Abstract
Owing to advances in genome sequencing and editing, a genome can now be redesigned, synthesized and introduced into cells as desired. The field of synthetic genomics not only aims to provide deeper understanding of how the genome functions but can also be harnessed for a wide range of synthetic biology and bioengineering applications, from rapid evolution and screening for favourable strains to biotechnological and bioproduction tool development. Although genome synthesis has been carried out mainly in simple unicellular organisms, plants and animals are now also being investigated. Compared with animals, plants have unique advantages, such as fewer ethical concerns, simpler experimental operations and easier regeneration from cells to organisms. In this Review, we focus on genome synthesis in plants, discuss the current research landscape and assess possible future directions.
Key points
-
Advances in genome sequencing, synthesis and editing technologies have enabled systematic redesign and chemical synthesis of plant genomes, establishing a new research discipline for functional genome exploration with potential for agricultural innovation and industrial bioproduction.
-
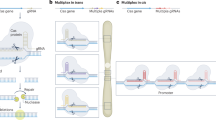
Top-down chromosomal engineering strategies focus on the modification of endogenous chromosomes, and bottom-up strategies use de novo DNA assembly techniques to generate artificial chromosomes with distinct structural and functional properties.
-
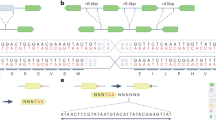
De novo synthesis of plant chromosome segments follows a stage-gated workflow involving computational design, modular DNA assembly, transformation, targeted integration and verification, plant regeneration and phenotypic validating, with post-analysis data driving iterative optimization cycles for genome design refinement.
-
Accurate integration of large DNA fragments remains a technical bottleneck in seed plant synthetic genomics, requiring breakthroughs in homology-directed repair or nonhomologous end joining-mediated integration efficiency and engineered recombinase systems to improve plant genome engineering platforms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
McKusick, V. A. & Ruddle, F. H. Toward a complete map of the human genome. Genomics 1, 103–106 (1987).
Sinyakov, A. N., Ryabinin, V. A. & Kostina, E. V. Application of array-based oligonucleotides for synthesis of genetic designs. Mol. Biol. 55, 487–500 (2021).
Casini, A., Storch, M., Baldwin, G. S. & Ellis, T. Bricks and blueprints: methods and standards for DNA assembly. Nat. Rev. Mol. Cell Biol. 16, 568–576 (2015).
He, B. et al. YLC-assembly: large DNA assembly via yeast life cycle. Nucleic Acids Res. 51, 8283–8292 (2023).
Cello, J., Paul, A. V. & Wimmer, E. Chemical synthesis of poliovirus cDNA: generation of infectious virus in the absence of natural template. Science 297, 1016–1018 (2002). This study established the first fully synthetic genome, validating template-independent de novo genome construction through chemical synthesis methodologies.
Smith, H. O., Hutchison, C. A., Pfannkoch, C. & Venter, J. C. Generating a synthetic genome by whole genome assembly: φX174 bacteriophage from synthetic oligonucleotides. Proc. Natl Acad. Sci. USA 100, 15440–15445 (2003).
Gibson, D. G. et al. Creation of a bacterial cell controlled by a chemically synthesized genome. Science 329, 52–56 (2010).
Fredens, J. et al. Total synthesis of Escherichia coli with a recoded genome. Nature 569, 514–518 (2019).
Hutchison, C. A. et al. Design and synthesis of a minimal bacterial genome. Science 351, aad6253 (2016). This study reports the excision of dispensable genes (47% reduction from 901 to 473) through systematic genome streamlining, resulting in the first functional minimal synthetic cell prototype.
Zhang, W., Mitchell, L. A., Bader, J. S. & Boeke, J. D. Synthetic genomes. Annu. Rev. Biochem. 89, 77–101 (2020).
Dymond, J. S. et al. Synthetic chromosome arms function in yeast and generate phenotypic diversity by design. Nature 477, 471–476 (2011).
Annaluru, N. et al. Total synthesis of a functional designer eukaryotic chromosome. Science 344, 55–58 (2014).
Richardson, S. M. et al. Design of a synthetic yeast genome. Science 355, 1040–1044 (2017). This study describes the Sc2.0 project, which delivered a definitive blueprint for synthetic eukaryotic genomics, incorporating sequence-editing strategies, synthetic telomere design and inducible evolution systems.
Zhang, W. et al. Mouse genome rewriting and tailoring of three important disease loci. Nature 623, 423–431 (2023).
Chen, L.-G. et al. A designer synthetic chromosome fragment functions in moss. Nat. Plants 10, 228–239 (2024). This study is the first demonstration of partial chromosome arm synthesis in plants.
Boeke, J. D. et al. The genome project-write. Science 353, 126–127 (2016).
Jiao, Y. & Wang, Y. Towards plant synthetic genomics. Biodes. Res. 5, 0020 (2023).
Blommaert, J. Genome size evolution: towards new model systems for old questions. Proc. Biol. Sci. 287, 20201441 (2020).
AGARWAL, K. L. et al. Total synthesis of the gene for an alanine transfer ribonucleic acid from yeast. Nature 227, 27–34 (1970).
Koonin, E. V., Dolja, V. V. & Krupovic, M. The logic of virus evolution. Cell Host Microbe 30, 917–929 (2022).
Racaniello, V. R. & Baltimore, D. Molecular cloning of poliovirus cDNA and determination of the complete nucleotide sequence of the viral genome. Proc. Natl Acad. Sci. USA 78, 4887–4891 (1981).
Venter, J. C., Glass, J. I., Hutchison, C. A. & Vashee, S. Synthetic chromosomes, genomes, viruses, and cells. Cell 185, 2708–2724 (2022).
Chan, L. Y., Kosuri, S. & Endy, D. Refactoring bacteriophage T7. Mol. Syst. Biol. 1, 2005.0018 (2005).
Thi Nhu Thao, T. et al. Rapid reconstruction of SARS-CoV-2 using a synthetic genomics platform. Nature 582, 561–565 (2020).
Dormitzer, P. R. et al. Synthetic generation of influenza vaccine viruses for rapid response to pandemics. Sci. Transl. Med. 5, 185ra68 (2013).
Gibson, D. G. et al. Complete chemical synthesis, assembly, and cloning of a Mycoplasma genitalium genome. Science 319, 1215–1220 (2008).
Pelletier, J. F. et al. Genetic requirements for cell division in a genomically minimal cell. Cell 184, 2430–2440.e16 (2021).
Ostrov, N. et al. Design, synthesis, and testing toward a 57-codon genome. Science 353, 819–822 (2016).
Wang, K. et al. Defining synonymous codon compression schemes by genome recoding. Nature 539, 59–64 (2016).
Goffeau, A. et al. Life with 6000 genes. Science 274, 546–567 (1996).
Mitchell, L. A. et al. Synthesis, debugging, and effects of synthetic chromosome consolidation: synVI and beyond. Science 355, eaaf4831 (2017).
Shen, Y. et al. Deep functional analysis of synII, a 770-kilobase synthetic yeast chromosome. Science 355, eaaf4791 (2017).
Wu, Y. et al. Bug mapping and fitness testing of chemically synthesized chromosome X. Science 355, eaaf4706 (2017).
Xie, Z.-X. et al. ‘Perfect’ designer chromosome V and behavior of a ring derivative. Science 355, eaaf4704 (2017).
Zhang, W. et al. Engineering the ribosomal DNA in a megabase synthetic chromosome. Science 355, eaaf3981 (2017).
Zhao, Y. et al. Debugging and consolidating multiple synthetic chromosomes reveals combinatorial genetic interactions. Cell 186, 5220–5236.e16 (2023).
Schindler, D. et al. Design, construction, and functional characterization of a tRNA neochromosome in yeast. Cell 186, 5237–5253.e22 (2023).
Admire, A. et al. Cycles of chromosome instability are associated with a fragile site and are increased by defects in DNA replication and checkpoint controls in yeast. Genes Dev. 20, 159–173 (2006).
Hamperl, S., Bocek, M. J., Saldivar, J. C., Swigut, T. & Cimprich, K. A. Transcription–replication conflict orientation modulates R-loop levels and activates distinct DNA damage responses. Cell 170, 774–786.e19 (2017).
Mularoni, L. et al. Retrotransposon Ty1 integration targets specifically positioned asymmetric nucleosomal DNA segments in tRNA hotspots. Genome Res. 22, 693–703 (2012).
Dai, J., Boeke, J. D., Luo, Z., Jiang, S. & Cai, Y. Sc3.0: revamping and minimizing the yeast genome. Genome Biol. 21, 205 (2020).
Birchler, J. A., Graham, N. D., Swyers, N. C., Cody, J. P. & McCaw, M. E. Plant minichromosomes. Curr. Opin. Biotechnol. 37, 135–142 (2016).
Shay, J. W. & Wright, W. E. Telomeres and telomerase: three decades of progress. Nat. Rev. Genet. 20, 299–309 (2019).
Yu, W., Lamb, J. C., Han, F. & Birchler, J. A. Telomere-mediated chromosomal truncation in maize. Proc. Natl Acad. Sci. USA 103, 17331–17336 (2006).
Carlson, W. R. & Phillips, R. L. The B chromosome of maize. Crit. Rev. Plant Sci. 3, 201–226 (1986).
Gaeta, R. T. et al. In vivo modification of a maize engineered minichromosome. Chromosoma 122, 221–232 (2013).
Teo, C. H. et al. Induction of telomere-mediated chromosomal truncation and stability of truncated chromosomes in Arabidopsis thaliana. Plant J. 68, 28–39 (2011).
Murata, M. Minichromosomes and artificial chromosomes in Arabidopsis. Chr. Res. 22, 167–178 (2014).
Xu, C., Cheng, Z. & Yu, W. Construction of rice mini-chromosomes by telomere-mediated chromosomal truncation. Plant J. Cell Mol. Biol. 70, 1070–1079 (2012).
Kapusi, E. et al. Telomere-mediated truncation of barley chromosomes. Chromosoma 121, 181–190 (2012).
Kouprina, N. et al. Human artificial chromosome with regulated centromere: a tool for genome and cancer studies. ACS Synth. Biol. 7, 1974–1989 (2018).
Jiang, S. et al. Efficient de novo assembly and modification of large DNA fragments. Sci. China Life Sci. 65, 1445–1455 (2022).
Cove, D. The moss Physcomitrella patens. Annu. Rev. Genet. 39, 339–358 (2005).
Rensing, S. A., Goffinet, B., Meyberg, R., Wu, S.-Z. & Bezanilla, M. The moss Physcomitrium (Physcomitrella) patens: a model organism for non-seed plants. Plant Cell 32, 1361–1376 (2020).
de Keijzer, J., Freire Rios, A. & Willemsen, V. Physcomitrium patens: a single model to study oriented cell divisions in 1D to 3D patterning. Int. J. Mol. Sci. 22, 2626 (2021).
Bi, G. et al. Near telomere-to-telomere genome of the model plant Physcomitrium patens. Nat. Plants 10, 327–343 (2024).
Schaefer, D. G. & Zrÿd, J.-P. Efficient gene targeting in the moss Physcomitrella patens. Plant J. 11, 1195–1206 (1997).
Charlot, F., Goudounet, G., Nogué, F. & Perroud, P.-F. in Protoplast Technology: Methods and Protocols (eds Wang, K. & Zhang, F.) 3–19 (Humana Press, 2022).
Widiez, T. et al. The chromatin landscape of the moss Physcomitrella patens and its dynamics during development and drought stress. Plant J. 79, 67–81 (2014).
Lang, D. et al. The Physcomitrella patens chromosome-scale assembly reveals moss genome structure and evolution. Plant J. 93, 515–533 (2018).
Khairul Ikram, N. K., Zakariya, A. M., Saiman, M. Z., Kashkooli, A. B. & Simonsen, H. T. Heterologous production of artemisinin in Physcomitrium patens by direct in vivo assembly of multiple DNA fragments. Bio-Protocol 13, e4719 (2023).
Reski, R., Parsons, J. & Decker, E. L. Moss-made pharmaceuticals: from bench to bedside. Plant Biotechnol. J. 13, 1191–1198 (2015).
Decker, E. L., Parsons, J. & Reski, R. Glyco-engineering for biopharmaceutical production in moss bioreactors. Front. Plant Sci. 5, 346 (2014).
Yu, W. et al. Designing a synthetic moss genome using GenoDesigner. Nat. Plants 10, 848–856 (2024). This study reports a systematic framework to govern synthesized chromosome in Physcomitrella patens, integrating sequence modularization and orthogonal design constraints.
Gao, D. Introduction of plant transposon annotation for beginners. Biology 12, 1468 (2023).
Elliott, T. A., Linquist, S. & Gregory, T. R. Conceptual and empirical challenges of ascribing functions to transposable elements. Am. Nat. 184, 14–24 (2014).
Doolittle, W. F., Brunet, T. D. P., Linquist, S. & Gregory, T. R. Distinguishing between ‘function’ and ‘effect’ in genome biology. Genome Biol. Evol. 6, 1234–1237 (2014).
Gemmell, N. J. Repetitive DNA: genomic dark matter matters. Nat. Rev. Genet. 22, 342 (2021).
Buckley, P. T., Khaladkar, M., Kim, J. & Eberwine, J. Cytoplasmic intron retention, function, splicing, and the sentinel RNA hypothesis. Wiley Interdiscip. Rev. RNA 5, 223–230 (2014).
Schmitz, U. et al. Intron retention enhances gene regulatory complexity in vertebrates. Genome Biol. 18, 216 (2017).
Shaul, O. How introns enhance gene expression. Int. J. Biochem. Cell Biol. 91, 145–155 (2017).
Erikson, O., Hertzberg, M. & Näsholm, T. A conditional marker gene allowing both positive and negative selection in plants. Nat. Biotechnol. 22, 455–458 (2004).
Dymond, J. & Boeke, J. The Saccharomyces cerevisiae SCRaMbLE system and genome minimization. Bioeng. Bugs 3, 168–171 (2012).
Comai, L., Maheshwari, S. & Marimuthu, M. P. A. Plant centromeres. Curr. Opin. Plant Biol. 36, 158–167 (2017).
Melters, D. P. et al. Comparative analysis of tandem repeats from hundreds of species reveals unique insights into centromere evolution. Genome Biol. 14, R10 (2013).
McKinley, K. L. & Cheeseman, I. M. The molecular basis for centromere identity and function. Nat. Rev. Mol. Cell Biol. 17, 16–29 (2016).
Carroll, C. W., Milks, K. J. & Straight, A. F. Dual recognition of CENP-A nucleosomes is required for centromere assembly. J. Cell Biol. 189, 1143–1155 (2010).
Harrington, J. J., Bokkelen, G. V., Mays, R. W., Gustashaw, K. & Willard, H. F. Formation of de novo centromeres and construction of first-generation human artificial microchromosomes. Nat. Genet. 15, 345–355 (1997).
Ebersole, T. et al. Rapid generation of long synthetic tandem repeats and its application for analysis in human artificial chromosome formation. Nucleic Acids Res. 33, e130 (2005).
Noskov, V. N., Lee, N. C., Larionov, V. & Kouprina, N. Rapid generation of long tandem DNA repeat arrays by homologous recombination in yeast to study their function in mammalian genomes. Biol. Proc. Online 13, 8 (2011).
Nakano, M. et al. Inactivation of a human kinetochore by specific targeting of chromatin modifiers. Dev. Cell 14, 507–522 (2008).
Kouprina, N. et al. Organization of synthetic alphoid DNA array in human artificial chromosome (HAC) with a conditional centromere. ACS Synth. Biol. 1, 590–601 (2012).
Logsdon, G. A. et al. Human artificial chromosomes that bypass centromeric DNA. Cell 178, 624–639.e19 (2019).
Phan, B. H. et al. Transformation of rice with long DNA-segments consisting of random genomic DNA or centromere-specific DNA. Transgenic Res. 16, 341–351 (2007).
Dawe, R. K. Charting the path to fully synthetic plant chromosomes. Exp. Cell Res. 390, 111951 (2020).
Gent, J. I., Dong, Y., Jiang, J. & Dawe, R. K. Strong epigenetic similarity between maize centromeric and pericentromeric regions at the level of small RNAs, DNA methylation and H3 chromatin modifications. Nucleic Acids Res. 40, 1550–1560 (2012).
Teo, C. H., Lermontova, I., Houben, A., Mette, M. F. & Schubert, I. De novo generation of plant centromeres at tandem repeats. Chromosoma 122, 233–241 (2013).
Dawe, R. K. et al. Synthetic maize centromeres transmit chromosomes across generations. Nat. Plants 9, 433–441 (2023). This article describes the engineering of LexA–CENH3 chimaeras to mediate accurate recruitment of centromeric histones to LexO repeats, establishing de novo kinetochore complexes that triggered programmable chromosome breakage.
Zhang, H. & Dawe, R. K. Total centromere size and genome size are strongly correlated in ten grass species. Chromosome Res. 20, 403–412 (2012).
Plačková, K., Bureš, P. & Zedek, F. Centromere size scales with genome size across eukaryotes. Sci. Rep. 11, 19811 (2021).
Kumawat, S. & Choi, J. Y. No end in sight: mysteries of the telomeric variation in plants. Am. J. Bot. 110, e16244 (2023).
Zvereva, M. I., Shcherbakova, D. M. & Dontsova, O. A. Telomerase: structure, functions, and activity regulation. Biochemistry (Mosc) 75, 1563–1583 (2010).
Heacock, M., Spangler, E., Riha, K., Puizina, J. & Shippen, D. E. Molecular analysis of telomere fusions in Arabidopsis: multiple pathways for chromosome end‐joining. EMBO J. 23, 2304–2313 (2004).
Kosuri, S. & Church, G. M. Large-scale de novo DNA synthesis: technologies and applications. Nat. Methods 11, 499–507 (2014).
Beaucage, S. L. & Caruthers, M. H. Deoxynucleoside phosphoramidites — a new class of key intermediates for deoxypolynucleotide synthesis. Tetrahedron Lett. 22, 1859–1862 (1981).
LeProust, E. M. et al. Synthesis of high-quality libraries of long (150mer) oligonucleotides by a novel depurination controlled process. Nucleic Acids Res. 38, 2522–2540 (2010).
Matzas, M. et al. High-fidelity gene synthesis by retrieval of sequence-verified DNA identified using high-throughput pyrosequencing. Nat. Biotechnol. 28, 1291–1294 (2010).
Palluk, S. et al. De novo DNA synthesis using polymerase–nucleotide conjugates. Nat. Biotechnol. 36, 645–650 (2018).
Barthel, S., Palluk, S., Hillson, N. J., Keasling, J. D. & Arlow, D. H. Enhancing terminal deoxynucleotidyl transferase activity on substrates with 3′ terminal structures for enzymatic de novo DNA synthesis. Genes 11, 102 (2020).
Benoit, R. M. et al. Seamless insert-plasmid assembly at high efficiency and low cost. PLoS ONE 11, e0153158 (2016).
Gibson, D. G. et al. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 6, 343–345 (2009).
Engler, C., Gruetzner, R., Kandzia, R. & Marillonnet, S. Golden Gate shuffling: a one-pot DNA shuffling method based on type IIs restriction enzymes. PLOS ONE 4, e5553 (2009).
Gibson, D. G. Gene and genome construction in yeast. Curr. Protoc. Mol. Biol. Ch. 3, Unit3.22 (2011).
Muller, H. et al. Assembling large DNA segments in yeast. Methods Mol. Biol. 852, 133–150 (2012).
Gelvin, S. B. Integration of Agrobacterium T-DNA into the plant genome. Annu. Rev. Genet. 51, 195–217 (2017).
Hwang, H.-H., Yu, M. & Lai, E.-M. Agrobacterium-mediated plant transformation: biology and applications. Arab. Book 15, e0186 (2017).
Altpeter, F. et al. Particle bombardment and the genetic enhancement of crops: myths and realities. Mol. Breed. 15, 305–327 (2005).
Lowe, B. A. et al. Enhanced single copy integration events in corn via particle bombardment using low quantities of DNA. Transgenic Res. 18, 831–840 (2009).
Hamilton, C. M., Frary, A., Lewis, C. & Tanksley, S. D. Stable transfer of intact high molecular weight DNA into plant chromosomes. Proc. Natl Acad. Sci. USA 93, 9975–9979 (1996).
Liu, J. et al. Genome-scale sequence disruption following biolistic transformation in rice and maize. Plant Cell 31, 368–383 (2019).
Ikeuchi, M., Sugimoto, K. & Iwase, A. Plant callus: mechanisms of induction and repression. Plant Cell 25, 3159–3173 (2013).
Duarte, P., Ribeiro, D., Carqueijeiro, I., Bettencourt, S. & Sottomayor, M. in Biotechnology of Plant Secondary Metabolism: Methods and Protocols (ed. Fett-Neto, A. G.) 137–148 (Humana Press, 2016).
Li, L. & Blankenstein, T. Generation of transgenic mice with megabase-sized human yeast artificial chromosomes by yeast spheroplast–embryonic stem cell fusion. Nat. Protoc. 8, 1567–1582 (2013).
Brown, D. M. et al. Efficient size-independent chromosome delivery from yeast to cultured cell lines. Nucleic Acids Res. 45, e50–e50 (2017).
Wu, F.-H., Yuan, Y.-H., Hsu, C.-T., Cheng, Q.-W. & Lin, C.-S. in Protoplast Technology: Methods and Protocols (eds Wang, K. & Zhang, F.) 49–64 (Humana Press, 2022).
Jones, A. M. P., Shukla, M. R., Biswas, G. C. G. & Saxena, P. K. Protoplast-to-plant regeneration of American elm (Ulmus americana). Protoplasma 252, 925–931 (2015).
Jeong, Y. Y., Lee, H.-Y., Kim, S. W., Noh, Y.-S. & Seo, P. J. Optimization of protoplast regeneration in the model plant Arabidopsis thaliana. Plant Methods 17, 21 (2021).
Lowe, K. et al. Morphogenic regulators Baby boom and Wuschel improve monocot transformation. Plant Cell 28, 1998–2015 (2016).
Debernardi, J. M. et al. A GRF–GIF chimeric protein improves the regeneration efficiency of transgenic plants. Nat. Biotechnol. 38, 1274–1279 (2020).
Yu, Y. et al. Enhancing wheat regeneration and genetic transformation through overexpression of TaLAX1. Focus Issue Wheat Biol. 5, 100738 (2024).
Liu, X. et al. Uncovering the transcriptional regulatory network involved in boosting wheat regeneration and transformation. Nat. Plants 9, 908–925 (2023).
Xu, M., Du, Q., Tian, C., Wang, Y. & Jiao, Y. Stochastic gene expression drives mesophyll protoplast regeneration. Sci. Adv. 7, eabg8466 (2021).
Yang, W. et al. Peptide REF1 is a local wound signal promoting plant regeneration. Cell 187, 3024–3038.e14 (2024).
Wang, N. et al. Leaf transformation for efficient random integration and targeted genome modification in maize and sorghum. Nat. Plants 9, 255–270 (2023).
Sun, Y. et al. Engineering herbicide-resistant rice plants through CRISPR/Cas9-mediated homologous recombination of acetolactate synthase. Mol. Plant 9, 628–631 (2016).
Schmidt, C., Pacher, M. & Puchta, H. in Transgenic Plants: Methods and Protocols (eds Kumar, S. et al.) 237–266 (Humana Press, 2019).
Fauser, F. et al. In planta gene targeting. Proc. Natl Acad. Sci. USA 109, 7535–7540 (2012).
Mali, P. et al. RNA-guided human genome engineering via Cas9. Science 339, 823–826 (2013).
Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013).
Zhao, Y. et al. An alternative strategy for targeted gene replacement in plants using a dual-sgRNA/Cas9 design. Sci. Rep. 6, 23890 (2016).
Rees, H. A., Yeh, W.-H. & Liu, D. R. Development of hRad51–Cas9 nickase fusions that mediate HDR without double-stranded breaks. Nat. Commun. 10, 2212 (2019).
Chauhan, V. P., Sharp, P. A. & Langer, R. Altered DNA repair pathway engagement by engineered CRISPR–Cas9 nucleases. Proc. Natl Acad. Sci. USA 120, e2300605120 (2023).
Schreiber, T. et al. Efficient scar-free knock-ins of several kilobases in plants by engineered CRISPR–Cas endonucleases. Mol. Plant 17, 824–837 (2024).
Ali, Z. et al. Fusion of the Cas9 endonuclease and the VirD2 relaxase facilitates homology-directed repair for precise genome engineering in rice. Commun. Biol. 3, 44 (2020).
Morton, J., Davis, M. W., Jorgensen, E. M. & Carroll, D. Induction and repair of zinc-finger nuclease-targeted double-strand breaks in Caenorhabditis elegans somatic cells. Proc. Natl Acad. Sci. USA 103, 16370–16375 (2006).
Maruyama, T. et al. Increasing the efficiency of precise genome editing with CRISPR–Cas9 by inhibition of nonhomologous end joining. Nat. Biotechnol. 33, 538–542 (2015).
Qi, Y. et al. Increasing frequencies of site-specific mutagenesis and gene targeting in Arabidopsis by manipulating DNA repair pathways. Genome Res. 23, 547–554 (2013).
Song, J. et al. RS-1 enhances CRISPR/Cas9- and TALEN-mediated knock-in efficiency. Nat. Commun. 7, 10548 (2016).
Karasu, M. E. et al. Removal of TREX1 activity enhances CRISPR–Cas9-mediated homologous recombination. Nat. Biotechnol. https://doi.org/10.1038/s41587-024-02356-3 (2024).
Lu, Y. et al. Targeted, efficient sequence insertion and replacement in rice. Nat. Biotechnol. 38, 1402–1407 (2020).
Liu, P. et al. Transposase-assisted target-site integration for efficient plant genome engineering. Nature 631, 593–600 (2024).
Kühn, R. & Torres, R. M. in Transgenesis Techniques: Principles and Protocols (ed. Clarke, A. R.) 175–204 (Springer, 2002).
Sun, C. et al. Precise integration of large DNA sequences in plant genomes using PrimeRoot editors. Nat. Biotechnol. 42, 316–327 (2024).
Rönspies, M., Dorn, A., Schindele, P. & Puchta, H. CRISPR–Cas-mediated chromosome engineering for crop improvement and synthetic biology. Nat. Plants 7, 566–573 (2021).
Rönspies, M., Schindele, P., Wetzel, R. & Puchta, H. CRISPR–Cas9-mediated chromosome engineering in Arabidopsis thaliana. Nat. Protoc. 17, 1332–1358 (2022).
Schmidt, C. et al. Changing local recombination patterns in Arabidopsis by CRISPR/Cas mediated chromosome engineering. Nat. Commun. 11, 4418 (2020).
Rönspies, M. et al. Massive crossover suppression by CRISPR–Cas-mediated plant chromosome engineering. Nat. Plants 8, 1153–1159 (2022).
Beying, N., Schmidt, C., Pacher, M., Houben, A. & Puchta, H. CRISPR–Cas9-mediated induction of heritable chromosomal translocations in Arabidopsis. Nat. Plants 6, 638–645 (2020).
Godwin, J. & Farrona, S. Plant epigenetic stress memory induced by drought: a physiological and molecular perspective. Methods Mol. Biol. 2093, 243–259 (2020).
Hemenway, E. A. & Gehring, M. Epigenetic regulation during plant development and the capacity for epigenetic memory. Annu. Rev. Plant Biol. 74, 87–109 (2023).
Gjaltema, R. A. F. & Rots, M. G. Advances of epigenetic editing. Curr. Opin. Chem. Biol. 57, 75–81 (2020).
Srivastava, V. & Thomson, J. Gene stacking by recombinases. Plant Biotechnol. J. 14, 471–482 (2016).
Acknowledgements
Work in the authors’ laboratory is funded by the National Key R&D Program of China (2023YFE0101100 and 2019YFA0903900), the Natural Science Foundation of China (32230010 and 32270345), the Qidong-SLS Innovation Fund, and the Guangdong Provincial Key Laboratory of Synthetic Genomics (2023B1212060054).
Author information
Authors and Affiliations
Contributions
The manuscript was written by T.L. All the authors contributed to discussion of the content and review and editing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Citation diversity statement
We acknowledge that papers authored by scholars from historically excluded groups are systematically under-cited. Here, we have made every attempt to reference relevant papers in a manner that is equitable in terms of racial, ethnic, gender and geographical representation.
Peer review
Peer review information
Nature Reviews Bioengineering thanks Ilha Lee, Yiping Qi, who co-reviewed with Rushil Mandlik, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lan, T., Chen, LG., Wang, Y. et al. Genome synthesis in plants. Nat Rev Bioeng (2025). https://doi.org/10.1038/s44222-025-00326-1
Accepted:
Published:
DOI: https://doi.org/10.1038/s44222-025-00326-1