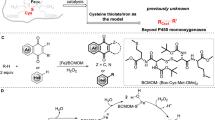
Abstract
The stereoconvergent reduction of alkenes for efficient synthesis of chiral compounds is a challenge in synthetic chemistry, even for exquisite enzymes in nature. Natural ene-reductases for example generally catalyse the reduction of alkenes in an enantiodivergent or resolution fashion. Here we report the repurposing of non-haem iron-based dioxygenases to catalyse the stereoconvergent reduction of alkenes through an iron hydride intermediate, by introducing silanes to the biocatalytic system. Directed evolution of gentisate 1,2-dioxygenase led to iron-based ene-reductases with high efficiency (up to 99% yield), excellent enantioselectivity (23 examples with >99% e.e.) and compatibility with a structurally diverse range of substrates. Experimental studies suggest the formation of iron hydride species in the enzyme and support the divalency of iron during the catalytic process. Computational studies show that the reaction is energetically feasible through an iron hydride mechanism and reveal the molecular mechanism of stereoconvergence.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All of the data needed to support the conclusions of this study are available in the main text and Supplementary Information.
References
Wu, S., Zhou, Y. & Li, Z. Biocatalytic selective functionalisation of alkenes via single-step and one-pot multi-step reactions. Chem. Commun. 55, 883–896 (2019).
Knowles, W. S. Asymmetric hydrogenations (Nobel lecture). Angew. Chem. Int. Ed. 41, 1998–2007 (2002).
Noyori, R. Asymmetric catalysis: science and opportunities (Nobel lecture). Angew. Chem. Int. Ed. 41, 2008–2022 (2002).
Massaro, L., Zheng, J., Margarita, C. & Andersson, P. G. Enantioconvergent and enantiodivergent catalytic hydrogenation of isomeric olefins. Chem. Soc. Rev. 49, 2504–2522 (2020).
Winkler, C. K., Faber, K. & Hall, M. Biocatalytic reduction of activated CC-bonds and beyond: emerging trends. Curr. Opin. Chem. Biol. 43, 97–105 (2018).
Hollmann, F., Opperman, D. J. & Paul, C. E. Biocatalytic reduction reactions from a chemist’s perspective. Angew. Chem. Int. Ed. 60, 5644–5665 (2021).
Mansell, D. J. et al. Biocatalytic asymmetric alkene reduction: crystal structure and characterization of a double bond reductase from Nicotiana tabacum. ACS Catal. 3, 370–379 (2013).
Turrini, N. G. et al. Biocatalytic access to nonracemic γ-oxo esters via stereoselective reduction using ene-reductases. Green Chem. 19, 511–518 (2017).
Durchschein, K. et al. Nicotinamide-dependent ene reductases as alternative biocatalysts for the reduction of activated alkenes. Eur. J. Org. Chem. 2012, 4963–4968 (2012).
Mueller, N. J. et al. The substrate spectra of pentaerythritol tetranitrate reductase, morphinone reductase, N-ethylmaleimide reductase and estrogen-binding protein in the asymmetric bioreduction of activated alkenes. Adv. Synth. Catal. 352, 387–394 (2010).
Winkler, C. K., Tasnádi, G., Clay, D., Hall, M. & Faber, K. Asymmetric bioreduction of activated alkenes to industrially relevant optically active compounds. J. Biotechnol. 162, 381–389 (2012).
Litman, Z. C., Wang, Y., Zhao, H. & Hartwig, J. F. Cooperative asymmetric reactions combining photocatalysis and enzymatic catalysis. Nature 560, 355–359 (2018).
Wang, Y., Huang, X., Hui, J., Vo, L. T. & Zhao, H. Stereoconvergent reduction of activated alkenes by a nicotinamide free synergistic photobiocatalytic system. ACS Catal. 10, 9431–9437 (2020).
Romero, E. O. et al. Enabling broader adoption of biocatalysis in organic chemistry. JACS Au 3, 2073–2085 (2023).
Arnold, F. H. Directed evolution: bringing new chemistry to life. Angew. Chem. Int. Ed. 57, 4143–4148 (2018).
Chen, K. & Arnold, F. H. Engineering new catalytic activities in enzymes. Nat. Catal. 3, 203–213 (2020).
Goldberg, N. W., Knight, A. M., Zhang, R. K. & Arnold, F. H. Nitrene transfer catalyzed by a non-heme iron enzyme and enhanced by non-native small-molecule ligands. J. Am. Chem. Soc. 141, 19585–19588 (2019).
Vila, M. A., Steck, V., Rodriguez Giordano, S., Carrera, I. & Fasan, R. C–H amination via nitrene transfer catalyzed by mononuclear non-heme iron-dependent enzymes. ChemBioChem 21, 1981–1987 (2020).
Yang, Y. & Arnold, F. H. Navigating the unnatural reaction space: directed evolution of heme proteins for selective carbene and nitrene transfer. Acc. Chem. Res. 54, 1209–1225 (2021).
Zhang, J. et al. Chemodivergent C(sp3)–H and C(sp2)–H cyanomethylation using engineered carbene transferases. Nat. Catal. 6, 152–160 (2023).
Mao, R. et al. Biocatalytic, stereoconvergent alkylation of (Z/E)-trisubstituted silyl enol ethers. Nat. Synth. 3, 256–264 (2024).
Huang, X. et al. Photoenzymatic enantioselective intermolecular radical hydroalkylation. Nature 584, 69–74 (2020).
Sandoval, B. A. et al. Photoenzymatic reductions enabled by direct excitation of flavin-dependent “ene”-reductases. J. Am. Chem. Soc. 143, 1735–1739 (2021).
Huang, X. et al. Photoinduced chemomimetic biocatalysis for enantioselective intermolecular radical conjugate addition. Nat. Catal. 5, 586–593 (2022).
Cheng, L. et al. Stereoselective amino acid synthesis by synergistic photoredox-pyridoxal radical biocatalysis. Science 381, 444–451 (2023).
Fu, H. & Hyster, T. K. From ground-state to excited-state activation modes: flavin-dependent “ene“-reductases catalyzed non-natural radical reactions. Acc. Chem. Res. 57, 1446–1457 (2024).
Xu, Y. et al. A light-driven enzymatic enantioselective radical acylation. Nature 625, 74–78 (2024).
Zhou, Q., Chin, M., Fu, Y., Liu, P. & Yang, Y. Stereodivergent atom-transfer radical cyclization by engineered cytochromes P450. Science 374, 1612–1616 (2021).
Rui, J. et al. Directed evolution of nonheme iron enzymes to access abiological radical-relay C(sp3)−H azidation. Science 376, 869–874 (2022).
Fu, W. et al. Enzyme-controlled stereoselective radical cyclization to arenes enabled by metalloredox biocatalysis. Nat. Catal. 6, 628–636 (2023).
Wang, B. et al. Repurposing iron- and 2-oxoglutarate-dependent oxygenases to catalyze olefin hydration. Angew. Chem. Int. Ed. 62, e202311099 (2023).
Zhao, Q. et al. Engineering non-haem iron enzymes for enantioselective C(sp3)–F bond formation via radical fluorine transfer. Nat. Synth. 3, 958–966 (2024).
Van Stappen, C. et al. Designing artificial metalloenzymes by tuning of the environment beyond the primary coordination sphere. Chem. Rev. 122, 11974–12045 (2022).
Schwizer, F. et al. Artificial metalloenzymes: reaction scope and optimization strategies. Chem. Rev. 118, 142–231 (2018).
Ji, P., Park, J., Gu, Y., Clark, D. S. & Hartwig, J. F. Abiotic reduction of ketones with silanes catalysed by carbonic anhydrase through an enzymatic zinc hydride. Nat. Chem. 13, 312–318 (2021).
Chen, R., Kayrouz, C. S., McAmis, E., Clark, D. S. & Hartwig, J. F. Carbonic anhydrase variants catalyze the reduction of dialkyl ketones with high enantioselectivity. Angew. Chem. Int. Ed. 63, e202407111 (2024).
Chen, D. et al. An evolved artificial radical cyclase enables the construction of bicyclic terpenoid scaffolds via an H-atom transfer pathway. Nat. Chem. 16, 1656–1664 (2024).
Waldron, K. J., Rutherford, J. C., Ford, D. & Robinson, N. J. Metalloproteins and metal sensing. Nature 460, 823–830 (2009).
Zhang, X. et al. Repurposing myoglobin into an abiological asymmetric ketoreductase. Chem 10, 2577–2589 (2024).
Nakashima, Y. et al. Structure function and engineering of multifunctional non-heme iron dependent oxygenases in fungal meroterpenoid biosynthesis. Nat. Commun. 9, 104 (2018).
Neugebauer, M. E. et al. Reaction pathway engineering converts a radical hydroxylase into a halogenase. Nat. Chem. Biol. 18, 171–179 (2022).
He, J.-B. et al. Enzymatic catalysis favours eight-membered over five-membered ring closure in bicyclomycin biosynthesis. Nat. Catal. 6, 637–648 (2023).
Ushimaru, R. & Abe, I. Unusual dioxygen-dependent reactions catalyzed by nonheme iron enzymes in natural product biosynthesis. ACS Catal. 13, 1045–1076 (2023).
Zhang, J., Wu, P., Zhang, X. & Wang, B. Coordination dynamics of iron is a key player in the catalysis of non-heme enzymes. ChemBioChem 24, e202300119 (2023).
McBride, M. J. et al. A peroxodiiron(iii/iii) intermediate mediating both N-hydroxylation steps in biosynthesis of the N-nitrosourea pharmacophore of streptozotocin by the multi-___domain metalloenzyme SznF. J. Am. Chem. Soc. 142, 11818–11828 (2020).
Wang, C. et al. Evidence that the fosfomycin-producing epoxidase, HppE, is a non-heme-iron peroxidase. Science 342, 991–995 (2013).
Wei, D. & Darcel, C. Iron catalysis in reduction and hydrometalation reactions. Chem. Rev. 119, 2550–2610 (2019).
Wen, J., Wang, F. & Zhang, X. Asymmetric hydrogenation catalyzed by first-row transition metal complexes. Chem. Soc. Rev. 50, 3211–3237 (2021).
Hu, M.-Y. et al. Ligands with 1,10-phenanthroline scaffold for highly regioselective iron-catalyzed alkene hydrosilylation. Nat. Commun. 9, 221 (2018).
Hou, X. et al. Discovery of the biosynthetic pathway of beticolin 1 reveals a novel non-heme iron-dependent oxygenase for anthraquinone ring cleavage. Angew. Chem. Int. Ed. 61, e202208772 (2022).
Meah, Y. & Massey, V. Old yellow enzyme: stepwise reduction of nitro-olefins and catalysis of aci-nitro tautomerization. Proc. Natl Acad. Sci. USA 97, 10733–10738 (2000).
Toogood, H. S. et al. A site-saturated mutagenesis study of pentaerythritol tetranitrate reductase reveals that residues 181 and 184 influence ligand binding, stereochemistry and reactivity. ChemBioChem 12, 738–749 (2011).
Lonsdale, R. & Reetz, M. T. Reduction of α,β-unsaturated ketones by old yellow enzymes: mechanistic insights from quantum mechanics/molecular mechanics calculations. J. Am. Chem. Soc. 137, 14733–14742 (2015).
Devine, P. N. et al. Extending the application of biocatalysis to meet the challenges of drug development. Nat. Rev. Chem. 2, 409–421 (2018).
Thiyagarajan, S., Diskin-Posner, Y., Montag, M. & Milstein, D. Manganese-catalyzed base-free addition of saturated nitriles to unsaturated nitriles by template catalysis. Chem. Sci. 15, 2571–2577 (2024).
Harpel, M. R. & Lipscomb, J. D. Gentisate 1,2-dioxygenase from Pseudomonas. Purification, characterization, and comparison of the enzymes from Pseudomonas testosteroni and Pseudomonas acidovorans. J. Biol. Chem. 265, 6301–6311 (1990).
Linford-Wood, T. G., Mahon, M. F., Grayson, M. N. & Webster, R. L. Iron-catalyzed H/D exchange of primary silanes, secondary silanes, and tertiary siloxanes. ACS Catal. 12, 2979–2985 (2022).
Trott, O. & Olson, A. J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 31, 455–461 (2010).
Case, D. A. et al. AMBER 2018 (Univ. California, San Francisco, 2018).
Wang, J. M., Wolf, R. M., Caldwell, J. W., Kollman, P. A. & Case, D. A. Development and testing of a general amber force field. J. Comput. Chem. 25, 1157–1174 (2004).
Bayly, C. I., Cieplak, P., Cornell, W. D. & Kollman, P. A. A well-behaved electrostatic potential based method using charge restraints for deriving atomic charges: the RESP model. J. Phys. Chem. 97, 10269–10280 (1993).
Li, P. & Merz, K. M. Jr. MCPB.py: a Python based metal center parameter builder. J. Chem. Inf. Model. 56, 599–604 (2016).
Maier, J. A. et al. ff14SB: improving the accuracy of protein side chain and backbone parameters from ff99SB. J. Chem. Theory Comput. 11, 3696–3713 (2015).
Sherwood, P. et al. QUASI: a general purpose implementation of the QM/MM approach and its application to problems in catalysis. J. Mol. Struct. THEOCHEM 632, 1–28 (2003).
Kaestner, J. et al. DL-FIND: an open-source geometry optimizer for atomistic simulations. J. Phys. Chem. A 113, 11856–11865 (2009).
Ahlrichs, R., Bar, M., Haser, M., Horn, H. & Kolmel, C. Electronic structure calculations on workstation computers: the program system turbomole. Chem. Phys. Lett. 162, 165–169 (1989).
Balasubramani, S. G. et al. TURBOMOLE: modular program suite for ab initio quantum-chemical and condensed-matter simulations. J. Chem. Phys. 152, 184107 (2020).
Becke, A. D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 38, 3098–3100 (1988).
Lee, C., Yang, W. & Parr, R. G. Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785–789 (1988).
Becke, A. D. Density‐functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993).
Stephens, P. J., Devlin, F. J., Chabalowski, C. F. & Frisch, M. J. Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 98, 11623–11627 (1994).
Weigend, F. & Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys. Chem. Chem. Phys. 7, 3297–3305 (2005).
Grimme, S. Accurate description of van der Waals complexes by density functional theory including empirical corrections. J. Comput. Chem. 25, 1463–1473 (2004).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H–Pu. J. Chem. Phys. 132, 154104 (2010).
Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 32, 1456–1465 (2011).
Acknowledgements
We thank staff at the Chemistry Instrumentation Center of Zhejiang University for instrumental support, including Q. He and X. Li for help with high-resolution mass spectrometry, X. Wang and Y. Wang for EPR analysis and M. Yu for assistance with NMR. We thank B. Shan’s group at Zhejiang University for help with obtaining ultraviolet–visible spectroscopy data. We further acknowledge Y. Rao’s group at Jiangnan University for providing plasmid DNA coding for BTG13. This work was supported by the National Natural Science Foundation of China (22172142 (P.J.), 22377107 (P.J.) and 22303043 (X.Z.)), the Zhejiang Provincial Outstanding Youth Science Foundation (LR22B030004 (P.J.)), Fundamental Research Funds for the Central Universities (226-2024-00003 (P.J.)), the Key-Area Research and Development Program of Guangdong Province (2022B1111080005 (R.W.)), the Ningbo Yongjiang Talent Programme (2023A-378-G (X.Z.)), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB0960201 (L.Y.)) and the Youth Innovation Promotion Association of the Chinese Academy of Sciences (2022455 (L.Y.)).
Author information
Authors and Affiliations
Contributions
P.J. and Z.W. initiated this project. Z.W. developed the biocatalytic system and performed most of the experiments. H.Z., Z.F. and W.W. assisted with the synthesis of compounds. L.Y. carried out the spectroscopic studies. Y.S. assisted with protein expression and purification. X.Z. and Z.X. performed the computational studies with R.W. providing guidance. Z.W., X.Z. and P.J. wrote the manuscript. All authors reviewed and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Hans Senn and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Thomas West, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–26, Tables 1–22, Experimental details and Characterization data.
Supplementary Data
Primer sequences for site-specific mutagenesis.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wan, Z., Zhang, X., Zhuang, H. et al. Stereoconvergent reduction of alkenes using a repurposed iron-based dioxygenase. Nat. Synth (2025). https://doi.org/10.1038/s44160-025-00788-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44160-025-00788-6