Abstract
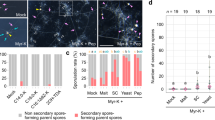
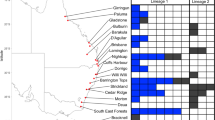
Symbioses are increasingly seen as dynamic ecosystems with multiple associates and varying fidelity. Symbiont specificity remains elusive in one of the most ecologically successful and economically damaging eukaryotic symbioses: the ambrosia symbiosis of wood-boring beetles and fungi. We used multiplexed pyrosequencing of amplified internal transcribed spacer II (ITS2) ribosomal DNA (rDNA) libraries to document the communities of fungal associates and symbionts inside the mycangia (fungus transfer organ) of three ambrosia beetle species, Xyleborus affinis, Xyleborus ferrugineus and Xylosandrus crassiusculus. We processed 93 beetle samples from 5 locations across Florida, including reference communities. Fungal communities within mycangia included 14–20 fungus species, many more than reported by culture-based studies. We recovered previously known nutritional symbionts as members of the core community. We also detected several other fungal taxa that are equally frequent but whose function is unknown and many other transient species. The composition of fungal assemblages was significantly correlated with beetle species but not with locality. The type of mycangium appears to determine specificity: two Xyleborus with mandibular mycangia had multiple dominant associates with even abundances; Xylosandrus crassiusculus (mesonotal mycangium) communities were dominated by a single symbiont, Ambrosiella sp. Beetle mycangia also carried many fungi from the environment, including plant pathogens and endophytes. The ITS2 marker proved useful for ecological analyses, but the taxonomic resolution was limited to fungal genus or family, particularly in Ophiostomatales, which are under-represented in our amplicons as well as in public databases. This initial analysis of three beetle species suggests that each clade of ambrosia beetles and each mycangium type may support a functionally and taxonomically distinct symbiosis.
Similar content being viewed by others
Login or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Accession codes
References
Abarenkov K, Henrik Nilsson R, Larsson KH, Alexander IJ, Eberhardt U, Erland S et al. (2010). The UNITE database for molecular identification of fungi—recent updates and future perspectives. New Phytol 186: 281–285.
Amend AS, Seifert KA, Bruns TD . (2010). Quantifying microbial communities with 454 pyrosequencing: does read abundance count? Mol Ecol 19: 5555–5565.
Anderson D . (1974). First record of Xyleborus semiopacus in the continental United States (Coleoptera, Scolytidae). Cooperative Economic Insect Report 24: 863–864.
Anderson MJ . (2001). A new method for non-parametric multivariate analysis of variance. Austral Ecol 26: 32–46.
Arfi Y, Buee M, Marchand C, Levasseur A, Record E . (2012). Multiple markers pyrosequencing reveals highly diverse and host-specific fungal communities on the mangrove trees Avicennia marina and Rhizophora stylosa. FEMS Microbiol Ecol 79: 433–444.
Baker JM, Norris DM . (1968). A complex of fungi mutualistically involved in the nutrition of the ambrosia beetle Xyleborus ferrugineus. J Invertebr Pathol 11: 246–250.
Balajee S, Borman A, Brandt M, Cano J, Cuenca-Estrella M, Dannaoui E et al. (2009). Sequence-based identification of Aspergillus, Fusarium, and Mucorales species in the clinical mycology laboratory: where are we and where should we go from here? J Clin Microbiol 47: 877–884.
Batra LR . (1963). Ecology of ambrosia fungi and their dissemination by beetles. Trans Kansas Acad Sci (1903-) 66: 213–236.
Batra LR . (1966). Ambrosia fungi: extent of specificity to ambrosia beetles. Science 153: 193–195.
Batra LR . (1967). Ambrosia fungi - a taxonomic revision and nutritional studies of some species. Mycologia 59: 976–1017.
Beaver RA . (1989). Insect–fungus relationships in the bark and ambrosia beetles. In: Wilding N, Collins NM, Hammond PM, Webber JF (eds). Insect–fungus Interactions. Academic Press: London, UK, pp 121–143.
Bellemain E, Carlsen T, Brochmann C, Coissac E, Taberlet P, Kauserud H . (2010). ITS as an environmental DNA barcode for fungi: an in silico approach reveals potential PCR biases. BMC Microbiol 10: 189.
Benjamini Y, Hochberg Y . (1995). Controlling the false discovery rate - a practical and powerful approach to multiple testing. J Roy Stat Soc B Met 57: 289–300.
Berry D, Ben Mahfoudh K, Wagner M, Loy A . (2011). Barcoded primers used in multiplex amplicon pyrosequencing bias amplification. Appl Environ Microbiol 77: 7846–7849.
Biedermann PH, Klepzig KD, Taborsky M . (2009). Fungus cultivation by ambrosia beetles: behavior and laboratory breeding success in three xyleborine species. Environ Entomol 38: 1096–1105.
Bruns TD, Shefferson RP . (2004). Evolutionary studies of ectomycorrhizal fungi: recent advances and future directions. Can J Bot 82: 1122–1132.
Caldera EJ, Poulsen M, Suen G, Currie CR . (2009). Insect symbioses: a case study of past, present, and future fungus-growing ant research. Environ Entomol 38: 78–92.
Calderon O, Berkov A . (2012). Midgut and fat body bacteriocytes in neotropical cerambycid beetles (Coleoptera: Cerambycidae). Environ Entomol 41: 108–117.
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK et al. (2010). QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7: 335–336.
Carrillo D, Duncan RE, Ploetz JN, Campbell AF, Ploetz RC, Peña JE . (2014). Lateral transfer of a phytopathogenic symbiont among native and exotic ambrosia beetles. Plant Pathol 63: 54–62.
Clarke KR . (1993). Non-parametric multivariate analyses of changes in community structure. Austral Ecology 18: 117–143.
Cognato AI, Hulcr J, Dole SA, Jordal BH . (2011). Phylogeny of haplo–diploid, fungus-growing ambrosia beetles (Curculionidae: Scolytinae: Xyleborini) inferred from molecular and morphological data. Zool Scripta 40: 174–186.
Doane RW, Gilliland OJ . (1929). Three California ambrosia-beetles. J Econ Entomol 22: 915–921.
Douglas AE . (2010) The Symbiotic Habit. Princeton University Press: Princeton, NJ, USA.
Dreaden TJ, Shin K, Smith JA . (2011). First report of Diplodia corticola causing branch cankers on live oak (Quercus virginiana) in Florida. Plant Dis 95: 1027–1027.
Edgar RC . (2010). Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26: 2460–2461.
Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R . (2011). UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27: 2194–2200.
Faircloth BC, Glenn TC . (2012). Not all sequence tags are created equal: designing and validating sequence identification tags robust to indels. PLoS One 7: e42543.
Farrell BD, Sequeira AS, O'Meara BC, Normark BB, Chung JH, Jordal BH . (2001). The evolution of agriculture in beetles (Curculionidae: Scolytinae and Platypodinae). Evolution 55: 2011–2027.
Fraedrich SW, Harrington TC, Rabaglia RJ, Ulyshen MD, Mayfield AE, Hanula JL et al. (2008). A fungal symbiont of the redbay ambrosia beetle causes a lethal wilt in redbay and other Lauraceae in the southeastern United States. Plant Dis 92: 215–224.
Francke-Grosmann H . (1956). Hautdrüsen als träger der pilzsymbiose bei ambrosiakäfern. Z Morph Oekol Tiere 45: 275–308.
Ganter PF . (2006). Yeast and invertebrate associations. In: Péter G, Rosa C (eds). Biodiversity and Ecophysiology of Yeasts. Springer: Berlin, Heidelberg, Germany, pp 303–370.
Gardes M, Bruns TD . (1993). ITS primers with enhanced specificity for basidiomycetes—application to the identification of mycorrhizae and rusts. Mol Ecol 2: 113–118.
Gebhardt H, Begerow D, Oberwinkler F . (2004). Identification of the ambrosia fungus of Xyleborus monographus and X. dryographus (Coleoptera: Curculionidae, Scolytinae). Mycol Progr 3: 95–102.
Gilbert SF, Sapp J, Tauber AI . (2012). A symbiotic view of life: we have never been individuals. Q Rev Biol 87: 325–341.
Harrington TC, Aghayeva DN, Fraedrich SW . (2010). New combinations in Raffaelea, Ambrosiella, and Hyalorhinocladiella, and four new species from the redbay ambrosia beetle, Xyleborus glabratus. Mycotaxon 111: 337–361.
Harrington TC, Fraedrich SW . (2010). Quantification of propagules of the laurel wilt fungus and other mycangial fungi from the redbay ambrosia beetle, Xyleborus glabratus. Phytopathology 100: 1118–1123.
Herrera Isla L, Grillo Ravelo H . (1989). Spathodea campanulata Beauv., new host plant of Ceratocystis fimbriata Hell & Halst and Xyleborus spp. Centro Agrícola 16: 91–93.
Hubbard HG . (1896). Ambrosia beetles. US Dep Agric Yearbook 1896: 421–430.
Hubbard HG . (1897). The ambrosia beetles of the United States. US Dep Agric Entomol Bull 7: 9–30.
Hulcr J, Kolarik M, Kirkendall LR . (2007). A new record of fungus-beetle symbiosis in Scolytodes bark beetles (Scolytinae, Curculionidae, Coleoptera). Symbiosis 43: 151–159.
Hulcr J, Cognato AI . (2010). Repeated evolution of crop theft in fungus-farming ambrosia beetles. Evolution 64: 3205–3212.
Hulcr J, Dunn RR . (2011). The sudden emergence of pathogenicity in insect-fungus symbioses threatens naive forest ecosystems. Proc Biol Sci 278: 2866–2873.
Ihrmark K, Bodeker IT, Cruz-Martinez K, Friberg H, Kubartova A, Schenck J et al. (2012). New primers to amplify the fungal ITS2 region—evaluation by 454-sequencing of artificial and natural communities. FEMS Microbiol Ecol 82: 666–677.
Jumpponen A, Jones KL . (2009). Massively parallel 454 sequencing indicates hyperdiverse fungal communities in temperate Quercus macrocarpa phyllosphere. New Phytol 184: 438–448.
Kajimura H, Hijii N . (1992). Dynamics of the fungal symbionts in the gallery system and the mycangia of the ambrosia beetle, Xylosandrus-Mutilatus (Blandford) (Coleoptera, Scolytidae) in relation to its life-history. Ecol Res 7: 107–117.
Kasson MT, O'Donnell K, Rooney AP, Sink S, Ploetz RC, Ploetz JN et al. (2013). An inordinate fondness for Fusarium: phylogenetic diversity of fusaria cultivated by ambrosia beetles in the genus Euwallacea on avocado and other plant hosts. Fungal Genet Biol 56: 147–157.
Kinuura H . (1995). Symbiotic fungi associated with ambrosia beetles. Jpn Agr Res Q 29: 57–63.
Koljalg U, Nilsson RH, Abarenkov K, Tedersoo L, Taylor AF, Bahram M et al. (2013). Towards a unified paradigm for sequence-based identification of fungi. Mol Ecol 22: 5271–5277.
Kunin V, Engelbrektson A, Ochman H, Hugenholtz P . (2010). Wrinkles in the rare biosphere: pyrosequencing errors can lead to artificial inflation of diversity estimates. Environ Microbiol 12: 118–123.
Kühnholz S . (2004) Chemical ecology and mechanisms of reproductive isolation in ambrosia beetles PhD thesis Simon Fraser University: Burnaby, BC, Canada.
Leach JG, Hodson AC, Chilton SJP, Christensen CM . (1940). Observations on two ambrosia beetles and their associated fungi. Phytopathology 30: 227–236.
Lynch S, Eskalen A, Zambino P, Scott T . (2013). First report of bot canker caused by Diplodia corticola on coast live oak (Quercus agrifolia) in California. Forest Ecol Manage 291: 30–42.
Magurran AE, Henderson PA . (2003). Explaining the excess of rare species in natural species abundance distributions. Nature 422: 714–716.
Massoumi Alamouti S, Tsui CK, Breuil C . (2009). Multigene phylogeny of filamentous ambrosia fungi associated with ambrosia and bark beetles. Mycol Res 113: 822–835.
Nilsson RH, Ryberg M, Abarenkov K, Sjokvist E, Kristiansson E . (2009). The ITS region as a target for characterization of fungal communities using emerging sequencing technologies. FEMS Microbiol Lett 296: 97–101.
Nilsson RH, Veldre V, Hartmann M, Unterseher M, Amend A, Bergsten J et al. (2010). An open source software package for automated extraction of ITS1 and ITS2 from fungal ITS sequences for use in high-throughput community assays and molecular ecology. Fungal Ecol 3: 284–287.
Norris DM . (1979). The mutualistic fungi of Xyleborini beetles. In: Batra LR (ed). Insect–Fungus Symbiosis: Nutrition, Mutualism, and Commensalism. Wiley: New York, NY, USA, pp 53–63.
O'Donnell K . (2000). Molecular phylogeny of the Nectria haematococca-Fusarium solani species complex. Mycologia 92: 919–938.
Ploetz R, Harrington T, Hulcr J, Fraedrich S, Smith J, Inch S et al. (2011). Recovery Plan for Laurel Wilt of Avocado (caused by Raffaelea lauricola). National Plant Disease Recovery System. Homeland Security Presidential Directive Number 9 (HSPD-9).
Quince C, Lanzen A, Curtis TP, Davenport RJ, Hall N, Head IM et al. (2009). Accurate determination of microbial diversity from 454 pyrosequencing data. Nat Methods 6: 639–641.
R Core Team. (2013) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria, URL http://www.R-projectorg.
Reeder J, Knight R . (2010). Rapidly denoising pyrosequencing amplicon reads by exploiting rank-abundance distributions. Nat Methods 7: 668–669.
Rey O, Estoup A, Facon B, Loiseau A, Aebi A, Duron O et al. (2013). Distribution of endosymbiotic reproductive manipulators reflects invasion process and not reproductive system polymorphism in the little fire ant Wasmannia auropunctata. PLoS One 8: e58467.
Rivera FN, Gonzalez E, Gomez Z, Lopez N, Hernández-Rodríguez C, Berkov A et al. (2009). Gut-associated yeast in bark beetles of the genus Dendroctonus Erichson (Coleoptera: Curculionidae: Scolytinae). Biol J Linnean Soc 98: 325–342.
Roeper R . (1996). Ambrosia beetles of the continental United States and Canada and status of knowledge of their associated primary symbiotic fungi. Mich Entomol Soc Newsl 41: 12–14.
Saunders JL, Knoke JK . (1967a). Diurnal emergence of Xyleborus ferrugineus (Coleoptera - Scolytidae) from cacao trunks in Ecuador and Costa Rica. Ann Entomol Soc Am 60: 1094–1096.
Saunders JL, Knoke JK . (1967b). Diets for rearing the ambrosia beetle Xyleborus ferrugineus (Fabricius) in vitro. Science 157: 460–463.
Schmidt PA, Balint M, Greshake B, Bandow C, Rombke J, Schmitt I . (2013). Illumina metabarcoding of a soil fungal community. Soil Biol Biochem 65: 128–132.
Schoch CL, Seifert KA, Huhndorf S, Robert V, Spouge JL, Levesque CA et al. (2012). Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc Natl Acad Sci USA 109: 6241–6246.
Six DL, Stone WD, de Beer ZW, Woolfolk SW . (2009). Ambrosiella beaveri, sp. nov., associated with an exotic ambrosia beetle, Xylosandrus mutilatus (Coleoptera: Curculionidae, Scolytinae), in Mississippi, USA. Antonie Van Leeuwenhoek 96: 17–29.
Unterseher M, Jumpponen A, Opik M, Tedersoo L, Moora M, Dormann CF et al. (2011). Species abundance distributions and richness estimations in fungal metagenomics—lessons learned from community ecology. Mol Ecol 20: 275–285.
Úrbez-Torres J, Peduto F, Rooney-Latham S, Gubler W . (2010). First report of Diplodia corticola causing grapevine (Vitis vinifera) cankers and trunk cankers and dieback of canyon live oak (Quercus chrysolepis) in California. Plant Dis 94: 785–785.
Valente P, Boekhout T, Landell MF, Crestani J, Pagnocca FC, Sette LD et al. (2012). Bandoniozyma gen. nov., a genus of fermentative and non-fermentative tremellaceous yeast species. PLoS One 7: e46060.
Van der Walt JP . (1972). The yeast genus Ambrosiozyma gen. nov.(Ascomycetes). Mycopathol Mycol Appl 46: 305–315.
Verrall AF . (1943). Fungi associated with certain ambrosia beetles. J Agric Res 66: 0135–0144.
Wang Q, Garrity GM, Tiedje JM, Cole JR . (2007). Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73: 5261–5267.
Weber BC, McPherson JE . (1984). The ambrosia fungus of Xylosandrus-Germanus (Coleoptera, Scolytidae). Can Entomol 116: 281–283.
White TJ, Bruns T, Lee S, Taylor J . (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protocols: A Guide to Methods and Applications 18: 315–322.
Wood SL, Bright DE . (1992). A Catalog of Scolytidae and Platypodidae (Coleoptera). Part 2: Taxonomic Index, Volumes A and B. Great Basin Naturalist Memoirs 13.
Zimmermann G, Butin H . (1973). Untersuchungen über die Hitze-und Trockenresistenz holzbewohneneder Pilze. Flora 162: 393–419.
Acknowledgements
The project was funded by the USDA Forest Service cooperative agreements 12-CA-11420004-042 and 11-CA-11330129-092, the National Science Foundation grant DEB 1256968, the NIFA grant 2009-51181-05915, grant 214232/F20 from the Norwegian Research Council and in-kind contributions. We thank Rob Dunn for support in the methods-development phase and Paul Kendra and Chris Gibbard for the supply of specimens.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on The ISME Journal website
Supplementary information
Rights and permissions
About this article
Cite this article
Kostovcik, M., Bateman, C., Kolarik, M. et al. The ambrosia symbiosis is specific in some species and promiscuous in others: evidence from community pyrosequencing. ISME J 9, 126–138 (2015). https://doi.org/10.1038/ismej.2014.115
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ismej.2014.115
This article is cited by
-
Fungi associated with the ambrosia beetle Xyleborus perforans (Coleoptera: Curculionidae: Scolytinae) on drought-stressed Pinus in New South Wales, Australia
Australasian Plant Pathology (2024)
-
Microorganisms Associated with the Ambrosial Beetle Xyleborus affinis with Plant Growth-Promotion Activity in Arabidopsis Seedlings and Antifungal Activity Against Phytopathogenic Fungus Fusarium sp. INECOL_BM-06
Microbial Ecology (2023)
-
Fungal symbiont community and absence of detectable mycangia in invasive Euplatypus ambrosia beetles
Symbiosis (2023)
-
Two Apriona Species Sharing a Host Niche Have Different Gut Microbiome Diversity
Microbial Ecology (2022)
-
The gut microbiome analysis of Anastrepha obliqua reveals inter-kingdom diversity: bacteria, fungi, and archaea
Archives of Microbiology (2022)