Abstract
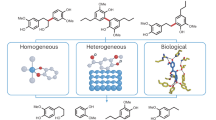
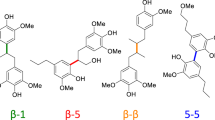
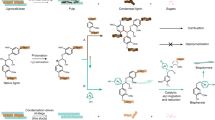
Carbon–carbon bonds, ubiquitous in lignin, limit monomer yields from current depolymerization strategies, which mainly target C–O bonds. Selective cleavage of the inherently inert σ-type C–C bonds without pre-functionalization remains challenging. Here we report the breaking of C–C bonds in lignin obtained upon initial disruption of labile C–O bonds, achieving monocyclic hydrocarbon yields up to an order of magnitude higher than previously reported. The use of a Pt (de)hydrogenation function leads to olefinic groups close to recalcitrant C–C bonds, which can undergo β-scission over zeolitic Brønsted acid sites. After confirming that this approach can selectively cleave common C–C linkages (5–5′, β–1′, β–5′ and β–β′) in lignin skeletons, we demonstrate its utility in the valorization of various representative lignins. A techno-economic analysis shows the promise of our method for producing gasoline- and jet-range cycloalkanes and aromatics, while a life-cycle assessment confirms its potential for CO2-neutral fuel production.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data are available within the manuscript and Supplementary Information. The atomic coordinates of the optimized computational models are provided in Supplementary Data 1. Source data are provided with this paper.
References
Questell-Santiago, Y. M., Galkin, M. V., Barta, K. & Luterbacher, J. S. Stabilization strategies in biomass depolymerization using chemical functionalization. Nat. Rev. Chem. 4, 311–330 (2020).
Liao, Y. et al. A sustainable wood biorefinery for low-carbon footprint chemicals production. Science 367, 1385–1390 (2020).
Li, C., Zhao, X., Wang, A., Huber, G. W. & Zhang, T. Catalytic transformation of lignin for chemicals and fuels. Chem. Rev. 115, 11559–11624 (2015).
Sun, Z. et al. Complete lignocellulose conversion with integrated catalyst recycling yielding valuable aromatics and fuels. Nat. Catal. 1, 82–92 (2018).
Tuck, C. O., Pérez, E., Horváth, I. T., Sheldon, R. A. & Poliakoff, M. Valorization of biomass: deriving more value from waste. Science 337, 695–699 (2012).
Ragauskas, A. J. et al. Lignin valorization: improving lignin processing in the biorefinery. Science 344, 1246843 (2014).
Rahimi, A., Ulbrich, A., Coon, J. J. & Stahl, S. S. Formic-acid-induced depolymerization of oxidized lignin to aromatics. Nature 515, 249–252 (2014).
Li, Y. et al. An ‘ideal lignin’ facilitates full biomass utilization. Sci. Adv. 4, eaau2968 (2018).
Meng, Q. et al. Sustainable production of benzene from lignin. Nat. Commun. 12, 4534 (2021).
Katahira, R., Elder, T. J. & Beckham, G. T. in A Brief Introduction to Lignin Structure. (ed Beckham, G. T.) Ch. 1 (Royal Society of Chemistry, 2018).
Zakzeski, J., Bruijnincx, P. C., Jongerius, A. L. & Weckhuysen, B. M. The catalytic valorization of lignin for the production of renewable chemicals. Chem. Rev. 110, 3552–3599 (2010).
Phongpreecha, T. et al. Predicting lignin depolymerization yields from quantifiable properties using fractionated biorefinery lignins. Green Chem. 19, 5131–5143 (2017).
Talebi Amiri, M., Dick, G. R., Questell-Santiago, Y. M. & Luterbacher, J. S. Fractionation of lignocellulosic biomass to produce uncondensed aldehyde-stabilized lignin. Nat. Protoc. 14, 921–954 (2019).
Biermann, C. J. Handbook of Pulping and Papermaking (Elsevier, 1996).
da Costa Sousa, L. et al. Next-generation ammonia pretreatment enhances cellulosic biofuel production. Energy Environ. Sci. 9, 1215–1223 (2016).
Kim, K. H. et al. Integration of renewable deep eutectic solvents with engineered biomass to achieve a closed-loop biorefinery. Proc. Natl Acad. Sci. USA 116, 13816–13824 (2019).
Luterbacher, J. S. et al. Nonenzymatic sugar production from biomass using biomass-derived γ-valerolactone. Science 343, 277–280 (2014).
Feghali, E., Carrot, G., Thuery, P., Genre, C. & Cantat, T. Convergent reductive depolymerization of wood lignin to isolated phenol derivatives by metal-free catalytic hydrosilylation. Energy Environ. Sci. 8, 2734–2743 (2015).
Deuss, P. J. et al. Phenolic acetals from lignins of varying compositions via iron (III) triflate catalysed depolymerisation. Green Chem. 19, 2774–2782 (2017).
Renders, T. et al. Lignin-first biomass fractionation: the advent of active stabilisation strategies. Energy Environ. Sci. 10, 1551–1557 (2017).
Wu, X. et al. Solar energy-driven lignin-first approach to full utilization of lignocellulosic biomass under mild conditions. Nat. Catal. 1, 772–780 (2018).
Schutyser, W. et al. Chemicals from lignin: an interplay of lignocellulose fractionation, depolymerisation and upgrading. Chem. Soc. Rev. 47, 852–908 (2018).
Rinaldi, R. et al. Paving the way for lignin valorisation: recent advances in bioengineering, biorefining and catalysis. Angew. Chem. Int. Ed. 55, 8164–8215 (2016).
Kim, S. et al. Computational study of bond dissociation enthalpies for a large range of native and modified lignins. J. Phys. Chem. Lett. 2, 2846–2852 (2011).
Subbotina, E. et al. Oxidative cleavage of C–C bonds in lignin. Nat. Chem. 13, 1118–1125 (2021).
Hemberger, P., Custodis, V. B., Bodi, A., Gerber, T. & van Bokhoven, J. A. Understanding the mechanism of catalytic fast pyrolysis by unveiling reactive intermediates in heterogeneous catalysis. Nat. Commun. 8, 15946 (2017).
Shuai, L. et al. Selective C-C bond cleavage of methylene-linked lignin models and kraft lignin. ACS Catal. 8, 6507–6512 (2018).
Zhu, J., Wang, J. & Dong, G. Catalytic activation of unstrained C(aryl)–C(aryl) bonds in 2,2′-biphenols. Nat. Chem. 11, 45–51 (2019).
Zhu, J., Xue, Y., Zhang, R., Ratchford, B. & Dong, G. Catalytic activation of unstrained C(aryl)–C(alkyl) bonds in 2,2′ -methylenediphenols. J. Am. Chem. Soc. 144, 3242–3249 (2022).
Wang, W. et al. Microwave-assisted catalytic cleavage of C–C bond in lignin models by bifunctional Pt/CDC-SiC. ACS Sustain. Chem. Eng. 8, 38–43 (2019).
Li, X. et al. Scission of C–O and C–C linkages in lignin over RuRe alloy catalyst. J. Energy Chem. 67, 492–499 (2022).
Dong, L. et al. Breaking the limit of lignin monomer production via cleavage of interunit carbon–carbon linkages. Chem 5, 1521–1536 (2019).
Luo, Z. et al. Hydrothermally stable Ru/HZSM-5-catalyzed selective hydrogenolysis of lignin-derived substituted phenols to bio-arenes in water. Green Chem. 18, 5845–5858 (2016).
Weitkamp, J. Catalytic hydrocracking—mechanisms and versatility of the process. ChemCatChem 4, 292–306 (2012).
Mirena, J. I. et al. Impact of the spatial distribution of active material on bifunctional hydrocracking. Ind. Eng. Chem. Res. 60, 6357–6378 (2021).
Chen, G. et al. Interfacial electronic effects control the reaction selectivity of platinum catalysts. Nat. Mater. 15, 564–569 (2016).
Huang, X., Korányi, T. I., Boot, M. D. & Hensen, E. J. Ethanol as capping agent and formaldehyde scavenger for efficient depolymerization of lignin to aromatics. Green Chem. 17, 4941–4950 (2015).
Sturgeon, M. R. et al. A mechanistic investigation of acid-catalyzed cleavage of aryl-ether linkages: implications for lignin depolymerization in acidic environments. ACS Sustain. Chem. Eng. 2, 472–485 (2014).
Shuai, L. et al. Formaldehyde stabilization facilitates lignin monomer production during biomass depolymerization. Science 354, 329–333 (2016).
Anderson, E. M. et al. Differences in S/G ratio in natural poplar variants do not predict catalytic depolymerization monomer yields. Nat. Commun. 10, 2033 (2019).
Humbird, D. et al. Process Design and Economics for Biochemical Conversion of Lignocellulosic Biomass to Ethanol: Dilute-Acid Pretreatment and Enzymatic Hydrolysis of Corn Stover (National Renewable Energy Laboratory, 2011); https://www.nrel.gov/docs/fy11osti/47764.pdf
Zhang, C. & Wang, F. Catalytic lignin depolymerization to aromatic chemicals. Acc. Chem. Res. 53, 470–484 (2020).
Moses, C. A. Comparative evaluation of semi-synthetic jet fuels. Contract 33415, 2299 (2008).
Rahmes, T., Kinder, J. & Crenfeldt, G. Sustainable bio-derived synthetic paraffinic kerosene (Bio-SPK) jet fuel flights and engine tests program results. In Proc. 9th AIAA Aviation Technology, Integration and Operations Conference (ATIO) and Aircraft Noise and Emissions Reduction Symposium (ANERS) 7002 (AIAA, 2009).
Enright, C. Aviation fuel standard takes flight. ASTM Stand. News 39, 5 (2011).
Standard Specification for Aviation Turbine Fuel Containing Synthesized Hydrocarbons. ASTM Standard D7566-14a (ASTM international, 2014).
Zijlstra, D. S. et al. Extraction of lignin with high β-O-4 content by mild ethanol extraction and its effect on the depolymerization yield. J. Vis. Exp 143, 58575 (2019).
Acknowledgements
This research was supported financially by the Chemelot Institute for Science and Technology awarded to E.J.M.H. Z.L. acknowledges support for the RCF experiments, TEA and LCA calculations from the National Natural Science Foundation of China (grant no. 52206236), the Natural Science Foundation of Jiangsu Province (grant no. BK20220837) and the Fundamental Research Funds for the Central Universities (3203002211A1). J.T.B.d.B. and J.S.L. were supported by the Swiss National Science Foundation through the National Competence Center Catalysis (grant no. 51NF40_180544). The contribution of A.R. was supported by the European Union’s Horizon 2020 research and innovation programme under grant agreement no. 883753 (IDEALFUEL).
Author information
Authors and Affiliations
Contributions
Z.L. and E.J.M.H. conceived the idea for lignin depolymerization. Z.L. and A.R. performed the reactions of lignin and lignin model compounds. C.L. conducted the DFT calculations. Y.W. and J.X. carried out the TEA and LCA calculations with guidance from H.Z. and R.X. P.D.K., M.D.B. and J.T.B.d.B., supervised by J.S.L., prepared the technical lignins. Z.L. and E.H. wrote the manuscript in close consultation with M.D.B., D.F.d.W., C.L., H.Z. and R.X. All authors contributed to the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Engineering thanks Changzhi Li, Joseph Samec, Yanqin Wang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Supplementary information
Supplementary Information
Supplementary Notes 1–5, Figs. 1–29, Tables 1–27 and references 1–40.
Supplementary Data 1
Atomic coordinates of the optimized computational models.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Luo, Z., Liu, C., Radu, A. et al. Carbon–carbon bond cleavage for a lignin refinery. Nat Chem Eng 1, 61–72 (2024). https://doi.org/10.1038/s44286-023-00006-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44286-023-00006-0
This article is cited by
-
Hydroxyl and nitrate co-upgrading to oxime via anode-cathode cascade electrolyzer
Nature Communications (2025)
-
Effect of gold slab layers relaxation on adsorption of alkanethiols on the (111) surface: a density functional theory study
Journal of Molecular Modeling (2025)
-
Theoretical study of 2D cancer drug nanocarriers based on calcium chloride
Journal of Molecular Modeling (2025)
-
Lignin-based plugging hydrogel with high-temperature resistance and adjustable gelation
Advanced Composites and Hybrid Materials (2025)
-
Nitrate-induced aerobic oxidative cleavage and esterification of carbon-carbon triple bonds
Science China Chemistry (2025)