Abstract
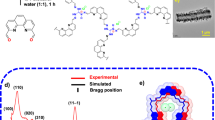
Perfluorooctanoic acid (PFOA) poses a substantial threat to human health due to its bioaccumulation and carcinogenic nature. Current remediation strategies typically focus on either adsorption or degradation, neglecting the potential of an integrated approach. Herein, we present a pyrazolate metal–organic framework (MOF), PCN-1003, featuring a lamellar structure with one-dimensional open channels. PCN-1003 exhibits exceptional stability across a wide pH range (1–12) in aqueous solutions, achieving efficient PFOA adsorption (642 mg g−1). Mechanistic studies revealed that a PFOA–acetate exchange process dominates, representing a remarkable example of such a mechanism and enabling efficient PFOA uptake. Notably, PCN-1003 greatly facilitates PFOA degradation at a much lower temperature (90 °C) than observed in previously reported methods, with an approximately threefold catalytic acceleration effect, attributed to the coordination of PFOA and the confined environment within PCN-1003. This study pioneers integrated PFOA concentration and degradation using a single MOF, presenting a promising avenue for treating water contaminated with per- and polyfluoroalkyl substances.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The X-ray crystallographic coordinates for PCN-1003 reported in this study have been deposited at the Cambridge Crystallographic Data Centre. These data can be obtained free of charge under deposition number 2358814. All other data supporting the findings of this study are available within the article and its Supplementary Information or from the corresponding author upon reasonable request.
References
Lim, X. Can the world leave ‘forever chemicals’ behind? Nature 620, 24–27 (2023).
Trang, B. et al. Low-temperature mineralization of perfluorocarboxylic acids. Science 377, 839–845 (2022).
Li, R., Adarsh, N. N., Lu, H. & Wriedt, M. Metal–organic frameworks as platforms for the removal of per- and polyfluoroalkyl substances from contaminated waters. Matter 5, 3161–3193 (2022).
Du, Z. et al. Removal of perfluorinated carboxylates from washing wastewater of perfluorooctanesulfonyl fluoride using activated carbons and resins. J. Hazard. Mater. 286, 136–143 (2015).
Du, Z. et al. Adsorption behavior and mechanism of perfluorinated compounds on various adsorbents—a review. J. Hazard. Mater. 274, 443–454 (2014).
Wang, W. et al. Cationic covalent organic framework for efficient removal of PFOA substitutes from aqueous solution. Chem. Eng. J. 412, 127509 (2021).
Ji, W. et al. Removal of GenX and perfluorinated alkyl substances from water by amine-functionalized covalent organic frameworks. J. Am. Chem. Soc. 140, 12677–12681 (2018).
Wang, S. et al. Photocatalytic degradation of perfluorooctanoic acid and perfluorooctane sulfonate in water: a critical review. Chem. Eng. J. 328, 927–942 (2017).
Gomez-Ruiz, B. et al. Photocatalytic degradation and mineralization of perfluorooctanoic acid (PFOA) using a composite TiO2–rGO catalyst. J. Hazard. Mater. 344, 950–957 (2018).
Duan, X. et al. Electrocatalytic degradation of perfluoroocatane sulfonate (PFOS) on a 3D graphene-lead dioxide (3DG-PbO2) composite anode: electrode characterization, degradation mechanism and toxicity. Chemosphere 260, 127587 (2020).
Pierpaoli, M. et al. Electrochemical oxidation of PFOA and PFOS in landfill leachates at low and highly boron-doped diamond electrodes. J. Hazard. Mater. 403, 123606 (2021).
Soriano, A., Gorri, D. & Urtiaga, A. Efficient treatment of perfluorohexanoic acid by nanofiltration followed by electrochemical degradation of the NF concentrate. Water Res. 112, 147–156 (2017).
Wang, J. et al. Perfluorooctane sulfonate and perfluorobutane sulfonate removal from water by nanofiltration membrane: the roles of solute concentration, ionic strength, and macromolecular organic foulants. Chem. Eng. J. 332, 787–797 (2018).
Kwon, B. G. et al. Biodegradation of perfluorooctanesulfonate (PFOS) as an emerging contaminant. Chemosphere 109, 221–225 (2014).
Albert, K., Hsieh, P. Y., Chen, T. H., Hou, C. H. & Hsu, H. Y. Diatom-assisted biomicroreactor targeting the complete removal of perfluorinated compounds. J. Hazard. Mater. 384, 121491 (2020).
Evich, M. G. et al. Per- and polyfluoroalkyl substances in the environment. Science 375, eabg9065 (2022).
Winchell, L. J. et al. Per- and polyfluoroalkyl substances thermal destruction at water resource recovery facilities: a state of the science review. Water Environ. Res. 93, 826–843 (2021).
Xiong, X. et al. Complete defluorination of perfluorooctanoic acid (PFOA) by ultrasonic pyrolysis towards zero fluoro-pollution. Water Res. 235, 119829 (2023).
Cheng, J., Vecitis, C. D., Park, H., Mader, B. T. & Hoffmann, M. R. Sonochemical degradation of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in groundwater: kinetic effects of matrix inorganics. Environ. Sci. Technol. 44, 445–450 (2010).
Schaefer, C. E., Andaya, C., Urtiaga, A., McKenzie, E. R. & Higgins, C. P. Electrochemical treatment of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) in groundwater impacted by aqueous film forming foams (AFFFs). J. Hazard. Mater. 295, 170–175 (2015).
Sharma, S., Shetti, N. P., Basu, S., Nadagouda, M. N. & Aminabhavi, T. M. Remediation of per- and polyfluoroalkyls (PFAS) via electrochemical methods. Chem. Eng. J. 430, 132895 (2022).
Chiang, S. D. et al. Supercritical water oxidation for the destruction of spent media wastes generated from PFAS treatment. J. Hazard. Mater. 460, 132264 (2023).
Li, J., Austin, C., Moore, S., Pinkard, B. R. & Novosselov, I. V. PFOS destruction in a continuous supercritical water oxidation reactor. Chem. Eng. J. 451, 139063 (2023).
Verma, S., Lee, T., Sahle-Demessie, E., Ateia, M. & Nadagouda, M. N. Recent advances on PFAS degradation via thermal and nonthermal methods. Chem. Eng. J. Adv. 13, 100421 (2023).
Watanabe, N., Takata, M., Takemine, S. & Yamamoto, K. Thermal mineralization behavior of PFOA, PFHxA, and PFOS during reactivation of granular activated carbon (GAC) in nitrogen atmosphere. Environ. Sci. Pollut. Res. 25, 7200–7205 (2018).
Wang, F., Lu, X., Li, X.-Y. & Shih, K. Effectiveness and mechanisms of defluorination of perfluorinated alkyl substances by calcium compounds during waste thermal treatment. Environ. Sci. Technol. 49, 5672–5680 (2015).
Alinezhad, A. et al. An investigation of thermal air degradation and pyrolysis of per- and polyfluoroalkyl substances and aqueous film-forming foams in soil. ACS EST Eng. 2, 198–209 (2022).
Liu, H., Yao, Y. & Samori, P. Taming multiscale structural complexity in porous skeletons: from open framework materials to micro/nanoscaffold architectures. Small Methods 7, e2300468 (2023).
McDonald, T. M. et al. Cooperative insertion of CO2 in diamine-appended metal–organic frameworks. Nature 519, 303–308 (2015).
Furukawa, H., Cordova, K. E., O’Keeffe, M. & Yaghi, O. M. The chemistry and applications of metal–organic frameworks. Science 341, 1230444 (2013).
Cui, Y., Yue, Y., Qian, G. & Chen, B. Luminescent functional metal–organic frameworks. Chem. Rev. 112, 1126–1162 (2012).
Zhou, H. C., Long, J. R. & Yaghi, O. M. Introduction to metal–organic frameworks. Chem. Rev. 112, 673–674 (2012).
Chen, Z., Kirlikovali, K. O., Li, P. & Farha, O. K. Reticular chemistry for highly porous metal–organic frameworks: the chemistry and applications. Acc. Chem. Res. 55, 579–591 (2022).
Herm, Z. R. et al. Separation of hexane isomers in a metal–organic framework with triangular channels. Science 340, 960–964 (2013).
Liang, R. R. et al. A robust pyrazolate metal–organic framework for efficient catalysis of dehydrogenative C–O cross coupling reaction. J. Am. Chem. Soc. 146, 14174–14181 (2024).
Wang, K. et al. Pyrazolate-based porphyrinic metal–organic framework with extraordinary base-resistance. J. Am. Chem. Soc. 138, 914–919 (2016).
Rieth, A. J., Wright, A. M. & Dincă, M. Kinetic stability of metal–organic frameworks for corrosive and coordinating gas capture. Nat. Rev. Mater. 4, 708–725 (2019).
Colombo, V. et al. High thermal and chemical stability in pyrazolate-bridged metal–organic frameworks with exposed metal sites. Chem. Sci. 2, 1311–1319 (2011).
Maji, T. K., Matsuda, R. & Kitagawa, S. A flexible interpenetrating coordination framework with a bimodal porous functionality. Nat. Mater. 6, 142–148 (2007).
Ok, K. M., Sung, J., Hu, G., Jacobs, R. M. J. & O’Hare, D. TOF-2: a large 1D channel thorium organic framework. J. Am. Chem. Soc. 130, 3762–3763 (2008).
Lü, J. et al. A robust binary supramolecular organic framework (SOF) with high CO2 adsorption and selectivity. J. Am. Chem. Soc. 136, 12828–12831 (2014).
Li, R. et al. Efficient removal of per- and polyfluoroalkyl substances from water with zirconium-based metal–organic frameworks. Chem. Mater. 33, 3276–3285 (2021).
Liu, K. et al. Understanding the adsorption of PFOA on MIL-101(Cr)-based anionic-exchange metal–organic frameworks: comparing DFT calculations with aqueous sorption experiments. Environ. Sci. Technol. 49, 8657–8665 (2015).
Yang, Y., Zheng, Z., Ji, W., Xu, J. & Zhang, X. Insights to perfluorooctanoic acid adsorption micro-mechanism over Fe-based metal organic frameworks: combining computational calculation with response surface methodology. J. Hazard. Mater. 395, 122686 (2020).
Liang, R.-R. et al. Exceptionally high perfluorooctanoic acid uptake in water by a zirconium-based metal–organic framework through synergistic chemical and physical adsorption. J. Am. Chem. Soc. 146, 9811–9818 (2024).
Frisch, M. J. et al. Gaussian 16, Revision C.01 (Gaussian Inc., 2016).
Kühne, T. D. et al. CP2K: An electronic structure and molecular dynamics software package - Quickstep: Efficient and accurate electronic structure calculations. J. Chem. Phys. 152, 194103 (2020).
Acknowledgements
We gratefully acknowledge the financial support from the US Department of Energy, Office of Fossil Energy (grant no. DE-FE0032108). Additional support was provided by the Robert A. Welch Foundation through a Welch Endowed Chair to H.-C.Z (A-0030). We also acknowledge the financial support of the US Department of Energy, Office of Fossil Energy/Pennsylvania State University (grant no. S000655-DOE). Y.F. gratefully acknowledges the support of GWK for funding this project by providing computing time through the Center for Information Services and HPC (ZIH) at TU Dresden.
Author information
Authors and Affiliations
Contributions
H.-C.Z. conceived the research idea. R.-R.L. performed most of the experiments and analysed the data. Y.F. conducted the calculations. Z.H. and Y.Y. solved the single-crystal data. V.I.B. conducted the solid-state NMR experiments and analysed the data. R.-R.L. and H.-C.Z. wrote the paper. Z.L. and J.R. collected and analysed the NMR data. All authors discussed the results and commented on the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Water thanks Lingxin Chen and Mario Wriedt for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
General methods and instruments, Supplementary Figs. 1–39 and Tables 1–3.
Supplementary Data 1
Crystallographic txt file.
Supplementary Data 2
Crystallographic checkcif report.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liang, RR., Fu, Y., Han, Z. et al. A robust pyrazolate metal–organic framework for integrated perfluorooctanoic acid concentration and degradation. Nat Water 2, 1218–1225 (2024). https://doi.org/10.1038/s44221-024-00343-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44221-024-00343-1
This article is cited by
-
The PFAS treatment evolution
Nature Water (2025)
-
PFAS concentration and destruction
Nature Water (2024)