Abstract
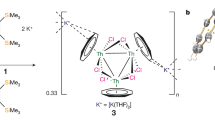
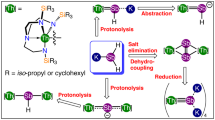
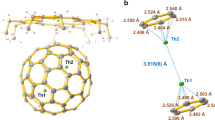
Theoretical studies predict that homoatomic metal–metal bonds of f-block actinide elements should be ubiquitous. Surprisingly, however, the isolation and characterization of compounds featuring an actinide–actinide bond has proven challenging and the field remains undeveloped. Here we report a well-defined thorium dimer featuring a Th–Th two-centre one-electron (2c-1e) σ bond and a 2c-1e π bond. This thorium dimer was synthesized by reducing a Th(IV) chloride complex with potassium metal in tetrahydrofuran. Magnetic measurements indicate that this thorium dimer features exceedingly strong antiferromagnetic coupling between the two formal Th(III) centres with a coupling constant J ≤ −1,200 cm−1 such that the Th–Th interaction has covalent bond character. Detailed computational investigations further support the existence of the Th–Th bond in this molecule. These results demonstrate that diactinide complexes with actinide–actinide bonds are accessible but require an appropriate ligand framework to stabilize low-valent actinide centres.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers CCDC 2182247 (2) and 2182248 (3). These CCDC data can be obtained free of charge via the CCDC at www.ccdc.cam.ac.uk/data_request/cif.
References
Cotton, F. A., Murillo, C. A. & Walton, R. A. Multiple Bonds Between Metal Atoms (Springer, 2005).
Liddle, S. T. Molecular Metal–Metal Bonds: Compounds, Synthesis, Properties (Wiley, 2015).
Green, S. P., Jones, C. & Stasch, A. Stable magnesium(I) compounds with Mg–Mg bonds. Science 318, 1754–1757 (2007).
Hill, M. S., Hitchcock, P. B. & Pongtavornpinyo, R. A linear homocatenated compound containing six indium centers. Science 311, 1904–1907 (2006).
Tokitoh, N., Arai, Y., Okazaki, R. & Nagase, S. Synthesis and characterization of a stable dibismuthene: evidence for a Bi–Bi double bond. Science 277, 78–80 (1997).
Su, J., Li, X. W., Crittendon, R. C. & Robinson, G. H. How short is a -Ga=Ga- triple bond? Synthesis and molecular structure of Na2[Mes*2C6H3-Ga=Ga-C6H3Mes*2] (Mes* = 2,4,6-i-Pr3C6H2): the first gallyne. J. Am. Chem. Soc. 119, 5471–5472 (1997).
Peng, Y., Ellis, B. D., Wang, X., Fettinger, J. C. & Power, P. P. Reversible reactions of ethylene with distannynes under ambient conditions. Science 325, 1668–1670 (2009).
Cotton, F. A. et al. Mononuclear and polynuclear chemistry of rhenium (III): Its pronounced homophilicity. Science 145, 1305–1307 (1964).
Kundig, E. P., Moskovits, M. & Ozin, G. A. Matrix synthesis and characterization of dichromium. Nature 254, 503–504 (1975).
Resa, I., Carmona, E., Gutierrez-Puebla, E. & Monge, A. Decamethyldizincocene, a stable compound of Zn(I) with a Zn–Zn bond. Science 305, 1136–1138 (2004).
Nguyen, T. et al. Synthesis of a stable compound with fivefold bonding between two chromium(I) centers. Science 310, 844–847 (2005).
Gould, C. A. et al. Ultrahard magnetism from mixed-valence dilanthanide complexes with metal–metal bonding. Science 375, 198–202 (2022).
Gagliardi, L. & Pyykkö, P. Theoretical search for very short metal–actinide bonds: NUIr and isoelectronic systems. Angew. Chem. Int. Ed. 43, 1573–1576 (2004).
Gagliardi, L. & Roos, B. O. Quantum chemical calculations show that the uranium molecule U2 has a quintuple bond. Nature 433, 848–851 (2005).
Gagliardi, L., Pyykkö, P. & Roos, B. O. A very short uranium–uranium bond: the predicted metastable U22+. Phys. Chem. Chem. Phys. 7, 2415–2417 (2005).
Straka, M. & Pyykkö, P. Linear HThThH: a candidate for a Th–Th triple bond. J. Am. Chem. Soc. 127, 13090–13091 (2005).
Roos, B. O. & Gagliardi, L. Quantum chemistry predicts multiply bonded diuranium compounds to be stable. Inorg. Chem. 45, 803–807 (2006).
La Macchia, G., Brynda, M. & Gagliardi, L. Quantum chemical calculations predict the diphenyl diuranium compound [PhUUPh] to have a stable 1Ag ground state. Angew. Chem. Int. Ed. 45, 6210–6213 (2006).
Roos, B. O., Malmqvist, P. Å. & Gagliardi, L. Exploring the actinide–actinide bond: theoretical studies of the chemical bond in Ac2, Th2, Pa2, and U2. J. Am. Chem. Soc. 128, 17000–17006 (2006).
Wang, C. Z. et al. Actinide (An = Th-Pu) dimetallocenes: promising candidates for metal–metal multiple bonds. Dalton Trans. 44, 17045–17053 (2015).
Hu, H. S. & Kaltsoyannis, N. The shortest Th–Th distance from a new type of quadruple bond. Phys. Chem. Chem. Phys. 19, 5070–5076 (2017).
Knecht, S., Jensen, H. J. A. & Saue, T. Relativistic quantum chemical calculations show that the uranium molecule U2 has a quadruple bond. Nat. Chem. 11, 40–44 (2019).
Ciborowski, S. M. et al. Metal–metal bonding in actinide dimers: U2 and U2−. J. Am. Chem. Soc. 143, 17023–17028 (2021).
Steimle, T., Kokkin, D. L., Muscarella, S. & Ma, T. Detection of the thorium dimer via two-dimensional fluorescence spectroscopy. J. Phys. Chem. A 119, 9281–9285 (2015).
Souter, P. F., Kushto, G. P. & Andrews, L. IR spectra of uranium hydride molecules isolated in solid argon. Chem. Commun. 21, 2401–2402 (1996).
Zhang, X. et al. U2@Ih(7)-C80: crystallographic characterization of a long-sought dimetallic actinide endohedral fullerene. J. Am. Chem. Soc. 140, 3907–3910 (2018).
Yan, Y. et al. Actinide–lanthanide single electron metal–metal bond formed in mixed-valence di-metallofullerenes. Nat. Commun. 14, 6637 (2023).
Zhuang, J. et al. Characterization of a strong covalent Th3+–Th3+ bond inside an Ih(7)-C80 fullerene cage. Nat. Commun. 12, 2372 (2021).
Sternal, R. S., Brock, C. P. & Marks, T. J. Metal–metal bonds involving actinides. synthesis and characterization of a complex having an unsupported actinide to transition metal bond. J. Am. Chem. Soc. 107, 8270–8272 (1985).
Napoline, J. W. et al. Tris(phosphinoamide)-supported uranium–cobalt heterobimetallic complexes featuring Co→U dative interactions. Inorg. Chem. 52, 12170–12177 (2013).
Ward, A. L., Lukens, W. W., Lu, C. C. & Arnold, J. Photochemical route to actinide–transition metal bonds: synthesis, characterization and reactivity of a series of thorium and uranium heterobimetallic complexes. J. Am. Chem. Soc. 136, 3647–3654 (2014).
Hlina, J. A., Pankhurst, J. R., Kaltsoyannis, N. & Arnold, P. L. Metal–metal bonding in uranium-group 10 complexes. J. Am. Chem. Soc. 138, 3333–3345 (2016).
Chi, C. et al. Preparation and characterization of uranium–iron triple‐bonded \({{\rm{UFe}}({\rm{CO}})}_{3}^{-}\) and \({{\rm{UFe}}({\rm{CO}})}_{3}^{-}\) complexes. Angew. Chem. Int. Ed. 56, 6932–6936 (2017).
Lu, E., Wooles, A. J., Gregson, M., Cobb, P. J. & Liddle, S. T. A very short uranium(IV)–rhodium(I) bond with net double-dative bonding character. Angew. Chem. Int. Ed. 57, 6587–6591 (2018).
Feng, G. et al. Transition-metal-bridged bimetallic clusters with multiple uranium–metal bonds. Nat. Chem. 11, 248–253 (2019).
Feng, G. et al. Identification of a uranium–rhodium triple bond in a heterometallic cluster. Proc. Natl Acad. Sci. USA 116, 17654–17658 (2019).
Xin, X. et al. Dinitrogen cleavage by a heterometallic cluster featuring multiple uranium–rhodium bonds. J. Am. Chem. Soc. 142, 15004–15011 (2020).
Feng, G., McCabe, K. N., Wang, S., Maron, L. & Zhu, C. Construction of heterometallic clusters with multiple uranium–metal bonds by dianionic nitrogen–phosphorus ligands. Chem. Sci. 11, 7585–7592 (2020).
Wang, P. et al. Selective hydroboration of terminal alkynes catalyzed by heterometallic clusters with uranium–metal triple bonds. Chem 8, 1361–1375 (2022).
Shen, J. et al. Complexes featuring a cis-[M⇉U⇇M] core (M = Rh, Ir): a new route to uranium–metal multiple bonds. Angew. Chem. Int. Ed. 62, e202303379 (2023).
Fang, W. et al. Oxidative addition of E–H (E = C, N) bonds to transient uranium(II) centers. Angew. Chem. Int. Ed. 63, e202407339 (2024).
Sheng, W., Rajeshkumar, T., Zhao, Y., Maron, L. & Zhu, C. Electronic delocalization and σ-aromaticity in heterometallic cluster with multiple thorium–palladium bonds. J. Am. Chem. Soc. 146, 12790–12798 (2024).
Boronski, J. T. et al. A crystalline tri-thorium cluster with σ-aromatic metal–metal bonding. Nature 598, 72–75 (2021).
Szczepanik, D. W. Bonding in a crystalline tri-thorium cluster: not σ-aromatic but still unique. Angew. Chem. Int. Ed. 61, e202204337 (2022).
Cuyacot, B. J. R. & Foroutan-Nejad, C. [{Th(C8H8)Cl2}3]2− is stable but not aromatic. Nature 603, E18–E20 (2022).
Badri, Z. & Foroutan-Nejad, C. On the aromaticity of actinide compounds. Nat. Rev. Chem. 8, 551–560 (2024).
Lin, X. & Mo, Y. On the bonding nature in the crystalline tri-thorium cluster: core–shell syngenetic σ-aromaticity. Angew. Chem. Int. Ed. 61, e202209658 (2022).
Zhu, Q., Fang, W., Maron, L. & Zhu, C. Heterometallic clusters with uranium–metal bonds supported by double-layer nitrogen–phosphorus ligands. Acc. Chem. Res. 55, 1718–1730 (2022).
Wang, P. et al. Facile dinitrogen and dioxygen cleavage by a uranium(III) complex: cooperativity between the non-innocent ligand and the uranium center. Angew. Chem. Int. Ed. 60, 473–479 (2021).
Pyykkö, P. & Atsumi, M. Molecular single-bond covalent radii for elements 1–118. Chem. Eur. J. 15, 186–197 (2009).
Pyykkö, P. Additive covalent radii for single-, double-, and triple-bonded molecules and tetrahedrally bonded crystals: a summary. J. Phys. Chem. A 119, 2326–2337 (2015).
Ephritikhine, M. Synthesis, structure, and reactions of hydride, borohydride, and aluminohydride compounds of the f-elements. Chem. Rev. 97, 2193–2242 (1997).
Booth, C. H. et al. Decamethylytterbocene complexes of bipyridines and diazabutadienes: multiconfigurational ground states and open-shell singlet formation. J. Am. Chem. Soc. 131, 6480–6491 (2009).
Booth, C. H. et al. Intermediate-valence tautomerism in decamethylytterbocene complexes of methyl-substituted bipyridines. J. Am. Chem. Soc. 132, 17537–17549 (2010).
Parry, J. S., Cloke, G. N., Coles, S. J. & Hursthouse, M. B. Synthesis and characterization of the first sandwich complex of trivalent thorium: a structural comparison with the uranium analogue. J. Am. Chem. Soc. 121, 6867–6871 (1999).
Langeslay, R. R., Fieser, M. E., Ziller, J. W., Furche, F. & Evans, W. J. Expanding thorium hydride chemistry through Th2+, including the synthesis of a mixed-valent Th4+/Th3+ hydride complex. J. Am. Chem. Soc. 138, 4036–4045 (2016).
Langeslay, R. R. et al. Synthesis, structure, and reactivity of the sterically crowded Th3+ complex (C5Me5)3Th including formation of the thorium carbonyl, [(C5Me5)3Th(CO)][BPh4]. J. Am. Chem. Soc. 139, 3387–3398 (2017).
Formanuik, A. et al. Actinide covalency measured by pulsed electron paramagnetic resonance spectroscopy. Nat. Chem. 9, 578–583 (2017).
Altman, A. B. et al. Chemical structure and bonding in a thorium(III)–aluminum heterobimetallic complex. Chem. Sci. 9, 4317–4324 (2018).
Huh, D. N., Roy, S., Ziller, J. W., Furche, F. & Evans, W. J. Isolation of a square-planar Th (III) complex: synthesis and structure of [Th(OC6H2tBu2-2,6-Me-4)4]1-. J. Am. Chem. Soc. 141, 12458–12463 (2019).
Stranger, R., Smith, P. W. & Grey, I. E. Magneto-structural correlations and metal–metal bonding in exchange-coupled A3Mo2X9 (X = Cl, Br, I) complexes. Inorg. Chem. 28, 1271–1278 (1989).
Duncan Lyngdoh, R. H., Schaefer, H. F. III & King, R. B. Metal–metal (MM) bond distances and bond orders in binuclear metal complexes of the first row transition metals titanium through zinc. Chem. Rev. 118, 11626–11706 (2018).
van Albada, G. A., Mutikainen, I., Turpeinen, U. & Reedijk, J. Crystal structure, magnetism and spectroscopy of two strongly antiferromagnetically coupled dinuclear Cu(II) paddlewheel-like compounds with 4-azabenzimidazole as a ligand. Polyhedron 25, 3278–3284 (2006).
Graziano, B. J. et al. One-electron bonds in copper–aluminum and copper–gallium complexes. Chem. Sci. 13, 6525–6531 (2022).
Shimajiri, T., Kawaguchi, S., Suzuki, T. & Ishigaki, Y. Direct evidence for a carbon–carbon one-electron σ-bond. Nature 634, 347–351 (2024).
Lepetit, C., Fau, P., Fajerwerg, K., Kahn, M. L. & Silvi, B. Topological analysis of the metal–metal bond: a tutorial review. Coord. Chem. Rev. 345, 150–181 (2017).
O’Connor, C. J. in Progress in Inorganic Chemistry, Vol. 29 (ed. Lippard, S. J.) 203–283 (John Wiley & Sons, Inc., 1982).
Weast, R. C. & Astle, M. J. CRC Handbook of Chemistry and Physics (CRC Press, 1979).
Bill, E. Max-Planck Institute for Chemical Energy Conversion.
Sheldrick, G. M. et al. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 71, 3–8 (2015).
Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 42, 339–341 (2009).
Becke, A. D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993).
Andrae, D., Häussermann, U., Dolg, M., Stoll, H. & Preuss, H. Energy-adjusted ab initio pseudopotentials for the second and third row transition elements. Theor. Chim. Acta 77, 123–141 (1990).
Martin, J. M. L. & Sundermann, A. Correlation consistent valence basis sets for use with the Stuttgart–Dresden–Bonn relativistic effective core potentials: the atoms Ga–Kr and In–Xe. J. Chem. Phys. 114, 3408–3420 (2001).
Höllwarth, A. et al. A set of d-polarization functions for pseudo-potential basis sets of the main group elements Al–Bi and f-type polarization functions for Zn, Cd, Hg. Chem. Phys. Lett. 208, 237–240 (1993).
Ditchfield, R., Hehre, W. J. & Pople, J. A. Self-consistent molecular-orbital methods. IX. An extended Gaussian-type basis for molecular-orbital studies of organic molecules. J. Chem. Phys. 54, 724–728 (1971).
Hehre, W. J., Ditchfield, R. & Pople, J. A. Self-consistent molecular orbital methods. XII. Further extensions of Gaussian-type basis sets for use in molecular orbital studies of organic molecules. J. Chem. Phys. 56, 2257–2261 (1972).
Hariharan, P. C. & Pople, J. A. The influence of polarization functions on molecular orbital hydrogenation energies. Theor. Chim. Acta 28, 213–222 (1973).
Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 32, 1456–1465 (2011).
Gaussian 09, Revision D.01 (Gaussian, 2016).
Acknowledgements
The work in Dalian is supported by the National Natural Science Foundation of China (grant nos. 92161204 and 92461311, S.Y.) and Dalian Institute of Chemical Physics, Chinese Academy of Sciences (grant no. DICP I202312, S.Y.). The work in Nanjing is supported by the National Key R&D Program of China (grant no. 2021YFA1502500, C.Z.), the National Natural Science Foundation of China (grant no. 22271138, C.Z.), the Natural Science Foundation of Jiangsu Province (grant no. BK20220065, C.Z.) and the Fundamental Research Funds for the Central Universities (grant no. 020514380329, C.Z.). L.M. is a member of the Institute Universitaire de France. Humboldt Foundation and Chinese Academy of Science are acknowledged for support. CalMip is also gratefully acknowledged for a generous grant of computing time.
Author information
Authors and Affiliations
Contributions
C.Z. conceived this project. W.S. performed the synthesis experiments. F.X., W.C. and Y.Z. performed the SQUID and X-ray diffraction experiments. W.S. and Y.J. performed HRMS experiments. C.Z. and S.Y. analysed the experimental data. T.R. conducted the theoretical computations, and L.M. analysed the results. C.Z., S.Y. and L.M. drafted the paper. All authors discussed the results and contributed to the preparation of the final paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Pekka Pyykkö, Weiqun Shi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Peter Seavill, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Experimental details, Supplementary Figs. 1–44 and Tables 1–18.
Supplementary Data 1
X-ray crystallographic data for 2, CCDC 2182247.
Supplementary Data 2
X-ray crystallographic data for 3, CCDC 2182248.
Source data
Source Data Fig. 3
Source data of unprocessed magnetometric measurements.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sheng, W., Xie, F., Rajeshkumar, T. et al. A crystalline dithorium complex with a Th–Th bond. Nat. Synth (2025). https://doi.org/10.1038/s44160-025-00789-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44160-025-00789-5