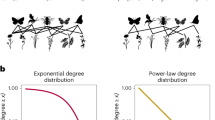
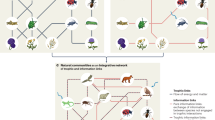
Abstract
Ecological interaction networks are an important tool for describing species interactions, but many network approaches are available, each with their strengths and weaknesses. In this Perspective, we describe how interaction networks can be differentiated in two main ways: by the extent of node aggregation (how species are lumped into groups) and by the type of information contained in links (potential versus realized interactions). We discuss the ecological questions that each network type can address, how measurements from different types of network should be interpreted, and their relative advantages. Networks with nodes aggregated to functional groups are suitable when focusing on ecosystem-level processes and ecosystem functions. Species-level networks provide information about the assembly of ecological communities or about how abiotic and biotic drivers influence species persistence. Networks with potential links are particularly useful for understanding ecological redundancy or for long-term or large-scale studies, where all potential interactions are likely to be realized. Networks of realized interactions provide access to finer mechanisms of the interplay between abiotic and biotic factors in determining ecological interactions. Identifying the advantages and limitations of different interaction networks will aid methodological decision making and increase the utility and applicability of ecological networks in biodiversity and conservation research programmes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Eschenbrenner, J. & Thébault, É. Diversity, food web structure and the temporal stability of total plant and animal biomasses. Oikos 2023, e08769 (2023).
Barnes, A. D. et al. Energy flux: the link between multitrophic biodiversity and ecosystem functioning. Trends Ecol. Evol. 33, 186–197 (2018).
Antunes, A. C. et al. Linking biodiversity and nature’s contributions to people (NCP): a macroecological energy flux perspective. Trends Ecol. Evol. 39, 427–434 (2024).
Giling, D. P. et al. A niche for ecosystem multifunctionality in global change research. Glob. Change Biol. 25, 763–774 (2019).
Martinez, N. D. Artifacts or attributes? Effects of resolution on the Little Rock Lake food web. Ecol. Monogr. 61, 367–392 (1991).
Renaud, E., Baudry, E. & Bessa-Gomes, C. Influence of taxonomic resolution on mutualistic network properties. Ecol. Evol. 10, 3248–3259 (2020).
Hines, J. et al. A meta food web for invertebrate species collected in a European grassland. Ecology 100, e02679 (2019).
Li, J. et al. A size-constrained feeding-niche model distinguishes predation patterns between aquatic and terrestrial food webs. Ecol. Lett. 26, 76–86 (2023).
McDonald-Madden, E. et al. Using food-web theory to conserve ecosystems. Nat. Commun. 7, 10245 (2016).
Valdovinos, F. S. et al. Species traits and network structure predict the success and impacts of pollinator invasions. Nat. Commun. 9, 2153 (2018).
Loeuille, N. & Loreau, M. Evolutionary emergence of size-structured food webs. Proc. Natl Acad. Sci. USA 102, 5761–5766 (2005).
Gauzens, B., Legendre, S., Lazzaro, X. & Lacroix, G. Food-web aggregation, methodological and functional issues. Oikos 122, 1606–1615 (2013).
Dunne, J. A. in Ecological Networks: Linking Structure to Dynamics in Food Webs (eds Pascual, M. & Dunne, J. A.) (Oxford Academic, 2005).
Paine, R. T. Road maps of interactions or grist for theoretical development? Ecology 69, 1648–1654 (1988).
Hulot, F. D., Lacroix, G., Lescher-Moutoué, F. & Loreau, M. Functional diversity governs ecosystem response to nutrient enrichment. Nature 405, 340–344 (2000).
Boit, A., Martinez, N. D., Williams, R. J. & Gaedke, U. Mechanistic theory and modelling of complex food-web dynamics in Lake Constance: mechanistic modelling of complex food web dynamics. Ecol. Lett. 15, 594–602 (2012).
Ersoy, Z. et al. Size-based interactions and trophic transfer efficiency are modified by fish predation and cyanobacteria blooms in Lake Mývatn, Iceland. Freshw. Biol. 62, 1942–1952 (2017).
Gauzens, B., Legendre, S., Lazzaro, X. & Lacroix, G. Intermediate predation pressure leads to maximal complexity in food webs. Oikos 125, 595–603 (2016).
Allesina, S. & Pascual, M. Food web models: a plea for groups. Ecol. Lett. 12, 652–662 (2009).
Gauzens, B., Thébault, E., Lacroix, G. & Legendre, S. Trophic groups and modules: two levels of group detection in food webs. J. R. Soc. Interf. 12, 20141176 (2015).
Galiana, N. et al. Can biomass distribution across trophic levels predict trophic cascades? Ecol. Lett. 24, 464–476 (2021).
Lane, P. A. A review of the trophic cascade concept using the lens of loop analysis: “the truth is the whole”. Food Webs 13, 1–11 (2017).
Barnes, A. D. et al. Biodiversity enhances the multitrophic control of arthropod herbivory. Sci. Adv. 6, eabb6603 (2020).
Geslin, B., Gauzens, B., Thébault, E. & Dajoz, I. Plant pollinator networks along a gradient of urbanisation. PLoS ONE 8, e63421 (2013).
Schwarz, B. et al. Warming alters energetic structure and function but not resilience of soil food webs. Nat. Clim. Change 7, 895–900 (2017).
Ropars, L., Affre, L., Thébault, É. & Geslin, B. Seasonal dynamics of competition between honey bees and wild bees in a protected Mediterranean scrubland. Oikos 2022, e08915 (2022).
Potapov, A. M. et al. Rainforest transformation reallocates energy from green to brown food webs. Nature 627, 116–122 (2024).
Bauer, B. et al. Biotic filtering by species’ interactions constrains food-web variability across spatial and abiotic gradients. Ecol. Lett. 25, 1225–1236 (2022).
Allhoff, K. T., Ritterskamp, D., Rall, B. C., Drossel, B. & Guill, C. Evolutionary food web model based on body masses gives realistic networks with permanent species turnover. Sci. Rep. 5, 10955 (2015).
Lomáscolo, S. B., Giannini, N., Chacoff, N. P., Castro-Urgal, R. & Vázquez, D. P. Inferring coevolution in a plant–pollinator network. Oikos 128, 775–789 (2019).
Gauzens, B., Rall, B. C., Mendonça, V., Vinagre, C. & Brose, U. Biodiversity of intertidal food webs in response to warming across latitudes. Nat. Clim. Change 10, 264–269 (2020).
Kratina, P., LeCraw, R. M., Ingram, T. & Anholt, B. R. Stability and persistence of food webs with omnivory: is there a general pattern? Ecosphere 3, art50 (2012).
Arroyo-Correa, B., Bartomeus, I. & Jordano, P. Individual-based plant–pollinator networks are structured by phenotypic and microsite plant traits. J. Ecol. 109, 2832–2844 (2021).
Woodward, G. et al. Individual-based food webs. Adv. Ecol. Res. 43, 211–266 (2010).
Blanchard, J. L., Heneghan, R. F., Everett, J. D., Trebilco, R. & Richardson, A. J. From bacteria to whales: using functional size spectra to model marine ecosystems. Trends Ecol. Evol. 32, 174–186 (2017).
Gauzens, B. et al. Flexible foraging behaviour increases predator vulnerability to climate change. Nat. Clim. Change 14, 387–392 (2024).
Toscano, B. J. & Griffen, B. D. Trait-mediated functional responses: predator behavioural type mediates prey consumption. J. Anim. Ecol. 83, 1469–1477 (2014).
Sporta Caputi, S. et al. Individual diet variability shapes the architecture of Antarctic benthic food webs. Sci. Rep. 14, 12333 (2024).
Cantwell-Jones, A., Tylianakis, J. M., Larson, K. & Gill, R. J. Using individual-based trait frequency distributions to forecast plant-pollinator network responses to environmental change. Ecol. Lett. 27, e14368 (2024).
Gårdmark, A. & Huss, M. Individual variation and interactions explain food web responses to global warming. Phil. Trans. R. Soc. B 375, 20190449 (2020).
Delmas, E. et al. Analysing ecological networks of species interactions. Biol. Rev. 94, 16–36 (2019).
Dickman, E. M., Newell, J. M., González, M. J. & Vanni, M. J. Light, nutrients, and food-chain length constrain planktonic energy transfer efficiency across multiple trophic levels. Proc. Natl. Acad. Sci. USA 105, 18408–18412 (2008).
Thébault, E. & Fontaine, C. Stability of ecological communities and the architecture of mutualistic and trophic networks. Science 329, 853–856 (2010).
Borzone Mas, D., Scarabotti, P. A., Alvarenga, P., Vaschetto, P. A. & Arim, M. Food web structure mediates positive and negative effects of diversity on ecosystem functioning in a large floodplain river. Am. Nat. https://doi.org/10.1086/735914 (2025).
Wang, S. & Brose, U. Biodiversity and ecosystem functioning in food webs: the vertical diversity hypothesis. Ecol. Lett. 21, 9–20 (2018).
Neutel, A.-M. et al. Reconciling complexity with stability in naturally assembling food webs. Nature 449, 599–602 (2007).
Gibert, J. P. Temperature directly and indirectly influences food web structure. Sci. Rep. 9, 5312 (2019).
Benadi, G., Hovestadt, T., Poethke, H.-J. & Blüthgen, N. Specialization and phenological synchrony of plant–pollinator interactions along an altitudinal gradient. J. Anim. Ecol. 83, 639–650 (2014).
Kortsch, S. et al. Food-web structure varies along environmental gradients in a high-latitude marine ecosystem. Ecography 42, 295–308 (2019).
Lazzaro, X., Lacroix, G., Gauzens, B., Gignoux, J. & Legendre, S. Predator foraging behaviour drives food-web topological structure. J. Anim. Ecol. 78, 1307–1317 (2009).
O’Gorman, E. J., Fitch, J. E. & Crowe, T. P. Multiple anthropogenic stressors and the structural properties of food webs. Ecology 93, 441–448 (2012).
Tylianakis, J. M. & Morris, R. J. Ecological networks across environmental gradients. Annu. Rev. Ecol. Evol. Syst. 48, 25–48 (2017).
Dormann, C. F. in Defining Agroecology — A Festschrift for Teja Tscharntke (eds Dormann, C. F. et al.) 143–159 (Tredition, 2023).
Blüthgen, N. Why network analysis is often disconnected from community ecology: a critique and an ecologist’s guide. Basic Appl. Ecol. 11, 185–195 (2010).
Timóteo, S. et al. Tripartite networks show that keystone species can multitask. Funct. Ecol. 37, 274–286 (2023).
Bianco, G., Manning, P. & Schleuning, M. A quantitative framework for identifying the role of individual species in nature’s contributions to people. Ecol. Lett. 27, e14371 (2024).
Allesina, S. & Pascual, M. Googling food webs: can an eigenvector measure species’ importance for coextinctions? PLoS Comput. Biol. 5, e1000494 (2009).
Memmott, J., Waser, N. M. & Price, M. V. Tolerance of pollination networks to species extinctions. Proc. R. Soc. Lond. B 271, 2605–2611 (2004).
Alger, S. A., Burnham, P. A., Boncristiani, H. F. & Brody, A. K. RNA virus spillover from managed honeybees (Apis mellifera) to wild bumblebees (Bombus spp.). PLoS ONE 14, e0217822 (2019).
Musseau, C. et al. Within-individual trophic variability drives short-term intraspecific trait variation in natural populations. J. Anim. Ecol. 89, 921–932 (2020).
Emer, C. & Memmott, J. Intraspecific variation of invaded pollination networks — the role of pollen-transport, pollen-transfer and different levels of biological organization. Persp. Ecol. Conserv. 21, 151–163 (2023).
Pires, M. M. et al. The indirect paths to cascading effects of extinctions in mutualistic networks. Ecology 101, e03080 (2020).
Stouffer, D. B. & Bascompte, J. Compartmentalization increases food-web persistence. Proc. Natl Acad. Sci. USA 108, 3648–3652 (2011).
Romanuk, T. N. et al. Predicting invasion success in complex ecological networks. Phil. Trans. R. Soc. B 364, 1743–1754 (2009).
Lurgi, M., Galiana, N., López, B. C., Joppa, L. N. & Montoya, J. M. Network complexity and species traits mediate the effects of biological invasions on dynamic food webs. Front. Ecol. Evol. 2, 36 (2014).
Trøjelsgaard, K., Heleno, R. & Traveset, A. Native and alien flower visitors differ in partner fidelity and network integration. Ecol. Lett. 22, 1264–1273 (2019).
Thompson, R. M. & Townsend, C. R. Is resolution the solution? The effect of taxonomic resolution on the calculated properties of three stream food webs. Freshw. Biol. 44, 413–422 (2000).
Patonai, K. & Jordán, F. Aggregation of incomplete food web data may help to suggest sampling strategies. Ecol. Model. 352, 77–89 (2017).
Llopis-Belenguer, C. et al. Sensitivity of bipartite network analyses to incomplete sampling and taxonomic uncertainty. Ecology 104, e3974 (2023).
Martinez, N. D. Effects of resolution on food web structure. Oikos 66, 403 (1993).
Guimarães, P. R. The structure of ecological networks across levels of organization. Annu. Rev. Ecol. Evol. Syst. 51, 433–460 (2020).
Valdovinos, F. S., Bodini, A. & Jordán, F. Connected interactions: enriching food web research by spatial and social interactions. Phil. Trans. R. Soc. B 379, 20230163 (2024).
Fontaine, C., Collin, C. L. & Dajoz, I. Generalist foraging of pollinators: diet expansion at high density. J. Ecol. 96, 1002–1010 (2008).
Brose, U. et al. Embedding information flows within ecological networks. Nat. Ecol. Evol. 9, 547–558 (2025).
Raimundo, R. L. G., Guimarães, P. R. & Evans, D. M. Adaptive networks for restoration ecology. Trends Ecol. Evol. 33, 664–675 (2018).
Andelman, S. J. & Fagan, W. F. Umbrellas and flagships: efficient conservation surrogates or expensive mistakes? Proc. Natl Acad. Sci. 97, 5954–5959 (2000).
Cuff, J. P. et al. Prey nutrient content is associated with the trophic interactions of spiders and their prey selection under field conditions. Oikos 2025, e10712 (2025).
Pomeranz, J. P. F., Thompson, R. M., Poisot, T. & Harding, J. S. Inferring predator–prey interactions in food webs. Methods Ecol. Evol. 10, 356–367 (2019).
Saito, V. S. et al. Untangling the complex food webs of tropical rainforest streams. J. Anim. Ecol. 93, 1022–1035 (2024).
Hernvann, P.-Y., Gascuel, D., Kopp, D., Robert, M. & Rivot, E. A hierarchical Bayesian model to combine stomach, biotracer, and literature data into diet matrix estimation. Ecol. Appl. 32, e2521 (2022).
Williams, R. J., Anandanadesan, A. & Purves, D. The probabilistic niche model reveals the niche structure and role of body size in a complex food web. PLoS ONE 5, e12092 (2010).
Brose, U. et al. Predator traits determine food-web architecture across ecosystems. Nat. Ecol. Evol. 3, 919–927 (2019).
Pires, M. M., Benchimol, M., Cruz, L. R. & Peres, C. A. Terrestrial food web complexity in Amazonian forests decays with habitat loss. Curr. Biol. 33, 389–396.e3 (2023).
Pawar, S., Dell, A. I. & Savage, VanM. Dimensionality of consumer search space drives trophic interaction strengths. Nature 486, 485–489 (2012).
Eklöf, A. et al. The dimensionality of ecological networks. Ecol. Lett. 16, 577–583 (2013).
Strydom, T. et al. A roadmap towards predicting species interaction networks (across space and time). Phil. Trans. R. Soc. B 376, 20210063 (2021).
Cherif, M. et al. The environment to the rescue: can physics help predict predator–prey interactions? Biol. Rev. 99, 1927–1947 (2024).
Portalier, S. M. J., Fussmann, G. F., Loreau, M. & Cherif, M. The mechanics of predator–prey interactions: first principles of physics predict predator–prey size ratios. Funct. Ecol. 33, 323–334 (2019).
Klecka, J. & Boukal, D. S. Foraging and vulnerability traits modify predator–prey body mass allometry: freshwater macroinvertebrates as a case study. J. Anim. Ecol. 82, 1031–1041 (2013).
Garibaldi, L. A. et al. Trait matching of flower visitors and crops predicts fruit set better than trait diversity. J. Appl. Ecol. 52, 1436–1444 (2015).
Pires, M. M. et al. Pleistocene megafaunal interaction networks became more vulnerable after human arrival. Proc. R. Soc. B 282, 20151367 (2015).
Williams, R. J. & Martinez, N. D. Simple rules yield complex food webs. Nature 404, 180–183 (2000).
Schneider, F. D., Brose, U., Rall, B. C. & Guill, C. Animal diversity and ecosystem functioning in dynamic food webs. Nat. Commun. 7, 12718 (2016).
Binzer, A., Guill, C., Rall, B. C. & Brose, U. Interactive effects of warming, eutrophication and size structure: impacts on biodiversity and food-web structure. Glob. Change Biol. 22, 220–227 (2016).
Gravel, D., Poisot, T., Albouy, C., Velez, L. & Mouillot, D. Inferring food web structure from predator–prey body size relationships. Methods Ecol. Evol. 4, 1083–1090 (2013).
Pichler, M., Boreux, V., Klein, A.-M., Schleuning, M. & Hartig, F. Machine learning algorithms to infer trait-matching and predict species interactions in ecological networks. Meth. Ecol. Evol. 11, 281–293 (2020).
Rodrigues, F. A., Peron, T., Connaughton, C., Kurths, J. & Moreno, Y. A machine learning approach to predicting dynamical observables from network structure. Proc. R. Soc. A 481, 20240435 (2025).
Biton, B., Puzis, R. & Pilosof, S. Inductive link prediction boosts data availability and enables cross-community link prediction in ecological networks. Preprint at EcoEvoRxiv https://doi.org/10.32942/X2JS75 (2024).
Ho, H.-C., Tylianakis, J. M., Zheng, J. X. & Pawar, S. Predation risk influences food-web structure by constraining species diet choice. Ecol. Lett. 22, 1734–1745 (2019).
Lesser, J. S., James, W. R., Stallings, C. D., Wilson, R. M. & Nelson, J. A. Trophic niche size and overlap decreases with increasing ecosystem productivity. Oikos 129, 1303–1313 (2020).
Sentis, A., Hemptinne, J.-L. & Brodeur, J. Towards a mechanistic understanding of temperature and enrichment effects on species interaction strength, omnivory and food-web structure. Ecol. Lett. 17, 785–793 (2014).
Leimberger, K. G., Hadley, A. S. & Betts, M. G. Plant–hummingbird pollination networks exhibit limited rewiring after experimental removal of a locally abundant plant species. J. Anim. Ecol. 92, 1680–1694 (2023).
Neves, M. P., Delariva, R. L., Perkins, D. M., Fialho, C. B. & Kratina, P. Trophic plasticity of omnivorous fishes in natural and human-dominated landscapes. Limnol. Oceanog. 69, 189–202 (2024).
Su, M., Ma, Q. & Hui, C. Adaptive rewiring shapes structure and stability in a three-guild herbivore–plant–pollinator network. Commun. Biol. 7, 103 (2024).
Kondoh, M. Foraging adaptation and the relationship between food-web complexity and stability. Science 299, 1388–1391 (2003).
Heckmann, L., Drossel, B., Brose, U. & Guill, C. Interactive effects of body-size structure and adaptive foraging on food-web stability: body size, adaptivity and food-web stability. Ecol. Lett. 15, 243–250 (2012).
Gilljam, D., Curtsdotter, A. & Ebenman, B. Adaptive rewiring aggravates the effects of species loss in ecosystems. Nat. Commun. 6, 8412 (2015).
Ho, H.-C., Pawar, S. & Tylianakis, J. M. Less is worse than none: ineffective adaptive foraging can destabilise food webs. Preprint at bioRxiv https://doi.org/10.1101/2021.11.28.470273 (2021).
CaraDonna, P. J. et al. Interaction rewiring and the rapid turnover of plant–pollinator networks. Ecol. Lett. 20, 385–394 (2017).
Lázaro, A. & Gómez-Martínez, C. Habitat loss increases seasonal interaction rewiring in plant–pollinator networks. Funct. Ecol. 36, 2673–2684 (2022).
Bartley, T. J. et al. Food web rewiring in a changing world. Nat. Ecol. Evol. 3, 345–354 (2019).
Pires, M. M. Rewilding ecological communities and rewiring ecological networks. Persp. Ecol. Conserv. 15, 257–265 (2017).
Peralta, G. et al. Predicting plant–pollinator interactions: concepts, methods, and challenges. Trends Ecol. Evol. 39, 494–505 (2024).
Petchey, O. L., Brose, U. & Rall, B. C. Predicting the effects of temperature on food web connectance. Phil. Trans. R. Soc. B 365, 2081–2091 (2010).
Petchey, O. L., Beckerman, A. P., Riede, J. O. & Warren, P. H. Size, foraging, and food web structure. Proc. Natl Acad. Sci. USA 105, 4191–4196 (2008).
Pringle, R. M. & Hutchinson, M. C. Resolving food-web structure. Annu. Rev. Ecol. Evol. Syst. 51, 55–80 (2020).
Brimacombe, C. et al. Publication-driven consistency in food web structures: implications for comparative ecology. Ecology 106, e4467 (2025).
Kleunen, L. V., Dee, L. E., Wootton, K. L., Massol, F. & Clauset, A. Predicting missing links in food webs using stacked models and species traits. Preprint at bioRxiv https://doi.org/10.1101/2024.11.22.624890 (2024).
Gray, C. et al. Joining the dots: an automated method for constructing food webs from compendia of published interactions. Food Webs 5, 11–20 (2015).
Van De Walle, R. et al. Arthropod food webs predicted from body size ratios are improved by incorporating prey defensive properties. J. Anim. Ecol. 92, 913–924 (2023).
Maiorano, L. et al. TETRA‐EU 1.0: a species‐level trophic metaweb of European tetrapods. Glob. Ecol. Biogeog. 29, 1452–1457 (2020).
Atakan, E. & Pehlívan, S. Attractiveness of various colored sticky traps to some pollinating insects in apple. Turk. J. Zool. 39, 474–481 (2015).
Simard, S. W., Beiler, K. J., Bingham, M. A., Deslippe, J. R., Philip, L. J., & Teste, F. P. Mycorrhizal networks: mechanisms, ecology and modelling. Fungal Biol. Rev. 26, 39–60 (2012).
Reid, R. E. B., Crowley, B. E. & Haupt, R. J. The prospects of poop: a review of past achievements and future possibilities in faecal isotope analysis. Biol. Rev. 98, 2091–2113 (2023).
Layman, C. A. et al. Applying stable isotopes to examine food-web structure: an overview of analytical tools. Biol. Rev. 87, 545–562 (2012).
O’Gorman, E. J. et al. Unexpected changes in community size structure in a natural warming experiment. Nat. Clim. Change 7, 659–663 (2017).
Novotny, A. et al. DNA metabarcoding highlights cyanobacteria as the main source of primary production in a pelagic food web model. Sci. Adv. 9, eadg1096 (2023).
Acknowledgements
The authors are supported by the German Centre for Integrative Biodiversity Research (iDiv) and its synthesis centre (sDiv) Halle-Jena-Leipzig, funded by the German Research Foundation grant FZT 118.
Author information
Authors and Affiliations
Contributions
All authors participated in the conceptualization of this study. B.G. wrote the first draft of the manuscript (with substantial contributions from J.H.). L.T. drew the figure. All authors contributed to the review and editing process.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Biodiversity thanks Fredric Windsor and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gauzens, B., Thouvenot, L., Srivastava, D.S. et al. Tailoring interaction network types to answer different ecological questions. Nat. Rev. Biodivers. (2025). https://doi.org/10.1038/s44358-025-00056-7
Accepted:
Published:
DOI: https://doi.org/10.1038/s44358-025-00056-7