passivation via ultrasmall-sized molecules. Sci. Adv. 10, eadn5683 (2024).
Ban, M. et al. Solution-processed perovskite light emitting diodes with efficiency exceeding 15% through additive-controlled nanostructure tailoring. Nat. Commun. 9, 3892 (2018).
Zhou, Y. et al. Self-powered perovskite photon-counting detectors. Nature 616, 712–718 (2023).
Zhao, J. et al. Perovskite-filled membranes for flexible and large-area direct-conversion X-ray detector arrays. Nat. Photon. 14, 612–617 (2020).
Zhao, L. et al. High-yield growth of FACsPbBr3 single crystals with low defect density from mixed solvents for gamma-ray spectroscopy. Nat. Photon. 17, 315–323 (2023).
Zhao, L. et al. Surface-defect-passivation-enabled near-unity charge collection efficiency in bromide-based perovskite gamma-ray spectrum devices. Nat. Photon. 18, 250–257 (2024).
Ni, Z. et al. Identification and suppression of point defects in bromide perovskite single crystals enabling gamma‐ray spectroscopy. Adv. Mater. 36, 2406193 (2024).
Petrov, A. A. et al. Crystal structure of DMF-intermediate phases uncovers the link between CH3NH3PbI3 morphology and precursor stoichiometry. J. Phys. Chem. C 121, 20739–20743 (2017).
Deng, Y. et al. Tailoring solvent coordination for high-speed, room-temperature blading of perovskite photovoltaic films. Sci. Adv. 5, eaax7537 (2019).
Zuo, L. et al. Morphology evolution of high efficiency perovskite solar cells via vapor induced intermediate phases. J. Am. Chem. Soc. 138, 15710–15716 (2016).
Kim, M. et al. Methylammonium chloride induces intermediate phase stabilization for efficient perovskite solar cells. Joule 3, 2179–2192 (2019).
McMeekin, D. P. et al. Intermediate-phase engineering via dimethylammonium cation additive for stable perovskite solar cells. Nat. Mater. 22, 73–83 (2023).
Miao, Y. et al. Green solvent enabled scalable processing of perovskite solar cells with high efficiency. Nat. Sustain. 6, 1465–1473 (2023).
Hamill, J. C. Jr, Schwartz, J. & Loo, Y.-L. Influence of solvent coordination on hybrid organic–inorganic perovskite formation. ACS Energy Lett. 3, 92–97 (2018).
Seok, S. I., Grätzel, M. & Park, N. Methodologies toward highly efficient perovskite solar cells. Small 14, 1704177 (2018).
Ma, C. et al. Unveiling facet-dependent degradation and facet engineering for stable perovskite solar cells. Science 379, 173–178 (2023).
Ma, C., Grätzel, M. & Park, N.-G. Facet engineering for stable, efficient perovskite solar cells. ACS Energy Lett. 7, 3120–3128 (2022).
Yan, K. et al. Hybrid halide perovskite solar cell precursors: colloidal chemistry and coordination engineering behind device processing for high efficiency. J. Am. Chem. Soc. 137, 4460–4468 (2015).
Ma, L. et al. A polymer controlled nucleation route towards the generalized growth of organic-inorganic perovskite single crystals. Nat. Commun. 12, 2023 (2021).
Liu, Y. et al. Ligand assisted growth of perovskite single crystals with low defect density. Nat. Commun. 12, 1686 (2021).
Burton, W. K., Cabrera, N. & Frank, F. C. The growth of crystals and the equilibrium structure of their surfaces. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 243, 299–358 (1951).
Nielsen, M. H., Aloni, S. & De Yoreo, J. J. In situ TEM imaging of CaCO3 nucleation reveals coexistence of direct and indirect pathways. Science 345, 1158–1162 (2014).
Schoeppler, V. et al. Crystallization by amorphous particle attachment: on the evolution of texture. Adv. Mater. 33, 2101358 (2021).
Zhong, X., Shtukenberg, A. G., Hueckel, T., Kahr, B. & Ward, M. D. Screw dislocation generation by inclusions in molecular crystals. Cryst. Growth Des. 18, 318–323 (2018).
Li, D. et al. Direction-specific interactions control crystal growth by oriented attachment. Science 336, 1014–1018 (2012).
Li, D. et al. Growth mechanism of highly branched titanium dioxide nanowires via oriented attachment. Cryst. Growth Des. 13, 422–428 (2013).
Shuangming, L. & Kuangdi, X. In The ECPH Encyclopedia of Mining and Metallurgy (ed. Xu, K.) 1–3 (Springer Nature Singapore, 2022); https://doi.org/10.1007/978-981-19-0740-1_810-1
Chen, Z. et al. Thin single crystal perovskite solar cells to harvest below-bandgap light absorption. Nat. Commun. 8, 1890 (2017).
Yoon, S. J., Stamplecoskie, K. G. & Kamat, P. V. How lead halide complex chemistry dictates the composition of mixed halide perovskites. J. Phys. Chem. Lett. 7, 1368–1373 (2016).
Shargaieva, O., Kuske, L., Rappich, J., Unger, E. & Nickel, N. H. Building blocks of hybrid perovskites: a photoluminescence study of lead-iodide solution species. ChemPhysChem 21, 2327–2333 (2020).
Cao, J. et al. Identifying the molecular structures of intermediates for optimizing the fabrication of high-quality perovskite films. J. Am. Chem. Soc. 138, 9919–9926 (2016).
Krautscheid, H. & Vielsack, F. [Pb18I44]8−–an iodoplumbate with an unusual structure. Angew. Chem. Int. Ed. 34, 2035–2037 (1995).
Fateev, S. A. et al. Solution processing of methylammonium lead iodide perovskite from γ-butyrolactone: crystallization mediated by solvation equilibrium. Chem. Mater. 30, 5237–5244 (2018).
Acknowledgements
This work was supported by the Center for Hybrid Organic Inorganic Semiconductors for Energy (CHOISE), an Energy Frontier Research Center funded by the Office of Basic Energy Sciences, Office of Science within the US Department of Energy.
Author information
Authors and Affiliations
Contributions
Z.S. and J.H. conceived the idea. Z.S. designed the experiments, Z.S. and H.L. carried out the FLIM-related measurements. H.J., M.L., L.Z. and Z.N. provided helpful suggestions about crystal growth and FLIM measurements. Z.S. and J.H. wrote the paper, and all authors reviewed the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Makhsud Saidaminov, Liang Shen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Alexandra Groves, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–11, Table 1 and Discussion 1.
Source data
Source Data Fig. 2
Source data.
Source Data Fig. 3
Source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shi, Z., Liu, H., Jiao, H. et al. Self-regulated facet stability during solution growth of perovskite crystals. Nat. Synth (2025). https://doi.org/10.1038/s44160-025-00786-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44160-025-00786-8