Abstract
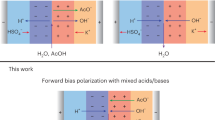
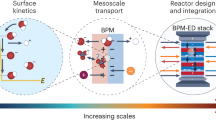
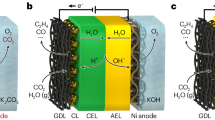
The ability for bipolar membranes (BPMs) to interconvert voltage and pH makes them attractive materials for use in energy conversion and storage. Reverse-biased BPMs, which use electrical voltage to dissociate water into acid and base, have become increasingly well studied. However, forward-biased BPMs (FB-BPMs), in which voltage is extracted from pH gradients through recombination, require further study. Here physics-based modeling elucidates how the complex coupling of transport and kinetics dictates the performance of FB-BPMs in electrochemical devices. Simulations reveal that the open-circuit potential of FB-BPMs is dictated by the balance of ion recombination and crossover, where recombination of buffering counter-ions attenuates the open-circuit potential. Counter-ion mass-transport limitations and uptake of ionic impurities limit achievable current densities by reducing the applied pH gradient or the available fixed-charge sites that mediate recombination. The model highlights the importance of selective ion management in mitigating energy losses and provides insight into the rational material design of FB-BPMs for energy applications.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data associated with the figures in this Article can be found in Supplementary Data. Source data are provided with this paper.
References
Pärnamäe, R. et al. Bipolar membranes: a review on principles, latest developments, and applications. J. Memb. Sci. 617, 118538 (2021).
Blommaert, M. A. et al. Insights and challenges for applying bipolar membranes in advanced electrochemical energy systems. ACS Energy Lett. 6, 2539–2548 (2021).
Bui, J. C. et al. Multi-scale physics of bipolar membranes in electrochemical processes. Nat. Chem. Eng. 1, 45–60 (2024).
Lucas, É. et al. Asymmetric bipolar membrane for high current density electrodialysis operation with exceptional stability. ACS Energy Lett. 9, 5596–5605 (2024).
Oener, S. Z., Foster, M. J. & Boettcher, S. W. Accelerating water dissociation in bipolar membranes and for electrocatalysis. Science 369, 1099–1103 (2020).
Toh, W. L., Dinh, H. Q., Chu, A. T., Sauvé, E. R. & Surendranath, Y. The role of ionic blockades in controlling the efficiency of energy recovery in forward bias bipolar membranes. Nat. Energy 8, 1405–1416 (2023).
Dinh, H. Q., Toh, W. L., Chu, A. T. & Surendranath, Y. Neutralization short-circuiting with weak electrolytes erodes the efficiency of bipolar membranes. ACS Appl. Mater. Interfaces 15, 4001–4010 (2023).
Gabrielsson, E. O., Tybrandt, K. & Berggren, M. Ion diode logics for pH control. Lab Chip 12, 2507–2513 (2012).
Blommaert, M. A. et al. Orientation of a bipolar membrane determines the dominant ion and carbonic species transport in membrane electrode assemblies for CO2 reduction. J. Mater. Chem. A 9, 11179–11186 (2021).
Peng, S. et al. A self-humidifying acidic–alkaline bipolar membrane fuel cell. J. Power Sources 299, 273–279 (2015).
Mitchell, J. B., Chen, L., Langworthy, K., Fabrizio, K. & Boettcher, S. W. Catalytic proton–hydroxide recombination for forward-bias bipolar membranes. ACS Energy Lett. 7, 3967–3973 (2022).
Yan, Z. et al. High-voltage aqueous redox flow batteries enabled by catalyzed water dissociation and acid-base neutralization in bipolar membranes. ACS Cent. Sci. 7, 1028–1035 (2021).
Chen, R. Redox flow batteries: mitigating cross-contamination via bipolar redox-active materials and bipolar membranes. Curr. Opin. Electrochem. 37, 101188 (2023).
Xi, D. et al. Mild pH-decoupling aqueous flow battery with practical pH recovery. Nat. Energy 9, 479–490 (2024).
Bui, J. C., Digdaya, I., Xiang, C., Bell, A. T. & Weber, A. Z. Understanding multi-ion transport mechanisms in bipolar membranes. ACS Appl. Mater. Interfaces 12, 52509–52526 (2020).
Newman, J. & Thomas-Alyea, K. E. Electrochemical Systems (John Wiley and Sons, 2004).
Pärnamäe, R. et al. Origin of limiting and overlimiting currents in bipolar membranes. Environ. Sci. Technol. 57, 9664–9674 (2023).
Crandall, B. S., Overa, S., Shin, H. & Jiao, F. Turning carbon dioxide into sustainable food and chemicals: how electrosynthesized acetate is paving the way for fermentation innovation. Acc. Chem. Res. 56, 1505–1516 (2023).
Ramdin, M. et al. High pressure electrochemical reduction of CO2 to formic acid/formate: a comparison between bipolar membranes and cation exchange membranes. Ind. Eng. Chem. Res. 58, 1834–1847 (2019).
Rabinowitz, J. A. & Kanan, M. W. The future of low-temperature carbon dioxide electrolysis depends on solving one basic problem. Nat. Commun. 11, 5231 (2020).
Peng, J., Roy, A. L., Greenbaum, S. G. & Zawodzinski, T. A. Effect of CO2 absorption on ion and water mobility in an anion exchange membrane. J. Power Sources 380, 64–75 (2018).
Yan, Z., Hitt, J. L., Zeng, Z., Hickner, M. A. & Mallouk, T. E. Improving the efficiency of CO2 electrolysis by using a bipolar membrane with a weak-acid cation exchange layer. Nat. Chem. 13, 33–40 (2021).
Bui, J. C. et al. Analysis of bipolar membranes for electrochemical CO2 capture from air and oceanwater. Energy Environ. Sci. 16, 5076–5095 (2023).
She, X. et al. Pure-water-fed, electrocatalytic CO2 reduction to ethylene beyond 1,000 h stability at 10 A. Nat. Energy 9, 81–91 (2024).
Fischer, R., Dessiex, M. A., Marone, F. & Buchi, F. N. Gas-induced structural damages in forward-bias bipolar membrane CO2 electrolysis studied by fast X‑ray tomography. ACS Appl. Energy Mater. 7, 3590–3601 (2024).
Bard, A. J. & Faulkner, L. R. Electrochemical Methods: Fundamentals and Applications (Wiley, 2001).
Corpus, K. R. M. et al. Coupling covariance matrix adaptation with continuum modeling for determination of kinetic parameters associated with electrochemical CO2 reduction. Joule 7, 1289–1307 (2023).
Hansen, N. The CMA evolution strategy: a tutorial. Preprint at https://arxiv.org/abs/1604.00772 (2023).
Pärnamäe, R. et al. The acid–base flow battery: sustainable energy storage via reversible water dissociation with bipolar membranes. Membranes 10, 1–20 (2020).
Yan, H. et al. Bipolar membrane-assisted reverse electrodialysis for high power density energy conversion via acid–base neutralization. J. Memb. Sci. 647, 120288 (2022).
Kitto, D. & Kamcev, J. The need for ion-exchange membranes with high charge densities. J. Memb. Sci. 677, 121608 (2023).
Kusoglu, A. & Weber, A. Z. New insights into perfluorinated sulfonic-acid ionomers. Chem. Rev. 117, 987–1104 (2017).
Lees, E. W., Bui, J. C., Song, D., Weber, A. Z. & Berlinguette, C. P. Continuum model to define the chemistry and mass transfer in a bicarbonate electrolyzer. ACS Energy Lett. 7, 834–842 (2022).
Jouny, M., Luc, W. & Jiao, F. High-rate electroreduction of carbon monoxide to multi-carbon products. Nat. Catal. 1, 748–755 (2018).
Li, T., Lees, E. W., Zhang, Z. & Berlinguette, C. P. Conversion of bicarbonate to formate in an electrochemical flow reactor. ACS Energy Lett. 5, 2624–2630 (2020).
van Linden, N., Bandinu, G. L., Vermaas, D. A., Spanjers, H. & van Lier, J. B. Bipolar membrane electrodialysis for energetically competitive ammonium removal and dissolved ammonia production. J. Clean. Prod. 259, 120788 (2020).
Wheaton, R. M. & Bauman, W. C. Properties of strongly basic anion exchange resins. Ind. Eng. Chem. 43, 1088–1093 (1951).
Crothers, A. R., Darling, R. M., Kushner, D. I., Perry, M. L. & Weber, A. Z. Theory of multicomponent phenomena in cation-exchange membranes: part III. Transport in vanadium redox-flow-battery separators. J. Electrochem. Soc. 167, 013549 (2020).
Andersen, M. B. et al. Current-induced membrane discharge. Phys. Rev. Lett. 109, 1–5 (2012).
Singh, S., Taketsugu, T. & Singh, R. K. Hydration, prediction of the pKa, and infrared spectroscopic study of sulfonated polybenzophenone (SPK) block-copolymer hydrocarbon membranes and comparisons with Nafion. ACS Omega 6, 32739–32748 (2021).
De Paul Nzuwah Nziko, V., Shih, J. L., Jansone-Popova, S. & Bryantsev, V. S. Quantum chemical prediction of pKa values of cationic ion-exchange groups in polymer electrolyte membranes. J. Phys. Chem. C 122, 2490–2501 (2018).
Krewer, U., Weinzierl, C., Ziv, N. & Dekel, D. R. Impact of carbonation processes in anion exchange membrane fuel cells. Electrochim. Acta 263, 433–446 (2018).
Keith, D. W., Holmes, G., St. Angelo, D. & Heidel, K. A process for capturing CO2 from the atmosphere. Joule 2, 1573–1594 (2018).
Suzuki, S., Muroyama, H., Matsui, T. & Eguchi, K. Influence of CO2 dissolution into anion exchange membrane on fuel cell performance. Electrochim. Acta 88, 552–558 (2013).
Bui, J. C. et al. Engineering catalyst—electrolyte microenvironments to optimize the activity and selectivity for the electrochemical reduction of CO2 on Cu and Ag. Acc. Chem. Res. 55, 484–494 (2022).
Tricker, A. W. et al. Engineering bipolar interfaces for water electrolysis using earth-abundant anodes. ACS Energy Lett. 8, 5275–5280 (2023).
Heßelmann, M. et al. Pure-water-fed forward-bias bipolar membrane CO2 electrolyzer. ACS Appl. Mater. Interfaces https://doi.org/10.1021/acsami.4c02799 (2024).
Kushner, D. I., Crothers, A. R., Kusoglu, A. & Weber, A. Z. Transport phenomena in flow battery ion-conducting membranes. Curr. Opin. Electrochem. 21, 132–139 (2020).
Lucas, É., Han, L., Sullivan, I., Atwater, H. A. & Xiang, C. Measurement of ion transport properties in ion exchange membranes for photoelectrochemical water splitting. Front. Energy Res. 10, 1–11 (2022).
Craig, N. P. Electrochemical Behavior of Bipolar Membranes: PhD thesis, Univ. of California, Berkeley (2013).
Onsager, L. & Fuoss, R. M. Irreversible processes in electrolytes. Diffusion, conductance, and viscous flow in arbitrary mixtures of strong electrolytes. J. Phys. Chem. 36, 2689–2778 (1932).
Onsager, L. Deviations from Ohm’s law in weak electrolytes. J. Chem. Phys. 2, 599–615 (1934).
Kaiser, V., Bramwell, S. T., Holdsworth, P. C. W. & Moessner, R. Onsager’s Wien effect on a lattice. Nat. Mater. 12, 1033–1037 (2013).
Bui, J. C., Corpus, K. R. M., Bell, A. T. & Weber, A. Z. On the nature of field enhanced water dissociation in bipolar membranes. J. Phys. Chem. C 125, 24974–24987 (2021).
Lin, M., Digdaya, I. A. & Xiang, C. Modeling the electrochemical behavior and interfacial junction profiles of bipolar membranes at solar flux relevant operating current densities. Sustain. Energy Fuels 5, 2149–2158 (2021).
Kamcev, J. et al. Partitioning of mobile ions between ion exchange polymers and aqueous salt solutions: Importance of counter-ion condensation. Phys. Chem. Chem. Phys. 18, 6021–6031 (2016).
Manning, G. S. Limiting laws and counterion condensation in polyelectrolyte solutions. 8. mixtures of counterions, species selectivity, and valence selectivity. J. Phys. Chem. 88, 6654–6661 (1984).
Purpura, G. et al. Modelling of selective ion partitioning between ion-exchange membranes and highly concentrated multi-ionic brines. J. Memb. Sci. 700, 122659 (2024).
Ramírez, P., Rapp, H. J., Reichle, S., Strathmann, H. & Mafé, S. Current–voltage curves of bipolar membranes. J. Appl. Phys. 72, 259–264 (1992).
Rodellar, C. G., Gisbert-Gonzalez, J. M., Sarabia, F., Roldan Cuenya, B. & Oener, S. Z. Ion solvation kinetics in bipolar membranes and at electrolyte-metal interfaces. Nat. Energy 9, 548–558 (2024).
Grew, K. N. & Chiu, W. K. S. A dusty fluid model for predicting hydroxyl anion conductivity in alkaline anion exchange membranes. J. Electrochem. Soc. 157, B327 (2010).
Acknowledgements
This material is based upon work supported by the US Department of Energy, Office of Science Energy Earthshot Initiative as part of the Center for Ionomer-based Water Electrolysis at Lawrence Berkeley National Laboratory under contract #DE-AC02-05CH11231. J.C.B. was supported in part by a fellowship award under contract FA9550-21-F-0003 through the National Defense Science and Engineering Graduate (NDSEG) Fellowship Program, sponsored by the Army Research Office (ARO). E.W.L acknowledges funding from the National Science and Engineering Research Council of Canada (NSERC). F.J.U.G. and T.N.S. acknowledge funding from the US Department of Energy, Office of Science Energy Earthshot Initiative as part of the Bipolar Membrane Science Foundations for the Energy Earthshot under contact #DE-SC0024713. T.N.S. acknowledges support from the National Science Foundation Graduate Research Fellowship (NSFGRFP) under grant no. DGE 2146752.
Author information
Authors and Affiliations
Contributions
J.C.B. conceived of the study, developed the continuum model and theory, and collected and analyzed simulation data. A.K.L. performed initial model calculations and validations. E.W.L., P.G. and F.J.U.G. assisted with theory development and provided modeling support. W.L.T. and T.N.S provided experimental data and assisted with data interpretation. J.C.B and E.W.L. prepared the initial draft of the manuscript. A.Z.W., A.T.B. and Y.S. supervised the project and assisted with data analysis and interpretation. All authors engaged in the writing and revision of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Engineering thanks Sebastian Oener and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Supplementary information
Supplementary Information
Supplementary Figs. 1-75, Tables 1–6, discussion and methods.
Source data
Source Data Fig. 2
Electrochemical simulation data for FB-BPM open circuit potential, experimental data for comparison.
Source Data Fig. 3
Electrochemical simulation data for FB-BPM limiting current density.
Source Data Fig. 4
Electrochemical simulation data for FB-BPM in mixed electrolytes, experimental data for comparison.
Source Data Fig. 5
Electrochemical simulation data of FB-BPMs with absorbed CO2.
Source Data Fig. 6
Sensitivity analysis data of FB-BPM performance.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bui, J.C., Lees, E.W., Liu, A.K. et al. Ion-specific phenomena limit energy recovery in forward-biased bipolar membranes. Nat Chem Eng 2, 63–76 (2025). https://doi.org/10.1038/s44286-024-00154-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44286-024-00154-x
This article is cited by
-
Modeling insights to navigate limitations in bipolar membranes
Nature Chemical Engineering (2024)