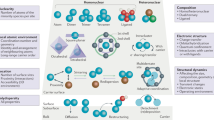
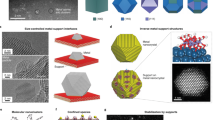
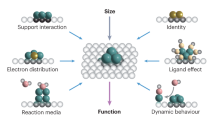
Abstract
Metal nanoparticle catalysts can undergo dynamic structural changes within chemical environments. Reaction processes and reactors are often designed to accommodate or exploit these changes to achieve desired performance targets. Consequently, controlling dynamic structural changes can lead to upgrading of reactors and reaction processes. This Perspective summarizes the characteristic dynamic behaviors of supported metal catalysts and their corresponding reactors in current industrial processes. We explore recent advancements in the programmable changes of metal catalysts by controlling reaction environments and metal–support interactions. These techniques offer avenues for upgrading reactors and reaction routes, aiming to improve efficiency and simplify production processes. The need to upgrade existing reactors also raises demands for managing catalyst dynamic structural changes. This Perspective emphasizes the importance of connecting atomic-scale changes in catalyst structure with industrial-scale reactions and reactors, which will advance research in catalysis and reaction engineering.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Dhashinamoorthy, A. & Garcia, H. Catalysis by metal nanoparticles embedded on metal–organic frameworks. Chem. Soc. Rev. 41, 5262–5284 (2012).
van Deelen, T. W., Mejía, C. H. & de Jong, K. P. Control of metal–support interactions in heterogeneous catalysts to enhance activity and selectivity. Nat. Catal. 2, 955–970 (2019).
Pacchioni, G. & Freund, H.-J. Controlling the charge state of supported nanoparticles in catalysis: lessons from model systems. Chem. Soc. Rev. 47, 8474–8502 (2018).
Liu, L. & Corma, A. Metal catalysts for heterogeneous catalysis: from single atoms to nanoclusters and nanoparticles. Chem. Rev. 118, 4981–5079 (2018).
Otroshchenko, T., Jiang, G., Kondratenko, V. A., Rodemerck, U. & Kondratenko, E. V. Current status and perspectives in oxidative, non-oxidative and CO2-mediated dehydrogenation of propane and isobutane over metal oxide catalysts. Chem. Soc. Rev. 50, 473–527 (2021).
Frey, H., Beck, A., Huang, X., van Bokhoven, J. A. & Willinger, M. G. Dynamic interplay between metal nanoparticles and oxide support under redox conditions. Science 376, 982–987 (2022).
Monai, M. et al. Restructuring of titanium oxide overlayers over nickel nanoparticles during catalysis. Science 380, 644–651 (2023).
Matsubu, J. C. et al. Adsorbate-mediated strong metal–support interactions in oxide-supported Rh catalysts. Nat. Chem. 9, 120–127 (2017).
Moliner, M. et al. Reversible transformation of Pt nanoparticles into single atoms inside high-silica chabazite zeolite. J. Am. Chem. Soc. 138, 15743–15750 (2016).
Tao, F. et al. Reaction-driven restructuring of Rh–Pd and Pt–Pd core–shell nanoparticles. Science 322, 932–934 (2008).
Xin, H. et al. Overturning CO2 hydrogenation selectivity with high activity via reaction-induced strong metal–support interactions. J. Am. Chem. Soc. 144, 4874–4882 (2022).
Jiang, D. et al. Dynamic and reversible transformations of subnanometre-sized palladium on ceria for efficient methane removal. Nat. Catal. 6, 618–627 (2023).
Yan, G. et al. Reaction product-driven restructuring and assisted stabilization of a highly dispersed Rh-on-ceria catalyst. Nat. Catal. 5, 119–127 (2022).
Tao, F. et al. Break-up of stepped platinum catalyst surfaces by high CO coverage. Science 327, 850–853 (2010).
Corma, A. et al. Exceptional oxidation activity with size-controlled supported gold clusters of low atomicity. Nat. Chem. 5, 775–781 (2013).
He, Y. et al. Size-dependent dynamic structures of supported gold nanoparticles in CO oxidation reaction condition. Proc. Natl Acad. Sci. USA 115, 7700–7705 (2018).
Liu, X. et al. Strong metal–support interactions between gold nanoparticles and ZnO nanorods in CO oxidation. J. Am. Chem. Soc. 134, 10251–10258 (2012).
Tang, H. et al. Classical strong metal–support interactions between gold nanoparticles and titanium dioxide. Sci. Adv. 3, e1700231 (2017).
Dong, J., Fu, Q., Jiang, Z., Mei, B. & Bao, X. Carbide-supported Au catalysts for water–gas shift reactions: a new territory for the strong metal–support interaction effect. J. Am. Chem. Soc. 140, 13808–13816 (2018).
Du, P. et al. Single-atom-driven dynamic carburization over Pd1–FeOx catalyst boosting CO2 conversion. Chem 8, 3252–3262 (2022).
Hansen, T. W., DeLaRiva, A. T., Challa, S. R. & Datye, A. K. Sintering of catalytic nanoparticles: particle migration or Ostwald ripening? Acc. Chem. Res. 46, 1720–1730 (2013).
Jones, J. et al. Thermally stable single-atom platinum-on-ceria catalysts via atom trapping. Science 353, 150–154 (2016).
Kosinov, N., Coumans, F. J. A. G., Uslamin, E., Kapteijn, F. & Hensen, E. J. M. Selective coke combustion by oxygen pulsing during Mo/ZSM-5-catalyzed methane dehydroaromatization. Angew. Chem. Int. Ed. 55, 15086–15090 (2016).
Li, F. et al. Interplay of electrochemical and electrical effects induces structural transformations in electrocatalysts. Nat. Catal. 4, 479–487 (2021).
Chen, H. et al. Photoinduced strong metal–support interaction for enhanced catalysis. J. Am. Chem. Soc. 143, 8521–8526 (2021).
Zhang, J., Zhu, D., Yan, J. & Wang, C.-A. Strong metal–support interactions induced by an ultrafast laser. Nat. Commun. 12, 6665 (2021).
Pham, H. N., Sattler, J. J. H. B., Weckhuysen, B. M. & Datye, A. K. Role of Sn in the regeneration of Pt/γ-Al2O3 light alkane dehydrogenation catalysts. ACS Catal. 6, 2257–2264 (2016).
Caspary, K. J., Gehrke, H., Heinrit-Adrian, M. & Schwefer, M. in Handbook of Heterogeneous Catalysis: Online (eds Ertl, G. et al.) 3206–3229 (Wiley-VCH, 2008).
Cottrell, P. R. & Fettis, M. E. Process for the dehydrogenation of hydrocarbons. US patent 5087792 (1992).
Williamson, R. R., Fettis, M. E. & Cottrell, P. R. Moving bed regeneration process with combined drying and dispersion steps. US patent 5457077 (1993).
Moore, M. A., Sechrist, P. A. & Glover, B. K. Processes and apparatuses for regenerating catalyst particles. US patent 20120322649 (2012).
Tao, F. & Salmeron, M. Surface restructuring and predictive design of heterogeneous catalysts. Science 386, eadq0102 (2024).
Zhong, L. et al. Cobalt carbide nanoprisms for direct production of lower olefins from syngas. Nature 538, 84–87 (2016).
Liu, L. et al. Dealuminated beta zeolite reverses Ostwald ripening for durable copper nanoparticle catalysts. Science 383, 94–101 (2024).
Frassoldati, A., Pinel, C. & Besson, M. Aerobic oxidation of secondary pyridine-derivative alcohols in the presence of carbon-supported noble metal catalysts. Catal. Today 203, 133–138 (2013).
Zhao, J. et al. Suppressing metal leaching in a supported Co/SiO2 catalyst with effective protectants in the hydroformylation reaction. ACS Catal. 10, 914–920 (2020).
Puskas, I. & James D. E. Process for preparation of palladium on carbon catalysts used in the purification of crude terephthalic acid. US patent 4421676 (1983).
Xu, Y. et al. Suppressing C–C bond dissociation for efficient ethane dehydrogenation over the isolated Co(II) sites in SAPO-34. ACS Catal. 11, 13001–13019 (2021).
Chung, K. et al. Non-oxidized bare copper nanoparticles with surface excess electrons in air. Nat. Nanotechnol. 7, 285–291 (2022).
Liu, Y. et al. Efficient catalytic production of hydrogen peroxide using tin-containing zeolite fixed palladium nanoparticles with oxidation resistance. Angew. Chem. Int. Ed. 62, e202312377 (2023).
Li, S., Lin, L., Wang, Z. & Ma, D. Direct utilization of crude and waste H2 via CO-tolerant hydrogenation. The Innovation 4, 100353 (2023).
Prieto, G., Zečević, J., Friedrich, H., de Jong, K. P. & de Jongh, P. E. Towards stable catalysts by controlling collective properties of supported metal nanoparticles. Nat. Mater. 12, 34–39 (2013).
Li, D. et al. Induced activation of the commercial Cu/ZnO/Al2O3 catalyst for the steam reforming of methanol. Nat. Catal. 5, 99–108 (2022).
Yao, D. et al. Scalable synthesis of Cu clusters for remarkable selectivity control of intermediates in consecutive hydrogenation. Nat. Commun. 14, 1123 (2023).
Otor, H. O., Steiner, J. B., García-Sancho, C. & Alba-Rubio, A. C. Encapsulation methods for control of catalyst deactivation: a review. ACS Catal. 10, 7630–7656 (2020).
Wang, L., Wang, L., Meng, X. & Xiao, F.-S. New strategies for the preparation of sinter-resistant metal-nanoparticle-based catalysts. Adv. Mater 31, 1901905 (2019).
Motagamwala, A. H., Almallahi, R., Wortman, J., Igenegbai, V. O. & Linic, S. Stable and selective catalysts for propane dehydrogenation operating at thermodynamic limit. Science 373, 217–222 (2021).
Pieck, C., Jablonski, E. L. & Parera, J. M. Sintering-redispersion of Pt-Re/Al2O3 during regeneration. Appl. Catal. 62, 47–60 (1990).
Kim, G. H. et al. Effect of oxychlorination treatment on the regeneration of Pt–Sn/Al2O3 catalyst for propane dehydrogenation. Res. Chem. Intermed. 42, 351–365 (2016).
Monai, M., Gambino, M., Wannakao, S. & Weckhuysen, B. M. Propane to olefins tandem catalysis: a selective route towards light olefins production. Chem. Soc. Rev. 50, 11503–11529 (2021).
Zhao, D. et al. In situ formation of ZnOx species for efficient propane dehydrogenation. Nature 599, 234–238 (2021).
Shao, H. et al. The active sites and catalytic properties of CrOx/Zn–Al2O3 catalysts for propane dehydrogenation. Appl. Catal. A 637, 118610 (2022).
Plessow, P. N. & Abild-Pedersen, F. Sintering of Pt nanoparticles via volatile PtO2: simulation and comparison with experiments. ACS Catal. 6, 7098–7108 (2016).
Wang, Y. et al. Boosting selectivity and stability on Pt/BN catalysts for propane dehydrogenation via calcination & reduction−mediated strong metal–support interaction. J. Energy Chem. 67, 451–457 (2022).
Xiong, H. et al. Thermally stable and regenerable platinum–tin clusters for propane dehydrogenation prepared by atom trapping on ceria. Angew. Chem. Int. Ed. 56, 8986–8991 (2017).
Goodman, E. D. et al. Catalyst deactivation via decomposition into single atoms and the role of metal loading. Nat. Catal. 2, 748–755 (2019).
Kwak, J. H. et al. Coordinatively unsaturated Al3+ centers as binding sites for active catalyst phases of platinum on γ-Al2O3. Science 325, 1670–1673 (2009).
Yao, H. C., Stepien, H. K. & Gandhi, H. S. Metal–support interaction in automotive exhaust catalysts: Rh–washcoat interaction. J. Catal. 61, 547–550 (1980).
Zhang, X. et al. Reversible loss of core–shell structure for Ni–Au bimetallic nanoparticles during CO2 hydrogenation. Nat. Catal. 3, 411–417 (2020).
Liu, L. et al. Generation of subnanometric platinum with high stability during transformation of a 2D zeolite into 3D. Nat. Mater. 16, 132–138 (2017).
Liu, L. et al. Direct assessment of confinement effect in zeolite-encapsulated subnanometric metal species. Nat. Commun. 13, 821 (2022).
Zhang, J. et al. Sinter-resistant metal nanoparticle catalysts achieved by immobilization within zeolite crystals via seed-directed growth. Nat. Catal. 1, 540–546 (2018).
Liu, L. et al. Regioselective generation and reactivity control of subnanometric platinum clusters in zeolites for high-temperature catalysis. Nat. Mater. 18, 866–873 (2017).
Liu, L. et al. Structural modulation and direct measurement of subnanometric bimetallic PtSn clusters confined in zeolites. Nat. Catal. 3, 628–638 (2020).
Dou, X. et al. Regioselective hydroformylation with subnanometre Rh clusters in MFI zeolite. Nat. Catal. 7, 666–677 (2024).
Liu, L. et al. Evolution and stabilization of subnanometric metal species in confined space by in situ TEM. Nat. Commun. 9, 574 (2018).
Farpón, M. G. et al. Rhodium single-atom catalyst design through oxide support modulation for selective gas-phase ethylene hydroformylation. Angew. Chem. Int. Ed. 62, e202214048 (2023).
Chen, J.-S., Vasiliev, A. N., Panarello, A. P. & Khinast, J. G. Pd-leaching and Pd-removal in Pd/C-catalyzed Suzuki couplings. Appl. Catal. A 325, 76–86 (2007).
Cantillo, D. & Oliver Kappe, C. Immobilized transition metals as catalysts for cross-couplings in continuous flow—a critical assessment of the reaction mechanism and metal leaching. ChemCatChem 6, 3286–3305 (2014).
Hebrard, F. & Kalck, P. Cobalt-catalyzed hydroformylation of alkenes: generation and recycling of the carbonyl species, and catalytic cycle. Chem. Rev. 109, 4272–4282 (2009).
Fassbach, T. A., Ji, J.-M., Vorholt, A. J. & Leitner, W. Recycling of homogeneous catalysts—basic principles, industrial practice, and guidelines for experiments and evaluation. ACS Catal. 14, 7289–7298 (2024).
Beller, M., Cornils, B., Frohning, C. D. & Kohlpaintner, C. W. Progress in hydroformylation and carbonylation. J. Mol. Catal. A 104, 17–85 (1995).
Cai, Z. et al. Hydroformylation of 1-hexene over ultrafine cobalt nanoparticle catalysts. J. Mol. Catal. A 330, 94–98 (2010).
Luo, S. et al. Light-induced dynamic restructuring of Cu active sites on TiO2 for low-temperature H2 production from methanol and water. J. Am. Chem. Soc. 145, 20530–20538 (2023).
Fan, Y. et al. Water-assisted oxidative redispersion of Cu particles through formation of Cu hydroxide at room temperature. Nat. Commun. 15, 3046 (2024).
Behrens, M. et al. The active site of methanol synthesis over Cu/ZnO/Al2O3 industrial catalysts. Science 336, 893–897 (2012).
Li, H. et al. Physical regulation of copper catalyst with a hydrophobic promoter for enhancing CO2 hydrogenation to methanol. The Innovation 4, 100445 (2023).
Xu, C. et al. Development of stable water−resistant Cu-based catalyst for methanol synthesis. Appl. Catal. A 623, 118299 (2021).
Sun, X., Wang, P., Shao, Z., Cao, X. & Hu, P. A first-principles microkinetic study on the hydrogenation of carbon dioxide over Cu(211) in the presence of water. Sci. China Chem. 62, 1686–1697 (2019).
Li, H. et al. Na+-gated water-conducting nanochannels for boosting CO2 conversion to liquid fuels. Science 367, 667–671 (2020).
Chen, S. et al. Defective TiOx overlayers catalyze propane dehydrogenation promoted by base metals. Science 385, 295–300 (2024).
Almallahi, R., Wortman, J. & Linic, S. Overcoming limitations in propane dehydrogenation by codesigning catalyst–membrane systems. Science 383, 1325–1331 (2024).
Morejudo, S. H. et al. Direct conversion of methane to aromatics in a catalytic co-ionic membrane reactor. Science 353, 563–566 (2016).
Tang, M. et al. Oscillatory active state of a Pd nanocatalyst identified by in situ capture of the instantaneous structure–activity change at the atomic scale. J. Am. Chem. Soc. 146, 18341–18349 (2024).
Cortés, E., Grzeschik, R., Maier, S. A. & Schlücker, S. Experimental characterization techniques for plasmon-assisted chemistry. Nat. Rev. Chem. 6, 259–274 (2022).
Zhai, H. & Alexandrova, A. N. Local fluxionality of surface-deposited cluster catalysts: the case of Pt7 on Al2O3. J. Phys. Chem. Lett. 9, 1696–1702 (2018).
Liu, Y. et al. Progress and challenges in structural, in situ and operando characterization of single-atom catalysts by X-ray-based synchrotron radiation techniques. Chem. Soc. Rev. 53, 11850–11887 (2024).
Xu, L. et al. Formation of active sites on transition metals through reaction-driven migration of surface atoms. Science 380, 70–76 (2023).
Wang, T. et al. Nature of metal–support interaction for metal catalysts on oxide supports. Science 386, 915–920 (2024).
Avanesian, T. et al. Quantitative and atomic-scale view of CO-induced Pt nanoparticle surface reconstruction at saturation coverage via DFT calculations coupled with in situ TEM and IR. J. Am. Chem. Soc. 139, 4551–4558 (2017).
Marimuthu, A., Zhang, J. & Linic, S. Tuning selectivity in propylene epoxidation by plasmon mediated photo-switching of Cu oxidation state. Science 339, 1590–1593 (2013).
Xie, B. et al. Synergistic ultraviolet and visible light photoactivation enables intensified low-temperature methanol synthesis over copper/zinc oxide/alumina. Nat. Commun. 11, 1615 (2020).
Yang, J. et al. Potential-driven restructuring of Cu single atoms to nanoparticles for boosting the electrochemical reduction of nitrate to ammonia. J. Am. Chem. Soc. 144, 12062–12071 (2022).
Yu, J. et al. Ultra-high thermal stability of sputtering reconstructed Cu-based catalysts. Nat. Commun. 12, 7209 (2021).
Gopeesingh, J. et al. Resonance-promoted formic acid oxidation via dynamic electrocatalytic modulation. ACS Catal. 10, 9932–9942 (2020).
Ardagh, M. A., Abdelrahman, O. A. & Dauenhauer, P. J. Principles of dynamic heterogeneous catalysis: surface resonance and turnover frequency response. ACS Catal. 9, 6929–6937 (2019).
Ardagh, M. A. et al. Catalytic resonance theory: parallel reaction pathway control. Chem. Sci. 11, 3501–3510 (2020).
Acknowledgements
We acknowledge support by the National Key Research and Development Program of China (2022YFA1503502), the National Natural Science Foundation of China (22288101 and 22241801), the Zhejiang Provincial Basic Public Welfare Project (LD24E030003) and Qizhen Funding of Zhejiang University (226-2023-00035, Fundamental Research Funds for the Central Universities).
Author information
Authors and Affiliations
Contributions
H. Wang and L.W. conceived the concept of this paper. Y.W., Q.L. and H. Wu provided helpful discussions. All authors participated in preparing the paper. F.-S.X. and L.W. supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Engineering thanks Lichen Liu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, H., Wu, Y., Luo, Q. et al. Managing dynamic catalyst changes to upgrade reactors and reaction processes. Nat Chem Eng 2, 169–180 (2025). https://doi.org/10.1038/s44286-025-00199-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44286-025-00199-6