Abstract
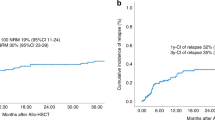
There is no consensus on second allogeneic stem cell transplantation (alloSCT) indications in patients with hematologic malignancies relapsing after a first alloSCT. In historic publications, a very high non-relapse mortality (NRM) has been described, arguing against performing a second alloSCT. We analysed the outcome of 3356 second alloSCTs performed 2011–21 following a hematologic malignancy relapse. Outcomes at two years after second alloSCT were: NRM 22%, relapse incidence 50%, overall survival 38%, and progression-free survival 28%. Key risk factors for increased NRM were: older age, low performance score, high disease-risk-index, early relapse after the first alloSCT, unrelated/haploidentical donor, and GVHD before second alloSCT. Any type of GVHD after first alloSCT was also important risk factor for acute GVHD and chronic GVHD after second alloSCT. There was a preferential use of a different donor (80%) at second alloSCT from first alloSCT. However, in multivariate analysis, the use of the same alloSCT donor for second alloSCT vs. a different donor was not associated with any of the survival or GVHD endpoints. We show considerably improved outcome as compared to historic reports. These current data support a wider use of second alloSCT and provide risk factors for NRM that need to be considered.
Similar content being viewed by others
Introduction
Relapse of the underlying malignancy after the first allogeneic stem cell transplantation (alloSCT) is a frequent problem, usually leading to consideration of a subsequent alloSCT, especially in younger and healthier patients. A previous European Society for Blood and Marrow transplantation (EBMT) analysis including patients with second transplants before 2009 found a high treatment-related mortality incidence of ~40% after second alloSCT [1]. Subsequently, similar outcome data was published in disease-specific subgroups [2,3,4,5]. These historic data showing high treatment-related mortality is the current basis for decision-making and argue against a second alloSCT in many patients.
We asked the question if this published data is still valid and up to date enough to be the foundation for clinical decision-making. Based on recent advances in the field, our assumption was that non-relapse mortality (NRM) after second alloSCT is considerably lower nowadays. Since there are no randomized studies or comprehensive recent large data file analyses available, we performed a retrospective EBMT data set analysis. We collected relevant data on second alloSCT-related mortality and other major outcome parameters, such as incidence of graft-versus-host disease (GVHD), relapse rates, and causes of deaths. Furthermore, we describe procedures of conditioning, as well as GVHD prophylaxis, which often differ between the first and second alloSCT. Our current analysis is intended to provide the data, which is required for evidence-based decision-making of a second alloSCT in patients with hematologic malignancies.
Patients and methods
Study design and data collection
This is a retrospective multicenter analysis using the data set of the EBMT registry. The EBMT is a voluntary working group of more than 600 transplant centers which are required to report regular follow-up on all consecutive stem cell transplantations. Audits are routinely performed to determine the accuracy of the data. The study was planned and approved by the Transplant Complications Working Party of the EBMT. All patients gave their written informed consent to use their personal information for research purposes. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines.
Eligibility criteria
Inclusion criteria:
-
Patients who underwent a second alloSCT between 2011 and 2021.
-
Types of donors included: identical sibling, matched unrelated donor, mismatched unrelated donor, and haploidentical donor.
-
Stem cell sources: bone marrow and peripheral blood.
-
Diagnoses: patients with hematologic malignancies including acute leukemia, chronic leukemia, myelodysplastic/myeloproliferative disorders, lymphoma, and plasma cell disorders.
Exclusion criteria:
-
Patients with a previous autologous SCT (autoSCT).
-
Non-calculable Disease Risk Index (DRI).
-
Incoherent disease status between the first and second alloSCT.
-
Evidence of non-engraftment of the first alloSCT.
-
Patients without a relapse between the first and second alloSCT.
-
Umbilical cord blood as donor source (due to low numbers).
Data collection
Data collected included recipient and donor characteristics (age, sex, cytomegalovirus serostatus, and Karnofsky Performance Status (KPS) score), diagnosis, status at transplant, and transplant-related factors, including conditioning regimen, stem cell source, and GVHD prophylaxis. GVHD grading was performed according to published criteria for acute GVHD [6] and chronic GVHD [7]. For the purpose of this study, all necessary data were collected according to the EBMT guidelines, using the EBMT Minimum Essential Data forms.
Statistical analysis
Median values and interquartile ranges, and minimum and maximum values were used to describe quantitative variables; frequency and percentage were used for categorical variables.
Study endpoints were NRM, overall survival (OS), progression-free survival (PFS), relapse incidence (RI), and incidence and severity of acute and chronic GVHD. The starting point of analyses was the date of second transplant for all endpoints. NRM was defined as death without relapse/progression, PFS was defined as survival without relapse or progression, RI was defined as disease recurrence. Probabilities of OS and PFS were calculated using the Kaplan–Meier method. Cumulative incidence was used to estimate NRM, RI, as well as acute and chronic GVHD in a competing risk setting, where death and relapse were considered as competing risks as appropriate [8]. Multivariate analyses were performed using the Cox cause-specific proportional-hazards model for all endpoints. All clinically known and potentially relevant risk factors were included in the multivariate models: patient age, year of transplant, patient and donor gender, donor to patient cytomegaly virus (CMV) combination, DRI, KPS, Donor Type, Same Donor in 1st alloSCT, stem cell source, GVHD between 1st and 2nd alloSCT, Delay between 1st alloSCT and 1st Relapse, Delay 1st Relapse to 2nd alloSCT, any level of total body irradiation (TBI) and conditioning intensity (reduced intensity conditioning (RIC) vs. myeloablative conditioning (MAC)). All factors were measured at the 2nd alloSCT. Center effect was taken into account by introducing a random effect or ‘frailty’ into all models. Results were expressed as the hazard ratio (HR) with the 95% confidence interval (95% CI). Statistical analyses were performed with R 4.3.0 software (R Development Core Team, Vienna, Austria) packages.
Data sharing statement
Individual participant data will not be shared because patients agreed to data sharing with EBMT as well as with publication of results, but not to share data with third parties.
Results
Patient- and treatment characteristics
We identified 3356 second alloSCTs that met the inclusion criteria. Patient- and treatment characteristics of first- and second alloSCTs are shown in Table 1. The main underlying malignancies were AML 60%, ALL 15%, and MDS 8%.
For first alloSCT, myeloablative conditioning was used in 64%. Most frequent donor types were identical siblings (39%), matched unrelated 10/10 (31%), and mismatched unrelated 9/10 (9%). A description of events and timelines between first- and second alloSCT is given in Table 2. Acute GVHD grades II-IV occurred in 16% and chronic GVHD occurred in 29% after first alloSCT. The median time from first alloSCT to second alloSCT was 1.73 years (Q1 0.93, Q3 3.41). The median time from relapse after first alloSCT to second alloSCT was 0.4 years (Q1 0.23, Q3 0.8).
The median age at second alloSCT was 48 years. For second alloSCT, myeloablative conditioning was used in 44%. About 49% of patients who received myeloablative conditioning during their first alloSCT did not receive it at their second alloSCT. About 32% of those who did not receive myeloablative conditioning during their first alloSCT had received it during their second alloSCT. Most frequent donor types were matched unrelated 10/10 (35%), haploidentical (22%), and identical siblings (18%). Median follow-up was 3.7 [CI 95%: (3.4–4)] years after second alloSCT. GVHD prophylaxis regimens are shown in Table 1. 79.1% of patients receiving second alloSCT from haploidentical donors received post-transplantation Cyclophosphamide as GVHD prophylaxis.
NRM, survival and relapse incidence after second alloSCT
Univariate outcomes are shown in Fig. 1 and Table 3. The results of the multivariate analyses are summarized in Table 4.
In multivariate analysis, several risk factors for NRM (2 years incidence: 22% [95% CI 20.6–23.6]) were identified: (a) the time of the second HSCT, with a HR of 1.07 [CI 95%: (1.04–1.11); p < 0.001] for every five-year increase; (b) low Karnofsky performance score [≥90 vs <90; HR: 0.64, CI 95%: (0.53–0.77); p < 0.001]; (c) sex—with females exhibiting a lower risk than males [HR: 0.83, CI 95%: (0.69–1); p = 0.048]; (d) donor type—with haploidentical donors [HR: 1.50, CI 95%: (1.02–2.21); p = 0.039] and unrelated donors (UD) [HR: 1.43, CI 95%: (1.01–2.02); p = 0.043] showing higher risks compared to matched sibling donors; (e) previous GVHD before the second alloSCT increased NRM risk [HR: 1.28, CI 95%: (1.07 to 1.54); p = 0.009]; (f) a very high DRI significantly elevated the NRM risk [HR: 1.97, CI 95%: (1.20–3.23); p = 0.007] relatively to Low DRI.; (g) longer delays from first alloSCT to relapse were associated with a decreased NRM risk [HR:0.94, CI 95%: (0.90 to 0.98); p = 0.002] for every year increase, as was recent years of transplant [HR {5 years increment}: 0.82, CI 95%: (0.70–0.96); p = 0.013]; and (h) TBI was also associated with a decreased risk of NRM [HR: HR:0.67, CI 95%: (0.53 to 0.84); p < 0.001].
RI was 50% [95% CI 48.1–51.8] at 2 years after second alloSCT and a high DRI relative to low was an important risk factor for relapse [HR {very high vs. low}: 3.33, CI 95%: (2.29–4.84); p-value < 0.001] and [HR {high vs. low}: 2.31, CI 95%: (1.64–3.26); p < 0.001]. OS was 38% [95% CI 36.4–39.9] and PFS 28% [95% CI 26.3–29.7] at 2 years after second alloSCT (Fig. 1, Table 3). Risk factors for reduced OS and PFS after second alloSCT were roughly similar to those described for NRM (Table 4).
Relapse of the underlying malignancy was the most frequent cause of death after second alloSCT, accounting for 63% (n = 1347) of total deaths. The median time to relapse after second alloSCT was 145 days (Q1, Q3 80, 325) [Min, Max 2–2408]. NRM causes of death were: infections 16% (n = 334), GVHD 11% (n = 232), and other alloSCT-related causes 7% (n = 144) of total deaths. Secondary malignancies contributed to ~0.5% of total deaths.
GVHD after second alloSCT
Univariate outcomes are shown in Fig. 2 and Table 3. The results of the multivariate analyses are summarized in Table 5.
The cumulative incidences of acute GVHD grades II–IV and III–IV at 100 days post second alloSCT were 24% [95% CI 22.2–25.4] and 10% [95% CI 9.3–11.5], respectively (Fig. 2, Table 3). In multivariate analysis, we identified several risk factors for acute GVHD: (a) the occurrence of any type of GVHD after first alloSCT, for grades II/IV [HR: 1.51, CI 95%: (1.26–1.81); p < 0.001], for grades III/IV [HR: 1.36, CI 95%: (1.03–1.79); p = 0.029]; (b) shorter delays from first alloSCT to first relapse, for grades II/IV [[HR: 0.94, CI 95%: (0.90–0.98); p = 0.003]] for every year of increase, for grades III/IV [HR: 0.89, CI 95%: (0.82–0.96); p = 0.002] for every year of increase; (c) low Karnofsky performance score, for grades II/IV [HR {≥90 vs. <90}: 0.73, CI 95%: (0.61–0.88); p = 0.001], for grades III/IV [HR {≥90 vs. <90}: 0.70, CI 95%: (0.53–0.93); p = 0.014] (Table 5).
The cumulative incidences of overall chronic GVHD and of extensive chronic GVHD at 2 years post alloSCT were 30% [95% CI 28.2–31.6] and 14% [95% CI 12.4–15], respectively (Fig. 2, Table 3). In multivariate analysis, we identified some risk factors for the development of chronic GVHD: The occurrence of any type of GVHD before second alloSCT and the type of donor used. Specifically, experiencing GVHD before the second alloSCT significantly increases the risk for chronic GVHD [HR: 1.60, CI 95%: (1.35–1.89); p < 0.001] and for extensive chronic GVHD [HR: 1.83, CI 95%: (1.45–2.31); p < 0.001].
Donor choice for second alloSCT
Regarding the donor choice for second alloSCT, both haploidentical and UD in second alloSCT were beneficial as compared to identical sibling donors. However, NRM was lower in recipients of unrelated donor grafts that were human leukocyte antigen (HLA)-mismatched vs. HLA-matched (Supplementary Tables 1 and 2). The use of haploidentical donors was associated to a lower risk of all grades chronic GVHD [HR: 0.60, CI 95%: (0.44–0.84); p = 0.003] compared to the use of identical-sibling donors. This effect was consistent for extensive chronic GVHD [0.61, CI 95%: (0.38–0.97); p = 0.036]. Furthermore, use of UD was associated with a reduced risk of all grades chronic GVHD [HR: 0.67, CI 95%: (0.51–0.88); p = 0.003] compared to the use of matched-sibling donors (Table 5). The lower GVHD rates in unrelated and haploidentical donors are possibly explained by different GVHD prophylaxis regimens: in our study 66% of patients receiving unrelated second alloSCT vs. 21% of patients receiving identical sibling second alloSCT received in vivo T cell depletion with ATG (Anti-T cell/anti-thymocyte globulin). In addition, most haploidentical alloSCTs were performed with post-transplantation Cyclophosphamide. Of note, the donor age was closely related to the donor type: in identical sibling donors the median age was 47.3 years (Q1, Q3 34.6, 56) vs. 38 years (Q1, Q3 27.7, 51.1) in haploidentical donors vs, 30.2 years (Q1, Q3 24.9, 37.6) in UD.
In 20% of cases the same stem cell donor was used in first- and second alloSCT and in 80% of cases a different donor was chosen. In multivariate analysis, the use of the same alloSCT donor for second alloSCT vs. a different donor was not associated with any of the survival endpoints or relapse (Table 4). Furthermore, use of the same vs. a different second alloSCT donor was not associated with the incidence or severity of GVHD (Table 5).
Discussion
This recent EBMT analysis of second alloSCT reports more patients as previous publications on the same topic [2,3,4, 9,10,11,12]. We found that NRM after second alloSCT was considerably lower as compared to previous cohorts [1,2,3,4, 9,10,11,12] but higher than NRM after first alloSCT [13,14,15,16,17].
The most important limitation of our registry data is the lack of information about how centers selected patients for second alloSCT. The current standard of care is to base second alloSCT decisions on the most important factors, including: (a) extent and aggressiveness of relapsed disease, including the presence of extramedullary involvement and cytogenetic/molecular risk factors; (b) patient’s overall health, organ function, and ability to tolerate conditioning regimens; (c) the duration between the first transplant and relapse; (d) availability of suitable donors; and (e) pre-existing comorbidities as well as prior transplant-related toxicities. The current dataset on second alloSCTs is probably a positive selection based on the above factors.
The 2 years PFS of 28% that we have observed in our study can be interpreted from different viewpoints. The positive view is that this is a good outcome for an extremely high-risk population for mortality and for relapse. The more negative view is the relatively low survival in this selected population of patients who received a second alloSCT. To better answer the question if a second alloSCT is preferable to an alternative therapy in a given situation, results from randomized prospective clinical studies are needed. These studies should include more extensive data on morbidity as well as patient-reported outcomes. OS, while a clear endpoint may underrepresent the morbidity associated with second alloSCT. In addition, we found that survival continues to fall after 2 years and thus longer-term follow-up may be needed to ultimately determine the successes of second alloSCTs. However, currently no such studies are underway and clinical decision-making has to be based on the available evidence.
Comparison of NRM to previous reports
In a previous report on second alloSCTs during the period of 1994–2009 the cumulative incidence of NRM was 33% at 1 year and 40% at 5 years. The lower NRM we found in our recent cohort is in line with a previous report of first alloSCT also finding a considerable reduction of NRM over time [16]. Our data needs to be put in perspective with reports on second alloSCTs in previous disease-specific analyses [2,3,4, 9,10,11,12, 18]. One example is a report on adult patients with acute leukemia who received a second reduced-intensity alloSCT between 2000 and 2012 [4]. At 2 years, the cumulative incidence of NRM was with 22% similar to our current data. However, OS at 2 years was low with 22%, with relapse being the main factor. This is in line with our study showing 50% relapse at 2 years after 2nd alloSCT. A recent report included acute leukemia patients receiving a second haploidentical alloSCT and found a 2 years NRM of 18% and OS of 34% [9]. This is in line with another recent publication showing 19% NRM at two years in n = 80 leukemia patients after second alloSCT [19].
NRM after second alloSCT compares unfavorably to NRM rates after first alloSCT as data from prospective studies [13,14,15] and registry analyses demonstrates [16]. A recent prospective study found 1 year NRM of 11% after first alloSCT in acute leukemia patients [17]. A registry analysis found 12% NRM in patients with hematologic malignancies undergoing the first alloSCT from a matched-related donor [16]. When looking at the reasons of death for NRM in the published studies of first alloSCT and in our current study on second alloSCT it becomes evident that there are no major differences with infections, GVHD, and other alloSCT-related causes being the main factors.
GVHD outcomes comparison and risk factor
A recent study in patients with hematologic malignancies undergoing a first alloSCT between 2011 and 2015 found a cumulative incidence of acute GVHD grades II–IV and III–IV of 28% and 11%, respectively [20]. These data is in a similar range of what we found after second alloSCT and suggest that the acute GVHD risk is not increased after second alloSCT. However, we found that the acute GVHD risk after second alloSCT mainly depends on the fact if GVHD (acute or chronic) occurred after first alloSCT. The presence of GVHD after first alloSCT as main risk factor for acute GVHD after second alloSCT has been described previously [1].
Influence of the same donor vs. different donor in second alloSCT
When the decision is made to perform a second alloSCT in patients with relapse of the hematologic malignancy after first alloSCT there is one difficult other decision to make: which donor to choose? Most physicians tend to choose a different donor from the one used in first alloSCT. Our current results confirm this clinical preference. One major reason why a different donor is used as opposed to the same donor that has already been used in first alloSCT is probably common sense, with the expectation that it may be better to change the donor when the use of the original donor has not resulted in cure of the underlying disease. However, we did not find any significant impact of second alloSCT donor selection on major outcomes. This aligns with the results of previous studies. Christopeit and co-workers found in n = 179 related or unrelated second alloSCT that selecting a new donor did not result in a relevant improvement nor deterioration of survival [21]. Ruutu et al. found that there was no difference in OS or relapse-free survival between transplantations from the same vs. another donor. The NRM was slightly lower and the relapse rate slightly higher (non-significant) when the donor was the same as at the first transplantation [1].
In conclusion, NRM and survival after second alloSCT in this recent EBMT cohort have shown considerable improvement when compared to historic reports. These data can be used as rationale for clinical decision-making on second alloSCT indications.
Data availability
Individual participant data will not be shared because patients agreed to data sharing with EBMT as well as with publication of results, but not to share data with third parties.
References
Ruutu T, de Wreede LC, van Biezen A, Brand R, Mohty M, Dreger P, et al. Second allogeneic transplantation for relapse of malignant disease: retrospective analysis of outcome and predictive factors by the EBMT. Bone Marrow Transpl. 2015;50:1542–50. https://doi.org/10.1038/bmt.2015.186.
Horstmann K, Boumendil A, Finke J, Finel H, Kanfer E, Milone G, et al. Second allo-SCT in patients with lymphoma relapse after a first allogeneic transplantation. A retrospective study of the EBMT Lymphoma Working Party. Bone Marrow Transpl. 2015;50:790–4. https://doi.org/10.1038/bmt.2015.12.
Nabergoj M, Mauff K, Robin M, Kroger N, Angelucci E, Poire X, et al. Outcomes following second allogeneic haematopoietic cell transplantation in patients with myelofibrosis: a retrospective study of the Chronic Malignancies Working Party of EBMT. Bone Marrow Transpl. 2021. https://doi.org/10.1038/s41409-021-01271-4.
Vrhovac R, Labopin M, Ciceri F, Finke J, Holler E, Tischer J, et al. Second reduced intensity conditioning allogeneic transplant as a rescue strategy for acute leukaemia patients who relapse after an initial RIC allogeneic transplantation: analysis of risk factors and treatment outcomes. Bone Marrow Transpl. 2016;51:186–93. https://doi.org/10.1038/bmt.2015.221.
Weisdorf D. The role of second transplants for leukemia. Best Pract Res Clin Haematol. 2016;29:359–64. https://doi.org/10.1016/j.beha.2016.10.011.
Harris AC, Young R, Devine S, Hogan WJ, Ayuk F, Bunworasate U, et al. International, multicenter standardization of acute graft-versus-host disease clinical data collection: a Report from the Mount Sinai Acute GVHD International Consortium. Biol Blood Marrow Transpl. 2016;22:4–10. https://doi.org/10.1016/j.bbmt.2015.09.001.
Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transpl. 2015;21:389–401.e381. https://doi.org/10.1016/j.bbmt.2014.12.001.
Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509.
Filippini Velazquez G, Labopin M, Tischer J, Raiola AM, Angelucci E, Kulagin AD, et al. Second haploidentical stem cell transplantation (HAPLO-SCT2) after relapse from a first HAPLO-SCT in acute leukaemia-a study on behalf of the Acute Leukaemia Working Party (ALWP) of the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transpl. 2023;58:907–15. https://doi.org/10.1038/s41409-023-01985-7.
Lu Y, Zhang JP, Zhao YL, Xiong M, Sun RJ, Cao XY, et al. Prognostic factors of second hematopoietic allogeneic stem cell transplantation among hematological malignancy patients relapsed after first hematopoietic stem cell transplantation: a single center study. Front Immunol. 2022;13:1066748. https://doi.org/10.3389/fimmu.2022.1066748.
Orti G, Sanz J, Bermudez A, Caballero D, Martinez C, Sierra J, et al. Outcome of second allogeneic hematopoietic cell transplantation after relapse of myeloid malignancies following allogeneic hematopoietic cell transplantation: a retrospective cohort on behalf of the Grupo Espanol de Trasplante Hematopoyetico. Biol Blood Marrow Transpl. 2016;22:584–8. https://doi.org/10.1016/j.bbmt.2015.11.012.
Poon L, Bassett R Jr, Rondon G, Hamdi A, Qazilbash M, Hosing C, et al. Outcomes of second allogeneic hematopoietic stem cell transplantation for patients with acute lymphoblastic leukemia. Bone marrow Transplant. 2013;48:666–70.
Finke J, Schmoor C, Bethge WA, Ottinger H, Stelljes M, Volin L, et al. Long-term outcomes after standard graft-versus-host disease prophylaxis with or without anti-human-T-lymphocyte immunoglobulin in haemopoietic cell transplantation from matched unrelated donors: final results of a randomised controlled trial. Lancet Haematol. 2017;4:e293–301. https://doi.org/10.1016/S2352-3026(17)30081-9.
Penack O, Luft T, Peczynski C, Benner A, Sica S, Arat M, et al. Endothelial Activation and Stress Index (EASIX) to predict mortality after allogeneic stem cell transplantation: a prospective study. J Immunother Cancer. 2024;12. https://doi.org/10.1136/jitc-2023-007635.
Penack O, Peczynski C, Mohty M, Yakoub-Agha I, de la Camara R, Glass B, et al. Association of pre-existing comorbidities with outcome of allogeneic hematopoietic cell transplantation. A retrospective analysis from the EBMT. Bone Marrow Transpl. 2022;57:183–90. https://doi.org/10.1038/s41409-021-01502-8.
Penack O, Peczynski C, Mohty M, Yakoub-Agha I, Styczynski J, Montoto S, et al. How much has allogeneic stem cell transplant-related mortality improved since the 1980s? A retrospective analysis from the EBMT. Blood Adv. 2020;4:6283–90. https://doi.org/10.1182/bloodadvances.2020003418.
Penack O, Tridello G, Salmenniemi U, Martino R, Khanna N, Perruccio K, et al. Influence of invasive aspergillosis during acute leukaemia treatment on survival after allogeneic stem cell transplantation: a prospective study of the EBMT Infectious Diseases Working Party. eClinicalMedicine. 2024;67:102393. https://doi.org/10.1016/j.eclinm.2023.102393.
Kharfan-Dabaja MA, Labopin M, Bazarbachi A, Ciceri F, Finke J, Bruno B, et al. Comparing outcomes of a second allogeneic hematopoietic cell transplant using HLA-matched unrelated versus T-cell replete haploidentical donors in relapsed acute lymphoblastic leukemia: a study of the Acute Leukemia Working Party of EBMT. Bone Marrow Transpl. 2021;56:2194–202. https://doi.org/10.1038/s41409-021-01317-7.
Choi Y, Choi EJ, Lee JH, Lee KH, Jo JC, Park HS, et al. Second allogeneic hematopoietic stem cell transplantation in patients with acute leukemia relapsed after allogeneic hematopoietic stem cell transplantation. Clin Transplant. 2021;35:e14199.
Greinix HT, Eikema DJ, Koster L, Penack O, Yakoub-Agha I, Montoto S, et al. Improved outcome of patients with graft-versus-host disease after allogeneic hematopoietic cell transplantation for hematologic malignancies over time: an EBMT mega-file study. Haematologica. 2022;107:1054–63. https://doi.org/10.3324/haematol.2020.265769.
Christopeit M, Kuss O, Finke J, Bacher U, Beelen DW, Bornhauser M, et al. Second allograft for hematologic relapse of acute leukemia after first allogeneic stem-cell transplantation from related and unrelated donors: the role of donor change. J Clin Oncol. 2013;31:3259–71. https://doi.org/10.1200/JCO.2012.44.7961.
Funding
The authors thank the following funding agencies for supporting their work: José Carreras Leukämie-Stiftung (3R/2019, 23R/2021), Deutsche Krebshilfe (70113519), Deutsche Forschungsgemeinschaft (PE 1450/7-1, PE 1450/9-1) and Stiftung Charité BIH (BIH_PRO_549, Focus Group Vascular Biomedicine). Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
MA and CP performed statistical analyses; OP and ZP designed the study and wrote the manuscript; WB collected and analysed data; NK, RZ, FC, TS, PD, JP, JS, MS, IWB, G-NF, KR, HS and IM performed research. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
HS: personal fees from Incyte, Janssen, Novartis, Sanofi, and from the Belgian Hematological Society (BHS), as well as research grants from Novartis and the BHS, all paid to her institution and not directly related to this work. She has also received non-financial support (travel grants) from Gilead, Pfizer, the EBMT (European Society for Blood and Marrow transplantation), and the CIBMTR (Center for International Bone Marrow Transplantation Research). OP has no COIs directly related to this work. OP has received honoraria or travel support from Gilead, Jazz, MSD, Neovii, Novartis, Pfizer, and Therakos. He has received research support from Incyte and Priothera. He is member of advisory boards to Equillium Bio, Jazz, Gilead, Novartis, MSD, Omeros, Priothera, Sanofi, Shionogi, and SOBI. RZ: Honorarium from Novartis, Incyte, MNK (Therakos), medac, Neovii. The remaining authors declare no competing interests.
Ethical approval
The study was approved by the EBMT review board. Patients had to sign an informed consent document that permitted sharing of clinical data according to national rules.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Penack, O., Abouqateb, M., Peczynski, C. et al. How risky is a second allogeneic stem cell transplantation?. Leukemia 38, 1799–1807 (2024). https://doi.org/10.1038/s41375-024-02318-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-024-02318-3
This article is cited by
-
Survival outcomes and risk factors of second allogeneic hematopoietic cell transplantation in non-remission status for acute myeloid leukemia
Bone Marrow Transplantation (2025)
-
A randomized controlled trial of conventional GVHD prophylaxis with or without teprenone for the prevention of severe acute GVHD
Annals of Hematology (2025)
-
Offering patients a second chance: what is the minimum cure rate needed to justify allogeneic hematopoietic cell transplantation?
Leukemia (2024)