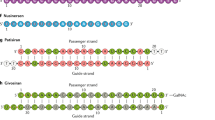
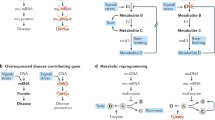
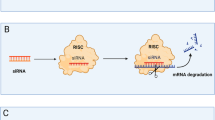
Abstract
Oligonucleotide therapeutics are revolutionizing disease treatment by regulating molecules at the genetic level, offering the possibility of treating conditions that were once considered ‘undruggable’. However, delivering oligonucleotides to tissues beyond the liver remains a key challenge, limiting their clinical applications thus far to niche indications. To achieve broader applicability, extensive biomolecular engineering is necessary to enhance the stability, tissue targetability, pharmacokinetics and pharmacodynamics of these structures. The intricate design of these molecules also demands sophisticated process-engineering techniques. Here we provide a collaborative Perspective from academia and industry on the pivotal role of chemical engineering in expanding the use of therapeutic oligonucleotides to treat a wider range of diseases. We discuss how the interplay between biomolecular and process engineering impacts the developability of next-generation oligonucleotide therapeutics as well as their translation from bench to bedside.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Roberts, T. C., Langer, R. & Wood, M. J. A. Advances in oligonucleotide drug delivery. Nat. Rev. Drug Discov. 19, 673–694 (2020).
Jadhav, V., Vaishnaw, A., Fitzgerald, K. & Maier, M. A. RNA interference in the era of nucleic acid therapeutics. Nat. Biotechnol. 42, 394–405 (2024).
Winkle, M., El-Daly, S. M., Fabbri, M. & Calin, G. A. Noncoding RNA therapeutics—challenges and potential solutions. Nat. Rev. Drug Discov. 20, 629–651 (2021).
Spradlin, J. N., Zhang, E. & Nomura, D. K. Reimagining druggability using chemoproteomic platforms. Acc. Chem. Res. 54, 1801–1813 (2021).
Janssen, K. et al. Exploiting the intrinsic misfolding propensity of the KRAS oncoprotein. Proc. Natl Acad. Sci. USA 120, e2214921120 (2023).
Egli, M. & Manoharan, M. Chemistry, structure and function of approved oligonucleotide therapeutics. Nucleic Acids Res. 51, 2529–2573 (2023).
Senior, M. Fresh from the biotech pipeline: record-breaking FDA approvals. Nat. Biotechnol. 42, 355–361 (2024).
Obexer, R., Nassir, M., Moody, E. R., Baran, P. S. & Lovelock, S. L. Modern approaches to therapeutic oligonucleotide manufacturing. Science 384, eadl4015 (2024).
Tang, Q. & Khvorova, A. RNAi-based drug design: considerations and future directions. Nat. Rev. Drug Discov. 23, 341–364 (2024).
Qin, S. et al. mRNA-based therapeutics: powerful and versatile tools to combat diseases. Signal Transduct. Target. Ther. 7, 166 (2022).
Rohner, E., Yang, R., Foo, K. S., Goedel, A. & Chien, K. R. Unlocking the promise of mRNA therapeutics. Nat. Biotechnol. 40, 1586–1600 (2022).
Geary, R. S., Norris, D., Yu, R. & Bennett, C. F. Pharmacokinetics, biodistribution and cell uptake of antisense oligonucleotides. Adv. Drug Deliv. Rev. 87, 46–51 (2015).
Bost, J. P. et al. Delivery of oligonucleotide therapeutics: chemical modifications, lipid nanoparticles and extracellular vesicles. ACS Nano 15, 13993–14021 (2021).
Baker, Y. R. et al. An LNA-amide modification that enhances the cell uptake and activity of phosphorothioate exon-skipping oligonucleotides. Nat. Commun. 13, 4036 (2022).
Judge, A. D., Bola, G., Lee, A. C. H. & MacLachlan, I. Design of noninflammatory synthetic siRNA mediating potent gene silencing in vivo. Mol. Ther. 13, 494–505 (2006).
Pollak, A. J. et al. Insights into innate immune activation via PS-ASO–protein–TLR9 interactions. Nucleic Acids Res. 50, 8107–8126 (2022).
Bijsterbosch, M. K. et al. In vivo fate of phosphorothioate antisense oligodeoxynucleotides: predominant uptake by scavenger receptors on endothelial liver cells. Nucleic Acids Res. 25, 3290–3296 (1997).
Hammond, S. M. et al. Antibody-oligonucleotide conjugate achieves CNS delivery in animal models for spinal muscular atrophy. JCI Insight 7, e154142 (2022).
Barker, S. J. et al. Targeting the transferrin receptor to transport antisense oligonucleotides across the mammalian blood-brain barrier. Sci. Transl. Med. 16, eadi2245 (2024).
Vinjamuri, B. P., Pan, J. & Peng, P. A review on commercial oligonucleotide drug products. J. Pharm. Sci. 113, 1749–1768 (2024).
Kim, J., Eygeris, Y., Ryals, R. C., Jozić, A. & Sahay, G. Strategies for non-viral vectors targeting organs beyond the liver. Nat. Nanotechnol. 19, 428–447 (2024).
Nagata, T. et al. Cholesterol-functionalized DNA/RNA heteroduplexes cross the blood-brain barrier and knock down genes in the rodent CNS. Nat. Biotechnol. 39, 1529–1536 (2021).
Li, X. et al. Enhanced in vivo blood–brain barrier penetration by circular tau-transferrin receptor bifunctional aptamer for tauopathy therapy. J. Am. Chem. Soc. 142, 3862–3872 (2020).
Malecova, B. et al. Targeted tissue delivery of RNA therapeutics using antibody–oligonucleotide conjugates (AOCs). Nucleic Acids Res. 51, 5901–5910 (2023).
Shu, D. et al. Systemic delivery of anti-miRNA for suppression of triple negative breast cancer utilizing RNA nanotechnology. ACS Nano 9, 9731–9740 (2015).
Beck, C. et al. Trimannose-coupled antimiR-21 for macrophage-targeted inhalation treatment of acute inflammatory lung damage. Nat. Commun. 14, 4564 (2023).
Brown, K. M. et al. Expanding RNAi therapeutics to extrahepatic tissues with lipophilic conjugates. Nat. Biotechnol. 40, 1500–1508 (2022).
Nair, J. K. et al. Multivalent N-acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. J. Am. Chem. Soc. 136, 16958–16961 (2014).
Ebrahimi, S. B., Samanta, D. & Mirkin, C. A. DNA-based nanostructures for live-cell analysis. J. Am. Chem. Soc. 142, 11343–11356 (2020).
Chen, Q. et al. Lipophilic siRNAs mediate efficient gene silencing in oligodendrocytes with direct CNS delivery. J. Control. Release 144, 227–232 (2010).
Poecheim, J. et al. Development of stable liquid formulations for oligonucleotides. Eur. J. Pharm. Biopharm. 129, 80–87 (2018).
Mitchell, M. J. et al. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 20, 101–124 (2021).
Rosi, N. L. et al. Oligonucleotide-modified gold nanoparticles for infracellular gene regulation. Science 312, 1027–1030 (2006).
Jensen, S. A. et al. Spherical nucleic acid nanoparticle conjugates as an RNAi-based therapy for glioblastoma. Sci. Transl. Med. 5, 209ra152 (2013).
Cheng, Q. et al. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR-Cas gene editing. Nat. Nanotechnol. 15, 313–320 (2020).
Yan, Y. et al. Functional polyesters enable selective siRNA delivery to lung cancer over matched normal cells. Proc. Natl Acad. Sci. USA 113, E5702–E5710 (2016).
Huang, K. et al. Size-dependent localization and penetration of ultrasmall gold nanoparticles in cancer cells, multicellular spheroids and tumors in vivo. ACS Nano 6, 4483–4493 (2012).
Hu, B. et al. Thermostable ionizable lipid-like nanoparticle (iLAND) for RNAi treatment of hyperlipidemia. Sci. Adv. 8, eabm1418 (2022).
Mullard, A. FDA approves fifth RNAi drug—Alnylam’s next-gen hATTR treatment. Nat. Rev. Drug Discov. 21, 548–549 (2022).
Hoose, A., Vellacott, R., Storch, M., Freemont, P. S. & Ryadnov, M. G. DNA synthesis technologies to close the gene writing gap. Nat. Rev. Chem. 7, 144–161 (2023).
Moody, E. R., Obexer, R., Nickl, F., Spiess, R. & Lovelock, S. L. An enzyme cascade enables production of therapeutic oligonucleotides in a single operation. Science 380, 1150–1154 (2023).
Molina, A. G. & Sanghvi, Y. S. Liquid-phase oligonucleotide synthesis: past, present and future predictions. Curr. Protoc. Nucleic Acid Chem. 77, e82 (2019).
Palluk, S. et al. De novo DNA synthesis using polymerase-nucleotide conjugates. Nat. Biotechnol. 36, 645–650 (2018).
Wiegand, D. J. et al. Template-independent enzymatic synthesis of RNA oligonucleotides. Nat. Biotechnol. https://doi.org/10.1038/s41587-024-02244-w (2024).
Crameri, A. & Tew, D. G. Novel processes for the production of oligonucleotides. PCT patent WO2019121500A1 (2017).
Lobue, P. A., Jora, M., Addepalli, B. & Limbach, P. A. Oligonucleotide analysis by hydrophilic interaction liquid chromatography-mass spectrometry in the absence of ion-pair reagents. J. Chromatogr. A 1595, 39–48 (2019).
Muslehiddinoglu, J. et al. Technical considerations for use of oligonucleotide solution API. Nucleic Acid Ther. 30, 189–197 (2020).
Iwamoto, N. et al. Control of phosphorothioate stereochemistry substantially increases the efficacy of antisense oligonucleotides. Nat. Biotechnol. 35, 845–851 (2017).
Danaei, M. et al. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 10, 57 (2018).
Herrmann, I. K., Wood, M. J. A. & Fuhrmann, G. Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 16, 748–759 (2021).
Huang, Y. et al. A P(V) platform for oligonucleotide synthesis. Science 373, 1265–1270 (2021).
Li, H. et al. Molecular spherical nucleic acids. Proc. Natl Acad. Sci. USA 115, 4340–4344 (2018).
Sych, T. et al. High-throughput measurement of the content and properties of nano-sized bioparticles with single-particle profiler. Nat. Biotechnol. 42, 587–590 (2024).
Rentel, C. et al. Determination of oligonucleotide deamination by high resolution mass spectrometry. J. Pharm. Biomed. Anal. 173, 56–61 (2019).
DeCollibus, D. P. et al. Considerations for the terminal sterilization of oligonucleotide drug products. Nucleic Acid Ther. 33, 159–177 (2023).
Rentel, C. et al. Assay, purity and impurity profile of phosphorothioate oligonucleotide therapeutics by ion pair-HPLC-MS. Nucleic Acid Ther. 32, 206–220 (2022).
Pavc, D. et al. Understanding self-assembly at molecular level enables controlled design of DNA G-wires of different properties. Nat. Commun. 13, 1062 (2022).
Ebrahimi, S. B. & Samanta, D. Engineering protein-based therapeutics through structural and chemical design. Nat. Commun. 14, 2411 (2023).
Tao, L., Faig, A. & Uhrich, K. E. Liposomal stabilization using a sugar-based, PEGylated amphiphilic macromolecule. J. Colloid Interface Sci. 431, 112–116 (2014).
Hadley, P. et al. Precise surface functionalization of PLGA particles for human T cell modulation. Nat. Protoc. 18, 3289–3321 (2023).
Cheng, F. et al. Research advances on the stability of mRNA vaccines. Viruses 15, 668 (2023).
Zhang, L. et al. Effect of mRNA-LNP components of two globally-marketed COVID-19 vaccines on efficacy and stability. npj Vaccines 8, 156 (2023).
Li, Y. et al. Aromatized liposomes for sustained drug delivery. Nat. Commun. 14, 6659 (2023).
Doan, T. N. K., Davis, M. M. & Croyle, M. A. Identification of film-based formulations that move mRNA lipid nanoparticles out of the freezer. Mol. Ther. Nucleic Acids 35, 102179 (2024).
Ruppl, A. et al. Don’t shake it! Mechanical stress testing of mRNA-lipid nanoparticles. Eur. J. Pharm. Biopharm. 198, 114265 (2024).
Miller, M. A., Engstrom, J. D., Ludher, B. S. & Johnston, K. P. Low viscosity highly concentrated injectable nonaqueous suspensions of lysozyme microparticles. Langmuir 26, 1067–1074 (2010).
Deokar, V., Sharma, A., Mody, R. & Volety, S. M. Comparison of strategies in development and manufacturing of low viscosity, ultra-high concentration formulation for IgG1 antibody. J. Pharm. Sci. 109, 3579–3589 (2020).
Watt, R. P., Khatri, H. & Dibble, A. R. G. Injectability as a function of viscosity and dosing materials for subcutaneous administration. Int. J. Pharm. 554, 376–386 (2019).
Farzan, M., Ross, A., Müller, C. & Allmendinger, A. Liquid crystal phase formation and non-Newtonian behavior of oligonucleotide formulations. Eur. J. Pharm. Biopharm. 181, 270–281 (2022).
Maksudov, F. et al. Therapeutic phosphorodiamidate morpholino oligonucleotides: physical properties, solution structures and folding thermodynamics. Mol. Ther. Nucleic Acids 31, 631–647 (2023).
Bookbinder, L. H. et al. A recombinant human enzyme for enhanced interstitial transport of therapeutics. J. Control. Release 114, 230–241 (2006).
Li, B. et al. Accelerating ionizable lipid discovery for mRNA delivery using machine learning and combinatorial chemistry. Nat. Mater. 23, 1002–1008 (2024).
van Meer, L. et al. Injection site reactions after subcutaneous oligonucleotide therapy. Br. J. Clin. Pharmacol. 82, 340–351 (2016).
Kim, J. et al. Patient-customized oligonucleotide therapy for a rare genetic disease. N. Engl. J. Med. 381, 1644–1652 (2019).
The cost of getting personal. Nat. Med. 25, 1797–1797 (2019).
Lemaitre, M. M. Individualized antisense oligonucleotide therapies: how to approach the challenge of manufacturing these oligos from a chemistry, manufacturing and control-regulatory standpoint. Nucleic Acid Ther. 32, 101–110 (2022).
Andrews, B. I. et al. Sustainability challenges and opportunities in oligonucleotide manufacturing. J. Org. Chem. 86, 49–61 (2021).
Acknowledgements
Images were created using BioRender.com, the PyMOL Molecular Graphics System version 2.0 (Schrödinger, LLC) and Avogadro (an open-source molecular builder and visualization tool, version 1.2.0, http://avogadro.cc/). We acknowledge the use of OpenAI’s ChatGPT (version GPT-4) for proofreading the manuscript. D.S. acknowledges support from start-up funds from The University of Texas at Austin and the Welch Foundation (grant no. F-2209-20240404). Y.L. thanks the US National Institute of Health (GM141931) and the Welch Foundation (F-0020) for support. P.S.D. is supported by NSF grant CBET-1936696.
Author information
Authors and Affiliations
Contributions
S.B.E. and D.S. conceived the Perspective. S.B.E. and D.S. wrote the paper with input from H.B., S.S., D.F., P.S.D. and Y.L. S.B.E. and D.S. created the figures and tables. All authors reviewed and edited each section of the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Engineering thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ebrahimi, S.B., Bhattacharjee, H., Sonti, S. et al. Engineering considerations for next-generation oligonucleotide therapeutics. Nat Chem Eng 1, 741–750 (2024). https://doi.org/10.1038/s44286-024-00152-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44286-024-00152-z