Abstract
Background
Metastatic colorectal cancer (mCRC) patients in trials are selected. The aim was to study mCRC features population-based.
Methods
All 765 mCRC patients in the Uppsala region, Sweden, 2010–2020 were identified and analysed for RAS (n = 356/708) and BRAF-V600E (n = 123/708) mutations (mt) and deficient mismatch repair (dMMR, n = 58/643).
Results
Right colon primary tumours were associated with BRAF-V600Emt and dMMR and had worse median overall survival (mOS) than left colon or rectal mCRC. RAS&BRAF wildtype (wt) and proficient MMR were seen in 22%, 45%, and 31% of right colon, left colon, and rectum, respectively. Patients with right colon primaries received best supportive care only more often (34% vs 25% vs 24%) and metastasectomy less often (21% vs 31% vs 33%) than left colon and rectal primaries. In molecularly homogeneous subgroups (RAS&BRAFwt/RASmt/BRAF-V600Emt/dMMR) no difference in mOS were seen between right and left colon primaries, whereas rectal primaries had better mOS (26/15/8/9 vs 24/21/8/8 vs 32/23/6/NA months, respectively). This was also the case in homogenous treatment groups. Primary tumour ___location turned non-significant in multivariable OS analyses.
Conclusions
The high variation of BRAF-V600Emt, RASmt, dMMR, and treatment allocation population-based per primary tumour ___location explain the poor outcome in right-sided cancers.
Similar content being viewed by others

Introduction
Colorectal cancer (CRC) is the third most common cancer and the second leading cause for cancer death worldwide [1]. At diagnosis, distant metastases are found in about 25% of the patients and up to 20% will recur after curative surgery. Medical treatment for metastatic CRC (mCRC) has advanced substantially [2], resulting in marked survival prolongation, with recent clinical trials reporting a median overall survival (mOS) exceeding 30 months [2, 3]. Surgery for metastatic disease has also contributed to the improved outcome [2, 4].
This marked progress has not been reported in population-based patient series. Rather, mOS in the order of 12–15 months is reported [5,6,7,8,9,10]. Patients in clinical trials or treated at dedicated hospitals are younger, have better Eastern Cooperative Oncology Group performance status (ECOG-PS), fewer comorbidities, and underrepresentation of certain metastatic sites. Patients included in recent clinical trials have been molecularly selected and subgroups with poor prognosis, such as RAS mutations (mt), BRAF-V600Emt, and deficient mismatch repair (dMMR), are more frequent population-based than in trials/hospital-based series [11,12,13].
For decades, patients with mCRC from different parts of the colon and rectum were handled similarly since they had the same prognosis and responded similarly to chemotherapy and besides colon and rectum, primary tumour ___location was seldom reported [14]. After the introduction of biologic agents, studies have reported a worse prognosis in patients with right colon tumours indicating that they should be treated differently [2, 15, 16]. The latter particularly relates to the use of epidermal growth factor receptor (EGFR)-inhibitors, and recommendations not to use these in right-sided tumours have emerged [2, 17,18,19]. Studies have also suggested that the colorectum should be divided beyond sidedness [20,21,22,23].
To better understand the relevance of different prognostic factors, including primary tumour ___location, all patients with mCRC in a defined population were identified and clinicopathological information collected. Diagnostic tumour material was, whenever sufficient, analysed for RAS, BRAF, and PIK3CA mutations, and MMR-status. The aim was to describe in detail clinical characteristics, treatments used, and outcomes in a population-based mCRC cohort, where selection has been reduced to a minimum, with focus on the relevance of primary tumour ___location and molecular alterations.
Methods
Patients and clinicopathological data collection
All patients living in the Uppsala region (population 388,000 in 2020) diagnosed with a primary CRC between January 2010 and December 2020 have been prospectively registered in the Swedish Colorectal Cancer Registry (coverage virtually 100%) [24] where relevant clinical information is documented. Biomaterials (blood and tissue) were collected in the Uppsala-Umeå Comprehensive Cancer Consortium [25]. The clinical records at Akademiska sjukhuset, were searched annually for missed recurrences, and all diagnoses of synchronous disease re-evaluated [24].
All treatments and care activities were performed according to guidelines. Systemic therapy (treatment intent either palliative, neoadjuvant, conversion, or adjuvant after metastasectomy) was given according to the Swedish national cancer care programme, which closely adheres to the European Society for Medical Oncology (ESMO)-guidelines [2, 3, 26]. EGFR-inhibitors were not given if the tumour was RASmt or BRAF-V600Emt [2, 27, 28]. During the inclusion period, sidedness was generally not considered in the decision of whether to give EGFR-inhibitors.
Molecular analyses
Tumour tissue from biopsies and/or surgical specimens was collected from primary tumours and/or metastases. Molecular analyses were done in routine healthcare for KRAS (codons 12, 13, and 61) and BRAF-V600E mutations using pyrosequencing [29] prior to November 2014 (16 patients were tested only for KRAS- and BRAF-V600E mutations; they were included in the RAS&BRAF wildtype [wt] group). Since December 2014, next-generation sequencing (NGS) panels have been used to analyse hotspot mutations in KRAS and NRAS (codons 12, 13, 61, and 146), BRAF-V600E, and since autumn 2016 PIK3CA. MMR-status was analysed in clinical routine for all patients using immunohistochemistry (IHC) since 2020, and as targeted testing before this.
Diagnostic slides were re-examined to identify material for additional molecular testing for as many cases as possible. Whenever fresh frozen tumour material with ≥20% tumour cell content and matched blood or normal tissue were available, whole-genome sequencing (WGS) was performed [30]. The coding regions of the KRAS and NRAS genes, BRAF-V600E, and PIK3CA were considered here.
In the cases without frozen material, formalin-fixed paraffin-embedded (FFPE) material was used for DNA extraction, if tumour content ≥10%. It was not allowed to consume all tumour material because of potential clinical use. DNA was extracted from FFPE, using the NGEx® FFPE DNA purification kit (Oncodia, Sweden) and sequenced with Ion TorrentTM OncomineTM Tumour Mutation Load assay (Thermo-Fisher Scientific, MA, USA) covering 1.7 Mb across 409 cancer-driver genes, allowing for tumour mutation burden (TMB) assessment [31]. The coding regions of the KRAS and NRAS genes, BRAF-V600E, and PIK3CA were considered here. Genetic testing was part of clinical routine in 603 samples, using NGS in 419 (77 not analysed in clinical routine), and using WGS in 187 (10 not analysed in clinical routine or using NGS). Samples of 5 patients had no invasive tumour detected and for 52 patients the material was too scarce.
Microsatellite instability (MSI) was assessed with the TrueMarkTM MSI Assay kit (Thermo-Fisher Scientific, MA, USA) as a multiplex PCR with subsequent fragment analysis. If ≥4/13 markers were unstable the tumour was classified as MSI-high (denoted dMMR). WGS was used for determining MSI-status using MSIsensor2 tumour-normal paired mode [30, 32]. dMMR was assessed using IHC for MSH6, PMS2, MLH1, and MSH2 [12], with limited material only MSH6 and PMS2 were stained. For 155 patients, TMB > 10 by the Ion TorrentTM OncomineTM Tumour Mutation Load assay was used as a proxy for dMMR [33].
Statistical analyses
The STROBE-statement for cohort studies was adhered to [34].
Categorical data were presented as proportions, with percentages, and Chi-square or Fischer’s exact test used for comparisons. Mann–Whitney or Kruskal–Wallis’s tests were used for continuous variables. Two-tailed p-values < 0.05 were considered statistically significant.
OS was estimated from mCRC diagnosis to death or censored if alive at last follow-up (data cut-off 16 March 2023) using Kaplan–Meier. OS comparisons were performed using Cox regression models with 95% confidence intervals (95% CI). A multivariable Cox regression model was constructed where statistically significant and clinically relevant variables were included. Conditional landmark analysis for OS was used for assessing whether guarantee time bias influenced the effect of treatments. Proportional hazard assumption was checked visually using Schoenfeld residuals, no clear violations were seen. All statistical analyses were done using SPSS statistical software versions 27 and 29 (IBM Corporation, Armonk, NY, USA).
Results
Characteristics of the cohort
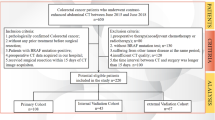
Between January 2010 and December 2020, 2,114 patients living in the Uppsala region were diagnosed with CRC. Of these, 510 (24%) had synchronous metastases and 255 (12%) developed metachronous metastases before March 2023. Totally, 765 mCRC patients constitute the final cohort.
Primary tumour ___location was right colon (caecum [including appendix] to transverse colon) in 291 (38%), left colon (splenic flexure to sigmoid colon) in 207 (27%), and rectum (distal 15 cm) in 262 (34%). Characteristics according to primary tumour ___location are shown in Table 1. Median age was higher for colon cancers (right colon 74 years, left colon 72 years) than for rectal cancers (70 years, p = 0.001). Right colon tumours were more often seen in females and their ECOG-PS at diagnosis of metastatic disease was worse. Synchronous disease was more common in left colon tumours than in right colon and rectal tumours. Liver and lung metastases were less common in right colon tumours than in left colon and rectal tumours, whereas peritoneal metastases were most frequent in right colon tumours.
Molecular characteristics of the tumours according to tumour site
All patients had a morphologically verified adenocarcinoma. Tumour grade was possible to characterise in 683 (89%) patients. High-grade tumours were more frequent in right colon than in left colon and rectum (Table 1), and in BRAF-V600Emt compared with RASmt and RAS&BRAFwt (Table 2).
Molecular analyses were performed for 708 (93%) cases, with similar frequencies for each primary tumour ___location. Not molecularly tested patients were older, had less primary tumour resections, synchronous presentation more often, poorer ECOG-PS, and received active treatment less often (Supplementary Table S1). Of the 708 tested patients, 123 (17%) had BRAF-V600Emt; being more common in right colon compared with left colon and rectum (Table 1). RASmt was seen in 363 (52%) tumours and was less common in right colon and left colon compared with rectum. PIK3CAmt was seen in 62/248 (25%) of right colon, in 35/185 (19%) of left colon, and in 29/235 (12%) of rectal tumours (p = 0.002).
Patient characteristics for each mutation group are shown in Table 2. No differences in age, presentation of metastases, or number of metastatic sites were seen. Female sex was more common and ECOG-PS worse among BRAF-V600Emt compared with RASmt and RAS&BRAFwt. Liver metastases were less common and peritoneal metastases more common in BRAF-V600Emt compared with RASmt and RAS&BRAFwt. Lung metastases were more common in RASmt compared with BRAF-V600Emt and RAS&BRAFwt.
MMR-status was assessed by IHC, PCR, or WGS in 488 or by TMB as a proxy in 155 tumours. Fifty-eight (9%) of the 643 (84%) tumours characterised for MMR had dMMR. dMMR was more common in right colon than left colon and rectum (Table 1, Fig. 1B), and in BRAF-V600Emt compared with RASmt and RAS&BRAFwt (Table 2). Characteristics according to MMR-status are presented in Table 3. dMMR patients were older, more often female, had worse ECOG-PS, and had high-grade tumours more often compared with pMMR. No difference in presentation of metastases or number of metastatic sites were seen. Peritoneal metastases were more common in dMMR compared with pMMR, whereas no differences were seen for other metastatic sites. Patients not tested for MMR were more similar to patients with pMMR. Results were in line if patients only analysed with TMB as a proxy for MMR were excluded (data not shown).
Molecular characteristics of the tumours according to a continuum in colon and rectum
When mutation- and MMR-status for primary tumour ___location were analysed beyond the trichotomous division, BRAF-V600Emt were more common from caecum to descending colon compared with sigmoid colon to rectum (27–48% vs 5–6%, p < 0.001). dMMR was more prevalent in caecum to the splenic flexure than in descending colon to rectum (15–33% vs 0–2%, p < 0.001, Supplementary Table S2).
Treatments according to clinical and molecular features
In the 510 patients with synchronous disease, the primary tumour was removed in 179 (35%) patients, more often in right and left colon than in rectum (89/187 [48%] vs 60/153 [39%] vs 30/166 [18%], p < 0.001). Age did not influence these proportions (data not shown).
Metastasectomy and/or local ablative therapy (LAT) with or without systemic therapy was the treatment for 213 (28%) patients (denoted “metastasectomy”), systemic therapy without metastasectomy for 334 (44%) patients (denoted systemic therapy), and no active tumour controlling therapy besides palliative radiotherapy for the remaining 217 (28%) patients (denoted best supportive care, BSC, Table 1). Metastasectomy rates among actively treated patients was 39% (32% right colon, 41% left colon, 43% rectal cancers (p = 0.062).
Patient characteristics according treatment groups are presented in Supplementary Table S3. Actively treated patients were younger than those cared for with BSC and had less right colon and high-grade tumours. The metastasectomy group had right colon primary tumours less often, less synchronous presentation, fewer metastatic sites, and better ECOG-PS compared with the other groups. Patients with BRAF-V600Emt tumours had metastasectomies less often than RASmt and RAS&BRAFwt (15% vs 31% vs 36%, p < 0.001, Table 2). Similarly, patients with dMMR tumours had metastasectomies less often than patients with pMMR tumours (17% vs 33%, p < 0.001, Table 3, Fig. 1B).
Chemotherapies according to primary tumour ___location are presented in Supplementary Table S4. Patients with right colon tumours received fewer treatment lines and more often had palliative intent of first-line chemotherapy compared with left colon and rectal tumours. First-line treatment was, thus, less intensive in right colon primaries, but more similar in further lines.
Chemotherapies according to mutation status are presented in Supplementary Table S5. Patients with BRAF-V600Emt tumours received fewer treatment lines, less intensive treatments, and the intent in first line was palliative more often compared with RASmt and RAS&BRAFwt. Differences in second-line treatment could also be seen between mutation groups.
Response to first-line chemotherapy was seen less often in BRAF-V600Emt compared with RASmt and RAS&BRAFwt (partial/complete response in 25% vs 49% vs 56%, p < 0.001, Supplementary Table S5). Thus, less responses were seen also in right colon cancers than in left colon and rectal cancers (Supplementary Table S4). No differences were seen in later lines.
In the RAS&BRAFwt subgroup a trend was seen for metastasectomies being performed more frequently in patients with rectal primaries compared with right and left colon primaries (Fig. 1, Supplementary Table S6). In this subgroup, number of treatment lines, intent of first-line treatment, or drugs used in different treatment lines did not differ according to primary tumour ___location. Of 156 patients with RAS&BRAFwt tumours treated with chemotherapy, 44 (28%) received an EGFR-inhibitor in first line, and 68 (44%) in later lines.
Among patients receiving chemotherapy, no differences according to MMR-status were seen in number of therapy lines, drugs used in different lines, or response to treatment (Supplementary Table S7). Three of 9 dMMR patients, alive and eligible for immune checkpoint inhibitors after approval, received this treatment and 2 patients received it earlier within a trial [35].
Thirty-seven patients underwent metastasectomies and two had curatively intended LAT without any systemic therapy. Among the patients only receiving BSC, the multifactorial reasons were recorded prospectively; poor ECOG-PS (49%), comorbidity (49%), and high age (40%) were the main reasons.
Treatment in subgroups according to primary tumour site and mutation and MMR status
Among nine subgroups defined according to primary tumour ___location and RAS-, and BRAF-V600E status, metastasectomy rates were the lowest among patients with BRAF-V600Emt right colon tumours (12%) and the highest among patients with RAS&BRAFwt rectal tumours (42%; Fig. 1A). Systemic therapy only rates varied between the subgroups (range 40–58%). BSC only rates were the lowest among RAS&BRAFwt rectal cancers (18%) and the highest among BRAF-V600Emt right colon cancers (40%; Fig. 1A).
Among ten subgroups (two <10 patients subgroups excluded) assembled according to primary tumour ___location and MMR-status, with the pMMR tumours further subdivided by RAS and BRAF status, the metastasectomy rates varied from 9% in BRAF-V600Emt/pMMR rectal cancers to 43% in RAS&BRAFwt/pMMR rectal cancers (Fig. 1B). Systemic therapy only rates varied less between the subgroups (range 41–64%). BSC rates were the lowest among RAS&BRAFwt/pMMR rectal cancers (15%) and the highest among dMMR right colon cancers (46%).
Fitness and eligibility for intensive therapy according to primary tumour ___location, mutation status and MMR-status
When retrospectively studying fitness for intensive therapy according to patient characteristics, based on ESMO-guidelines (exclusion criteria age >75 years and ECOG-PS 2–4, [2]), 44% were considered “fit for intensive therapy” (Supplementary Table S8A). This proportion varied widely according to primary tumour ___location, mutation status, and MMR-status, being lowest among BRAF-V600Emt and dMMR.
Of all, 36% were considered “eligible for intensive systemic therapy” (exclusion criteria unfit as above, receiving only one drug in one treatment line, or curative metastasectomy without perioperative therapy, and BSC only, Supplementary Table S8B). The highest eligibility of almost 50% was seen in rectal cancers, and the lowest 21% for dMMR patients.
Overall survival in all patients and according to presentation and treatment type
Among all patients mOS from diagnosis of metastatic disease was 15.1 months (95% CI 13.1–17.1). Patients with metachronous metastases did better than patients with synchronous metastases (mOS 20.4 vs 13.2 months, HR: 0.81 [95% CI 0.68–0.95], Supplementary Fig. S1A), being most evident in patients with rectal tumours (Supplementary Fig. S1B–D).
In actively treated patients, mOS was 23.0 months (95% CI 20.6–25.4). The best mOS was seen in those treated with metastasectomy, followed by systemic therapy only, and the shortest in the BSC group (mOS 63.2 vs 13.8 vs 4.3 months; HR: 0.18 [95% CI 0.14–0.23], reference, 2.60 [95% CI 2.18–3.11]; Supplementary Fig. S2A). These results withstood in conditional landmark analyses at 4 months and 3 weeks (Supplementary Fig. S2B, C).
Overall survival according to primary tumour ___location, mutation status, and mismatch repair status
Patients with right colon tumours had worse OS than patients with left colon and rectal tumours (mOS 10.8 vs 17.2 vs 21.6 months; Fig. 2A). When stratified by treatment, no statistically significant differences were seen between right and left colon primaries in any treatment group (Fig. 2B–D). In the metastasectomy group, rectal cancers did better than the other two locations (Fig. 2C). These results withstood in a conditional landmark analysis at 4 months (Supplementary Fig. S3).
Patients with BRAF-V600Emt tumours had an inferior OS compared with those that had RASmt or RAS&BRAFwt tumours (mOS 6.9 vs 19.0 vs 24.4 months; Fig. 3A). These differences remained statistically significant in analyses stratified by treatment (Fig. 3B–D).
PIK3CAmt status did not influence OS, neither in all patients (Supplementary Fig. S4), nor in analyses stratified by primary tumour ___location, RAS/BRAF-V600E mutation status, or treatment groups (data not shown).
OS for all tested patients according to primary tumour ___location stratified by mutation status is presented in Fig. 4. No OS differences were seen between primary tumour locations in the BRAF-V600Emt and RASmt subgroups (Fig. 4A, B). In the RAS&BRAFwt subgroup, patients with rectal primary tumours had the best OS, whereas no survival difference was seen between patients with right and left colon tumours (Fig. 4C). This was also seen when analysed separately in pMMR patients (Fig. 4D, F). Among RAS&BRAFwt and pMMR patients, no differences in OS were seen for systemic therapy or BSC only patients, whereas rectal cancers did the best in the metastasectomy group, with caveat of small subgroups (Supplementary Fig. S5A–C).
When studying OS for primary tumour ___location divided beyond the trichotomous division, some heterogeneity was observed between right colon locations (mOS 8.2–12.0 months), whereas greater variability was observed in subgroups of left colon with splenic flexure having shorter OS than descending colon and sigmoid colon (mOS 10.8 vs 17.2 vs 17.9 months), while rectum still had the best OS (mOS 21.6 months, Supplementary Fig. S6A). No OS differences were seen in the BRAF-V600Emt and RASmt subgroups (Supplementary Fig. S6B, C), whereas small differences were seen in the RAS&BRAFwt subgroup (Supplementary Fig. S6D), again with caveat of small subgroups.
Among nine subgroups defined according to primary tumour ___location, and RAS- and BRAF-status, mOS after metastasectomy was the shortest among patients with BRAF-V600Emt right colon cancers (29 months) and the longest among patients with RAS&BRAFwt rectal cancers (95 months; Fig. 1, Supplementary Fig. S7A–I). Among patients receiving systemic therapy only, mOS varied from 6 months in patients with BRAF-V600Emt left colon and rectal cancers to 23 months in patients with RAS&BRAFwt rectal cancers. In the BSC only group, mOS varied from 3 to 7 months, without significant differences between subgroups.
Patients with dMMR tumours had significantly worse OS than those with pMMR tumours (mOS 13.8 vs 22.9 months; Supplementary Fig. S8A). However, there were no OS differences between dMMR and pMMR patients in analyses stratified by treatment or mutational status (Supplementary Figs. S8B–D, S9A–C). Among five patients treated with checkpoint inhibitors, mOS was 64.0 months (OS 25+, 29, 30+, 47+, and 64 months).
Overall survival according to primary tumour ___location, mismatch repair status, mutation status, and treatment type
In ten subgroups assembled according to primary tumour ___location and MMR-status, with pMMR tumours further subdivided by RAS/BRAF mutation status, the shortest OS after metastasectomies was seen in patients in the RAS&BRAFwt/pMMR right colon subgroup (44 months) and the longest in patients with RAS&BRAFwt/pMMR rectal cancers (93 months; Fig. 1B). In the subgroups receiving systemic therapy only, the shortest mOS was seen among BRAF-V600Emt/pMMR rectal cancers (6 months) and the longest among RAS&BRAFwt/pMMR rectal cancers (23 months). BSC subgroups did poorly with mOS varying between 1 and 9 months.
Univariable and multivariable overall survival analyses
In the multivariable model, patients with BRAF-V600Emt tumours demonstrated a worse OS than patients with RASmt and RAS&BRAFwt tumours (Table 4). Number of metastatic sites, ECOG-PS, tumour grade, and type of treatment also remained statistically significant. Age, presentation of metastases, and MMR-status remained statistically significant, however, in an opposite direction, whereas interlinked primary tumour ___location turned out non-significant.
Discussion
This recently collected cohort of mCRC patients from a defined population, where in practice 100% of all cases diagnosed were identified, shows that certain molecular subgroups differ markedly in frequency from clinical trials/hospital-based series when all patients, i.e., also non-actively treated, BSC only, patients are included. Thus, higher proportions had BRAF-V600Emt (17%) and dMMR (9%) in this cohort compared with 4–12% and 3–5%, respectively, in clinical trials [36,37,38,39,40]. Patients with these characteristics are generally less fit and, thus, less often make it to a trial and are less often treated at hospitals reporting data in research publications. We could also show that more tumours were right-sided (38%) than reported in recent clinical trials (25–30%) [41,42,43] and confirm that right colon tumours have worse survival than left colon and rectal tumours [42, 44].
In line with previous studies [2, 11,12,13], both BRAF-V600Emt and dMMR were more common in right colon tumours than in left colon or rectal tumours. RASmt are more frequent in rectal cancers but seen throughout colon and rectum, and thus, much fewer patients with right colon tumours have RAS&BRAFwt tumours, in this cohort, only 22% compared with 44% for left colon tumours and 31% for rectal tumours (p < 0.001). When also MMR-status was considered (56% overlap between dMMR and BRAF-V600Emt), the differences became even more marked (RAS&BRAFwt/pMMR in 18% vs 41% vs 30%, p < 0.001), i.e., only a fraction of right colon tumours belong to a “treatment-sensitive group”. If PIK3CAmt also is included in the EGFR-inhibitor non-sensitive group [27, 45], the gap becomes even larger (RAS&BRAFwt/ pMMR/PIK3CAwt in 15% vs 41% vs 27%, p < 0.001).
When mutation status and MMR-status were studied in the CRC continuum, the higher prevalence of BRAF-V600Emt and dMMR, usually seen in right-sided tumours, continued beyond the transverse colon, an aspect also reported before [21, 23]. These findings indicate that dividing the colorectum into two or three parts might be too coarse for some applications [20], for example when predicting response to EGFR-inhibitors [2, 27]. Although numbers were limited in some subgroups, BRAF-V600Emt and dMMR were more common in ascending colon than in caecum, as also reported [23].
The OS differences according to primary tumour ___location and molecular subgroups were seen in the actively treated groups, but interestingly also in the BSC group, for BRAF-V600Emt. In virtually all reports about outcomes in mCRC patients in literature, patients have been actively treated [15, 17,18,19, 38, 43, 46]. Metastasectomy rates were lower with 21% in right colon primary tumours compared with 31% for left colon and 33% for rectum and their survival worse, in line with previous findings [16, 46, 47], whereas in actively treated patients, the proportions were more similar, or 32% vs 42% vs 44%, respectively. We show differences in metastatic sites for right colon primary tumour ___location with less liver and lung metastases and more peritoneal metastases, poorer ECOG-PS, older age, and more dMMR and BRAFmt tumours with a more aggressive clinical course. The wide variability in metastasectomy rates (13–43%) and survival (mOS 44–93 months) according to primary tumour ___location, MMR-status, and mutation status are most probably explained by the differences in RAS-, BRAF-, and MMR-status which, thus, seem most important. This aspect of combining primary tumour ___location, mutation status, and MMR-status has not previously been analysed in detail.
The systemic therapy only frequency was 41–64% according to primary tumour ___location, RAS-, BRAF-, and MMR-status, with a markedly variable mOS of 6–23 months, which cannot be explained by the minor differences in systemic therapy intensity in the subgroups. These outcomes contrast highly to the best mOS of 30–40 months in selected, RAS&BRAFwt and pMMR patients in recent clinical trials [2, 3, 43]. The BSC rate also varied highly 15–46%, and mOS was only a few months, in line with previous studies [5, 11].
Of all patients, 44% were fit for the most intensive therapy based on age ≤75 years and ECOG-PS 0-1 per ESMO-guidelines [2]. We are aware that this is a too crude way of defining fitness, but it has been used for example in triplet chemotherapy studies [2], and is a surrogate for significant co-morbidity, not recorded well enough to be used here. The proportion of ≤75-year-old not fit for intensive therapy is probably reasonably comparable to the proportion of >75-year-old fit for intensive therapy.
If the actual treatments given were considered, only about one-third or 36% were eligible for intensive therapy. Eleven percent were treated and potentially “cured” with metastasectomy only without receiving perioperative systemic therapy, 7% received only a single drug in the first-line situation, and 28% received BSC. A large proportion (94%) of those eligible for intensive therapy received combination chemotherapy with or without biologics (data not shown).
The comparisons of OS in homogenous mutation groups, even if hampered by few individuals in some subgroups, indicate that the relevance of primary tumour ___location is in large due to differences in clinical characteristics, metastatic sites, mutational- and MMR-status, and consequently, treatment allocation. When comparing primary tumour ___location, no significant differences in OS for patients with RASmt or BRAF-V600Emt tumours were noted, as also reported by others [48, 49]. When comparing patients with primary tumours in right and left colon, we saw no difference in OS, contrary to another study showing a worse prognosis for right colon tumours in the BRAFwt group [48]. Rectal RAS&BRAFwt tumours, however, had a superior OS compared with right colon RAS&BRAFwt tumours, which was also observed in the pMMR subset, this highlights the importance of analysing rectal cancers separately and not lumping them together with the other left-sided tumours.
In a multivariable analysis, primary tumour ___location was nonsignificant when adjusting for other factors including treatment. Contrary to our results, there was a difference in OS between right- and left-sided primaries in multivariable analyses of an Australian register-based study and between right and left colon in the PETACC3 mCRC subgroup [48, 50]. These two studies did, however, not separate right colon, left colon, and rectum and the study populations were selected.
The highest BSC only rate (46%) and ineligibility for intensive therapy (79%, with caveat that some >75-year-old may tolerate immune checkpoint inhibitors) was seen among dMMR tumours, a trend also seen in [13]. The prognosis of treatable dMMR tumours will markedly change in the future due to immune checkpoint inhibitors [35]. This could be seen already in this study (Supplementary Fig. S7B) even if these drugs were primarily used after approval in 2021 and only 5 dMMR patients received this treatment with impressive OS. A similar improvement will probably be seen also in other subgroups in the future after the introduction of other targeted drugs, for example the combination of encorafenib-cetuximab and chemotherapy [51].
The relevance of a particular treatment, for example EGFR-inhibition is best explored in randomised clinical trials [52]. In population-based cohorts, such as this study, heterogeneity is always present. Too few individuals were treated with an EGFR-inhibitor in first line to allow for comparisons according to tumour ___location. Interestingly, only 16% of population-based left-sided cancers were RAS&BRAFwt, pMMR, and eligible for intensive therapy. EGFR-inhibitors in any line were used in approximately 80% of patients eligible to intensive therapy, irrespective of sidedness in RAS&BRAF wild-type tumours in line with the Swedish guidelines [2, 3]. Recent trials comparing EGFR-inhibitors versus vascular endothelial growth factor inhibitors in combination with chemotherapy in RASwt mCRC have indicated benefit from EGFR-inhibitors in left-sided cancers but not in right-sided cancers, albeit not universally [17, 43, 46, 52].
PIK3CAmt was more common in right colon than in left colon and rectum, also reported by others [12, 53, 54]. In our cohort, PIK3CAmt did not affect OS, contrary to a meta-analysis indicating a worse OS after EGFR-inhibitor therapy [55]. The different distribution of PIK3CAmt according to primary tumour ___location and their relative resistance to EGFR-inhibitors [27], may partially explain survival differences according to primary tumour ___location.
Strengths and limitations
The strength is that the patient material is truly population-based without any selection meaning that the clinical presentation, treatments provided, and OS reflect a real-world situation where metastasectomies have been frequently used. Allocation to treatment groups and chemotherapies used were very reliably recorded making it possible to study these factors in detail. A very high 93% rate of molecularly analysed samples was achieved as all patients with sufficient material were analysed. Thus, the molecular analyses are representative also for the group of patients cared for with BSC, usually not tested or included in other studies.
However, sufficient tumour material for analyses is not always present (7% in this cohort) meaning that these results do not fully reflect the true incidence. Biopsies were sometimes too small or used up in clinical routine. We have previously noted that this drop-out most probably means that the groups with the poorest prognosis, BRAF-V600Emt and dMMR in reality are slightly higher than noted here, further increasing the gap to materials from clinical trials/hospital-based series [11, 12].
A further weakness is that different methods were used for the molecular analyses. Some analyses were done in clinical routine, whereas others were done for the purpose of this study, though with established methods. Preferably, MMR should have been analysed either using PCR or IHC but using TMB as a proxy to define MMR-status has been deemed sufficiently reliable [33], allowing for another 20% to be characterised for MMR. Congruence for RAS/BRAF/PIK3CA was usually seen between cases analysed by clinical routine and with the Oncomine/WGS platforms but differed for 31 cases (5%), the choice of another sample with lower tumour cell count, or methodological issues, are possible reasons.
Conclusions
This population-based cohort shows that primary tumour ___location, RAS/BRAF-status, and MMR-status affect clinical characteristics, treatability, and outcome of mCRC. High metastasectomy rates of 39% were achieved population-based among actively treated, but not all actively treated were fit for intensive systemic treatment and 28% received BSC only. Our data show that BRAF-V600Emt and dMMR are more common population-based and in right colon tumours, have fewer metastasectomies and more BSC only, and worse OS. This could explain the worse OS seen in right colon tumours, which is supported by the minor OS differences between right colon and left colon primary tumours in analyses stratified by treatment and mutation status, and that primary tumour ___location was non-significant in multivariable analyses. Therefore, we propose that molecular alterations are more important to consider than primary tumour ___location when it comes to evaluation of prognosis both overall and in treatment groups.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request, with approval by the steering committee.
References
Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–63.
Cervantes A, Adam R, Roselló S, Arnold D, Normanno N, Taïeb J, et al. Metastatic colorectal cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34:10–32.
Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386–422.
Adam R, Kitano Y. Multidisciplinary approach of liver metastases from colorectal cancer. Ann Gastroenterol Surg. 2019;3:50–6.
Sorbye H, Pfeiffer P, Cavalli-Bjorkman N, Qvortrup C, Holsen MH, Wentzel-Larsen T, et al. Clinical trial enrollment, patient characteristics, and survival differences in prospectively registered metastatic colorectal cancer patients. Cancer. 2009;115:4679–87.
Lemmens VE, de Haan N, Rutten HJ, Martijn H, Loosveld OJ, Roumen RM, et al. Improvements in population-based survival of patients presenting with metastatic rectal cancer in the south of the Netherlands, 1992-2008. Clin Exp Metastasis. 2011;28:283–90.
Sorbye H, Cvancarova M, Qvortrup C, Pfeiffer P, Glimelius B. Age-dependent improvement in median and long-term survival in unselected population-based Nordic registries of patients with synchronous metastatic colorectal cancer. Ann Oncol. 2013;24:2354–60.
Mitry E, Rollot F, Jooste V, Guiu B, Lepage C, Ghiringhelli F, et al. Improvement in survival of metastatic colorectal cancer: are the benefits of clinical trials reproduced in population-based studies? Eur J Cancer. 2013;49:2919–25.
Osterlund E, Glimelius B. Temporal development in survival, and gender and regional differences in the Swedish population of patients with synchronous and metachronous metastatic colorectal cancer. Acta Oncol. 2022;61:1278–88.
Hamers PAH, Elferink MAG, Stellato RK, Punt CJA, May AM, Koopman M, et al. Informing metastatic colorectal cancer patients by quantifying multiple scenarios for survival time based on real-life data. Int J Cancer. 2021;148:296–306.
Sorbye H, Dragomir A, Sundstrom M, Pfeiffer P, Thunberg U, Bergfors M, et al. High BRAF mutation frequency and marked survival differences in subgroups according to KRAS/BRAF mutation status and tumor tissue availability in a prospective population-based metastatic colorectal cancer cohort. PloS One. 2015;10:e0131046.
Nunes L, Aasebø K, Mathot L, Ljungström V, Edqvist PH, Sundström M, et al. Molecular characterization of a large unselected cohort of metastatic colorectal cancers in relation to primary tumor ___location, rare metastatic sites and prognosis. Acta Oncol. 2020;59:417–26.
Aasebø K, Dragomir A, Sundström M, Mezheyeuski A, Edqvist PH, Eide GE, et al. Consequences of a high incidence of microsatellite instability and BRAF-mutated tumors: a population-based cohort of metastatic colorectal cancer patients. Cancer Med. 2019;8:3623–35.
Meguid RA, Slidell MB, Wolfgang CL, Chang DC, Ahuja N. Is there a difference in survival between right- versus left-sided colon cancers? Ann Surg Oncol. 2008;15:2388–94.
Holch JW, Ricard I, Stintzing S, Modest DP, Heinemann V. The relevance of primary tumour ___location in patients with metastatic colorectal cancer: a meta-analysis of first-line clinical trials. Eur J Cancer. 2017;70:87–98.
Brouwer NPM, van der Kruijssen DEW, Hugen N, de Hingh I, Nagtegaal ID, Verhoeven RHA, et al. The impact of primary tumor ___location in synchronous metastatic colorectal cancer: differences in metastatic sites and survival. Ann Surg Oncol. 2020;27:1580–8.
Arnold D, Lueza B, Douillard JY, Peeters M, Lenz HJ, Venook A, et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol. 2017;28:1713–29.
Tejpar S, Stintzing S, Ciardiello F, Tabernero J, Van Cutsem E, Beier F, et al. Prognostic and predictive relevance of primary tumor ___location in patients with RAS wild-type metastatic colorectal cancer: retrospective analyses of the CRYSTAL and FIRE-3 trials. JAMA Oncol. 2017;3:194–201.
Karapetis CS, Liu H, Sorich MJ, Pederson LD, Van Cutsem E, Maughan T, et al. Fluoropyrimidine type, patient age, tumour sidedness and mutation status as determinants of benefit in patients with metastatic colorectal cancer treated with EGFR monoclonal antibodies: individual patient data pooled analysis of randomised trials from the ARCAD database. Br J Cancer. 2024;130:1269–78.
Yamauchi M, Lochhead P, Morikawa T, Huttenhower C, Chan AT, Giovannucci E, et al. Colorectal cancer: a tale of two sides or a continuum? Gut. 2012;61:794–7.
Yamauchi M, Morikawa T, Kuchiba A, Imamura Y, Qian ZR, Nishihara R, et al. Assessment of colorectal cancer molecular features along bowel subsites challenges the conception of distinct dichotomy of proximal versus distal colorectum. Gut. 2012;61:847–54.
Ugai T, Haruki K, Harrison TA, Cao Y, Qu C, Chan AT, et al. Molecular characteristics of early-onset colorectal cancer according to detailed anatomical locations: comparison with later-onset cases. Am J Gastroenterol. 2023;118:712–26.
Loree JM, Pereira AAL, Lam M, Willauer AN, Raghav K, Dasari A, et al. Classifying colorectal cancer by tumor ___location rather than sidedness highlights a continuum in mutation profiles and consensus molecular subtypes. Clin Cancer Res. 2018;24:1062–72.
Osterman E, Hammarström K, Imam I, Osterlund E, Sjöblom T, Glimelius B. Completeness and accuracy of the registration of recurrences in the Swedish Colorectal Cancer Registry (SCRCR) and an update of recurrence risk in colon cancer. Acta Oncol. 2021;60:842–9.
Glimelius B, Melin B, Enblad G, Alafuzoff I, Beskow A, Ahlström H, et al. U-CAN: a prospective longitudinal collection of biomaterials and clinical information from adult cancer patients in Sweden. Acta Oncol. 2018;57:187–94.
Van Cutsem E, Nordlinger B, Cervantes A, Group EGW. Advanced colorectal cancer: ESMO clinical practice guidelines for treatment. Ann Oncol. 2010;21:v93–7.
Therkildsen C, Bergmann TK, Henrichsen-Schnack T, Ladelund S, Nilbert M. The predictive value of KRAS, NRAS, BRAF, PIK3CA and PTEN for anti-EGFR treatment in metastatic colorectal cancer: a systematic review and meta-analysis. Acta Oncol. 2014;53:852–64.
Van Cutsem E, Bajetta E, Valle J, Kohne CH, Randolph Hecht J, Moore M, et al. Randomized, placebo-controlled, phase III study of oxaliplatin, fluorouracil, and leucovorin with or without PTK787/ZK 222584 in patients with previously treated metastatic colorectal adenocarcinoma. J Clin Oncol. 2011;29:2004–10.
Sundstrom M, Edlund K, Lindell M, Glimelius B, Birgisson H, Micke P, et al. KRAS analysis in colorectal carcinoma: analytical aspects of Pyrosequencing and allele-specific PCR in clinical practice. BMC Cancer. 2010;10:660.
Nunes L, Li F, Wu M, Luo T, Hammarström K, Torell E, et al. Prognostic genome and transcriptome signatures in colorectal cancers. Nature. 2024;633:137–46.
Hammarström K, Nunes L, Mathot L, Mezheyeuski A, Lundin E, Korsavidou Hult N, et al. Clinical and genetic factors associated with tumor response to neoadjuvant (chemo)radiotherapy, survival and recurrence risk in rectal cancer. Int J Cancer. 2024;155:40–53.
Niu B, Ye K, Zhang Q, Lu C, Xie M, McLellan MD, et al. MSIsensor: microsatellite instability detection using paired tumor-normal sequence data. Bioinformatics. 2014;30:1015–6.
Sha D, Jin Z, Budczies J, Kluck K, Stenzinger A, Sinicrope FA. Tumor mutational burden as a predictive biomarker in solid tumors. Cancer Discov. 2020;10:1808–25.
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335:806–8.
Diaz LA Jr, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, et al. Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): final analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 2022;23:659–70.
Koopman M, Kortman GA, Mekenkamp L, Ligtenberg MJ, Hoogerbrugge N, Antonini NF, et al. Deficient mismatch repair system in patients with sporadic advanced colorectal cancer. Br J Cancer. 2009;100:266–73.
Venderbosch S, Nagtegaal ID, Maughan TS, Smith CG, Cheadle JP, Fisher D, et al. Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: a pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clin Cancer Res. 2014;20:5322–30.
Seligmann JF, Fisher D, Smith CG, Richman SD, Elliott F, Brown S, et al. Investigating the poor outcomes of BRAF-mutant advanced colorectal cancer: analysis from 2530 patients in randomised clinical trials. Ann Oncol. 2017;28:562–8.
Yaeger R, Chatila WK, Lipsyc MD, Hechtman JF, Cercek A, Sanchez-Vega F, et al. Clinical sequencing defines the genomic landscape of metastatic colorectal cancer. Cancer Cell. 2018;33:125–36.e3.
Yaeger R, Cercek A, Chou JF, Sylvester BE, Kemeny NE, Hechtman JF, et al. BRAF mutation predicts for poor outcomes after metastasectomy in patients with metastatic colorectal cancer. Cancer. 2014;120:2316–24.
Venook AP, Niedzwiecki D, Lenz HJ, Innocenti F, Fruth B, Meyerhardt JA, et al. Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer: a randomized clinical trial. JAMA. 2017;317:2392–401.
Yin J, Cohen R, Jin Z, Liu H, Pederson L, Adams R, et al. Prognostic and predictive impact of primary tumor sidedness for previously untreated advanced colorectal cancer. J Natl Cancer Inst. 2021;113:1705–13.
Watanabe J, Muro K, Shitara K, Yamazaki K, Shiozawa M, Ohori H, et al. Panitumumab vs bevacizumab added to standard first-line chemotherapy and overall survival among patients with RAS wild-type, left-sided metastatic colorectal cancer: a randomized clinical trial. JAMA. 2023;329:1271–82.
Ahmed S, Pahwa P, Le D, Chalchal H, Chandra-Kanthan S, Iqbal N, et al. Primary tumor ___location and survival in the general population with metastatic colorectal cancer. Clin Colorectal Cancer. 2018;17:e201–e6.
Wu S, Gan Y, Wang X, Liu J, Li M, Tang Y. PIK3CA mutation is associated with poor survival among patients with metastatic colorectal cancer following anti-EGFR monoclonal antibody therapy: a meta-analysis. J Cancer Res Clin Oncol. 2013;139:891–900.
Bond MJG, Bolhuis K, Loosveld OJL, de Groot JWB, Droogendijk H, Helgason HH, et al. First-line systemic treatment strategies in patients with initially unresectable colorectal cancer liver metastases (CAIRO5): an open-label, multicentre, randomised, controlled, phase 3 study from the Dutch Colorectal Cancer Group. Lancet Oncol. 2023;24:757–71.
Uutela A, Osterlund E, Halonen P, Kallio R, Ålgars A, Salminen T, et al. Resectability, conversion, metastasectomy and outcome according to RAS and BRAF status for metastatic colorectal cancer in the prospective RAXO study. Br J Cancer. 2022;127:686–94.
Missiaglia E, Jacobs B, D’Ario G, Di Narzo AF, Soneson C, Budinska E, et al. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann Oncol. 2014;25:1995–2001.
Loupakis F, Schirripa M, Intini R, Bergamo F, Bertorelle R, Fassan M, et al. Another chapter of the right versus left story: is primary tumor ___location a prognostic feature in RAS mutant metastatic colorectal cancer? Oncologist. 2019;24:e77–e9.
Mendis S, Beck S, Lee B, Lee M, Wong R, Kosmider S, et al. Right versus left sided metastatic colorectal cancer: teasing out clinicopathologic drivers of disparity in survival. Asia Pacific J Clin Oncol. 2019;15:136–43.
Kopetz S, Yoshino T, Van Cutsem E, Eng C, Kim TW, Wasan HS, et al. Encorafenib, cetuximab and chemotherapy in BRAF-mutant colorectal cancer: a randomized phase 3 trial. Nat Med. 2025;31:901–8.
Shitara K, Muro K, Watanabe J, Yamazaki K, Ohori H, Shiozawa M, et al. Baseline ctDNA gene alterations as a biomarker of survival after panitumumab and chemotherapy in metastatic colorectal cancer. Nat Med. 2024;30:730–9.
Luo Q, Chen D, Fan X, Fu X, Ma T, Chen D. KRAS and PIK3CA bi-mutations predict a poor prognosis in colorectal cancer patients: a single-site report. Transl Oncol. 2020;13:100874.
Jin J, Shi Y, Zhang S, Yang S. PIK3CA mutation and clinicopathological features of colorectal cancer: a systematic review and meta-analysis. Acta Oncol. 2020;59:66–74.
Tan ES, Fan W, Knepper TC, Schell MJ, Sahin IH, Fleming JB, et al. Prognostic and predictive value of PIK3CA mutations in metastatic colorectal cancer. Targeted Oncol. 2022;17:483–92.
Acknowledgements
We thank Celina Österlund for graphical design.
Funding
This work was funded by grants from the Swedish Cancer Society (22 2054 Pj 01H), Lions Cancerforskningsfond Mellansverige, and an unrestricted start-up grant from Amgen. The funders had no role in the design of the study, nor in the analyses of data or the writing of this report. Open access funding provided by Uppsala University.
Author information
Authors and Affiliations
Contributions
Conceptualisation: EO, BG; methodology: EO, KH, LN, LM, AM, TS, BG; software: EO, KH, LN, LM, TS, BG; data curation: EO, KH, LN, LM, TS, BG; investigation: EO, KH, LN, BG; validation: EO, KH, LN, LM, TS, BG; formal analysis: EO; supervision: TS, BG; funding acquisition: EO, BG; visualisation: EO, BG; project administration: EO, KH, LN, LM, TS, BG; resources: EO, TS, BG; writing – original draft: EO and BG; writing – review & editing: EO, KH, LN, LM, AM, TS, BG. All authors have read and agreed with the decision to submit for publication.
Corresponding author
Ethics declarations
Competing interests
EO has been on an advisory board for Amgen, received lecture honoraria from Amgen, and travel reimbursements from Amgen, Merck KGaA, and Nordic Drugs. LN was employed by Oncodia AB at the time of the study. TS is co-founder and shareholder of Oncodia AB. BG has received an unrestricted research grant from Amgen. No other authors report any conflicts of interest.
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethical Review Board at Uppsala University (2010-198-0, 2015/419 and 2018/490). The patients included in the Uppsala-Umeå Comprehensive Cancer Consortium provided written informed consent, whereas the Swedish Colorectal Cancer Registry works with an opt-out principle.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Osterlund, E., Hammarström, K., Nunes, L. et al. Primary tumour ___location, molecular alterations, treatments, and outcome in a population-based metastatic colorectal cancer cohort. BJC Rep 3, 38 (2025). https://doi.org/10.1038/s44276-025-00156-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44276-025-00156-z